You're using an outdated browser. Please upgrade to a modern browser for the best experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ian Curthoys | + 1634 word(s) | 1634 | 2022-03-01 05:12:06 | | | |
| 2 | Conner Chen | -22 word(s) | 1612 | 2022-03-04 01:48:36 | | | | |
| 3 | Conner Chen | -89 word(s) | 1523 | 2022-03-11 07:42:05 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Curthoys, I. Bilateral Vestibular Dysfunction. Encyclopedia. Available online: https://encyclopedia.pub/entry/20132 (accessed on 26 December 2025).
Curthoys I. Bilateral Vestibular Dysfunction. Encyclopedia. Available at: https://encyclopedia.pub/entry/20132. Accessed December 26, 2025.
Curthoys, Ian. "Bilateral Vestibular Dysfunction" Encyclopedia, https://encyclopedia.pub/entry/20132 (accessed December 26, 2025).
Curthoys, I. (2022, March 03). Bilateral Vestibular Dysfunction. In Encyclopedia. https://encyclopedia.pub/entry/20132
Curthoys, Ian. "Bilateral Vestibular Dysfunction." Encyclopedia. Web. 03 March, 2022.
Copy Citation
Patients with dysfunction of both vestibular systems of the inner ear experience postural instability and gait disturbances. This condition is called Bilateral Vestibular Dysfunction (BVD).
bilateral vestibular dysfunction
otolith
saccular
utricular
postural stability
1. The Saccular Substitution Hypothesis
With this saccular ES, patients show improved postural stability and gait performance and so the question of central importance consists of how constant electrical stimulation of saccular afferents in these BVD patients could cause this improved performance. This maintained stimulation is generating a barrage of action potentials in the saccular nerve, which is substituting for the reduced or absent saccular afferent action potentials in these BVD patients. The saccular macula is unique in that saccular receptors are continuously stimulated by the force of gravity. There are a large number of primary saccular afferents (around 4000 in humans, [1][2]) and these saccular afferents have high resting discharge rates (probably around 50–100 spikes/s in humans [3]), so each second, a huge barrage of action potentials is continuously reaching the vestibular nuclei and the midline cerebellum and, indirectly projecting on to other structures, e.g., the basal ganglia. Bilateral vestibular loss will reduce or remove this sustained neural input, and it appears that the high-frequency electrical stimulation by the implanted vestibular electrode is substituting for this lost natural neural activation.
2. Saccular Projections
As is clear from the anatomical evidence of the direct and indirect central projections of saccular afferents, their neural activity has very widespread effects in motor control systems. Saccular afferents project to brainstem vestibular nuclei, to spinal cord, and to cerebellum and indirectly through the cerebellum to the basal ganglia and to other structures, which have been of great recent interest in motor control, especially the pedunculopontine nucleus (PPN) of the mesencephalic locomotor region (MLR). Therefore, the activation of saccular afferents is indirectly activating many motor control systems.
Saccular afferents travel in the inferior vestibular nerve, along with afferents from the posterior semicircular canal. Saccular afferent fibres branch and send a thinner collateral branch to the cerebellum, with the thicker afferent branch projecting to the vestibular nuclei. Saccular fibres terminate in the lateral and inferior vestibular nuclei. Anatomical evidence shows that saccular afferents project both to vestibular nuclei and the cerebellum, specifically to the anterior midline structures, including the uvula [4][5][6] and deep cerebellar nuclei [7] (see Figure 1).
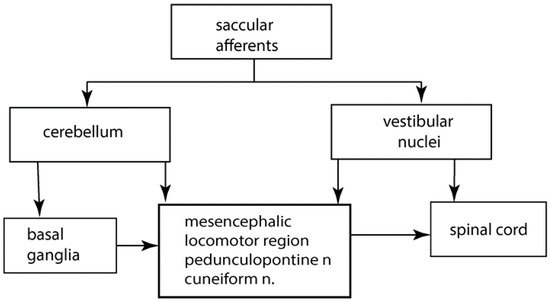
Figure 1. A greatly simplified schematic overview of some of the major structures involved in the control of gait and postural stability.
3. The Role of the Cerebellum in Posture and Gait
There is extensive evidence of the crucial role of the cerebellum in the control of balance and locomotion (see, e.g., [8]). Cerebellar circuits connect with many brain and spinal cord nuclei. Cerebellar activity is required for motor behaviours ranging from coordination to posture and balance and gait. This is clear from the deficits in these behaviours in human patients with localized cerebellar damage [8]. Projections from the deep cerebellar nuclei influence basal ganglia activity by afferents that project to the thalamic nuclei, which project to the basal ganglia primarily the striatum [8]. Dijkstra reported the cerebellum is involved in postural control as shown by image analysis [9]. Mori et al. [10] demonstrated in cats that stimulation of the midline cerebellar locomotor region (the fastigial nucleus) can independently induce locomotion. Neuroimaging suggests a similar region exists in humans. Studies with mental imagery of gait or foot pedals showed that active stepping during fMRI causes focal increases in the fastigial nucleus of the cerebellum and cerebellar vermis [9]. The cuneiform nucleus also appears to be of interest.
Many studies have suggested the cerebellum is implicated in aspects of the pathophysiology of Parkinson’s disease. One of the most characteristic signs of cerebellar damage is walking ataxia. Anterior cerebellar damage (to the so-called vestibulo-cerebellum) leads to increased postural sway [8][11][12]. Cerebellar damage is also associated with hypermetric postural responses to surface displacements and impaired ability to learn responses to predictable perturbations or step initiation [13]. Some of these deficits that are described for patients with cerebellar loss appear to be similar to those described for patients with bilateral vestibular loss.
4. Cerebellar Locomotor Region
The cerebellar locomotor region is in the midline of the cerebellum [14], and as noted above, it is this region where saccular afferents terminate. Neurons from the central cerebellum project back to the vestibular nuclei [1][4][5][6][7][15][16][17]. Physiological studies from animals suggest that cerebellar control of posture, equilibrium, and locomotion are tightly controlled and localised in the medial zone. There are cerebellar projections to vestibular and reticular nuclei and also to the thalamus. Thus, the medial cerebellar zone can integrate spinal and vestibular inputs and influence motor pathways for walking.
5. Cerebellum to Basal Ganglia
Manto elaborated on the short latency connections between the cerebellum and the basal ganglia [18][19][20]. These connections may explain the cerebellar involvement in disorders commonly associated with basal ganglia dysfunction, for example, Parkinson’s disease. Recently, cerebellar neurons are being stimulated in human patients by brain stimulation techniques, including transcranial magnetic stimulation and transcranial direct current stimulation, to alleviate disturbances of motor control [10][21][22][23][24]. In an animal model, Miterko reported that neuromodulation of the cerebellum rescues movement in a mouse model of ataxia [21].
6. The Pedunculopontine Nucleus (PPN)
The mesencephalic locomotor region (MLR) consists of the pedunculopontine tegmental nucleus (PPN), the cuneiform nucleus, and the sub-cuneiform nucleus. It receives input from cerebellar nuclei and basal ganglia [25][26][27]. Imaging of the PPN during imagined walking shows that it is involved in control of postural stability [28][29][30], and electrical stimulation of PPN is a target for treating locomotion deficits [31].
7. Vestibular Nuclei to Basal Ganglia
The most convincing evidence that there may be disynaptic projections from the vestibular nuclei to the basal ganglia was published by Lai et al. [32]. Using neuronal tracers, they reported that projections from the medial vestibular nucleus to the parafascicular nucleus (PFN) of the thalamus synapse on neurons that project to the dorsolateral putamen of the striatum. This anatomical evidence suggested the possibility of a disynaptic pathway between the vestibular nuclei and the striatum albeit from the medial vestibular nucleus rather than the inferior and lateral vestibular nuclei, where saccular afferents are known to terminate. The PFN is also strongly connected to the PPN [33]. Although there have been a small number of electrophysiological studies investigating whether electrical stimulation of the peripheral vestibular system can evoke field potentials and single-unit activity in the striatum [33][34], none of these can exclude the possibility that any responses arise via the cerebellum, and none of them are involved selective saccular stimulation. Nonetheless, there are many regions of the striatum that have not been explored, such the striatal tail, which is known to receive substantial visual and auditory sensory input and may also receive vestibular input [35]. Whether saccular information is transmitted to this multisensory integration centre remains to be determined but seems very likely.
8. Gait
Normal gait is a complex process that involves concomitant balance and locomotion processes. A hierarchy of supraspinal regions send signals to the central pattern generators (CPGs) of the spinal cord [30][36]. Supraspinal regions modify stereotyped locomotion in certain situations, such as initiating gait, turning, stopping, and avoiding obstacles. The locomotor network involves CPGs, mesencephalic locomotor region, the cerebellar locomotor areas, subthalamic locomotor region, and various cortical areas, including frontal and parietal supplementary motor and motor areas [30].
9. Contribution of the Otoliths to Spatial Awareness
Part of any qualitative improvement in BVD patients following ES may be attributable to its effects on higher centres of the brain concerned with the cognitive processing of vestibular information, especially spatial awareness and memory (“spatial cognition”). The effects of inferior vestibular nerve ES on spatial cognition have not been investigated quantitatively in humans; therefore, the only evidence available is from rodents.
There has been increasing evidence that the otoliths, independently of the semi-circular canals, may be important for spatial cognition. It is difficult to surgically manipulate the saccule in animal models without affecting the other vestibular sensors. Therefore, the only evidence available is restricted to mutant mice, which do not generate otoconia (e.g., tilted Het and Otop mice [34]). Inevitably, this means that the mice are devoid of both utricular and saccular function rather than just saccular function. However, due to its role in the perception of gravity, the saccule might be expected to be particularly important in providing a gravitational reference frame for other sensory information. A recent study in rats reported that selective electrical stimulation of the saccule resulted in widespread activation of the bilateral hippocampus, a structure that is important for spatial memory [37]. Quantitative gait analysis has not been performed on these otolith-deficient mouse models as yet; however, they do exhibit substantial deficits on the rotarod, in exploration and in performance in Y maze, radial arm maze, elevated plus maze, and place recognition tasks [34]. These results suggest that loss of otolithic function, including saccular function, results in deficits in spatial cognition. Studies of the first 10 days of development indicate that Het mice develop abnormally, exhibiting abnormal responses in the righting reflex, cliff drop aversion, and negative geotaxis tests [38]. There is also evidence that thalamic head direction cells and hippocampal place cells function abnormally in otolith-deficient mice [34]. Taken together, these studies suggest that the saccule is important for spatial awareness and orientation and that saccular ES may have a beneficial effect in BVD patients.
References
- Buttner-Ennever, J.A. A review of otolith pathways to brainstem and cerebellum. In Otolith Function in Spatial Orientation and Movement; Cohen, B., Hess, B.J.M., Eds.; The New York Academy of Science: New York, NY, USA, 1999; Volume 871, pp. 51–64.
- Won, S.L.; Suarez, C.; Honrubia, V.; Gomez, J. Morphological aspects of the human vestibular nerve. Laryngoscope 1990, 100, 756–764.
- Goldberg, J.M. Afferent diversity and the organization of central vestibular pathways. Exp. Brain Res. 2000, 130, 277–297.
- Maklad, A.; Fritzsch, B. Partial segregation of posterior crista and saccular fibers to the nodulus and uvula of the cerebellum in mice, and its development. Dev. Brain Res. 2003, 140, 223–236.
- Maklad, A.; Kamel, S.; Wong, E.; Fritzsch, B. Development and organization of polarity-specific segregation of primary vestibular afferent fibers in mice. Cell Tissue Res. 2010, 340, 303–321.
- Gacek, R.R. The course and central termination of first order neurons supplying vestibular endorgans in the cat. Acta Otolaryngol. Suppl. 1969, 254, 1–66.
- Barmack, N.H.; Baughman, R.W.; Errico, P.; Shojaku, H. Vestibular primary afferent projection to the cerebellum of the rabbit. J. Comp. Neurol. 1993, 327, 521–534.
- Morton, S.M.; Bastian, A.J. Cerebellar control of balance and locomotion. Neuroscientist 2004, 10, 247–259.
- Dijkstra, B.W.; Bekkers, E.M.J.; Gilat, M.; de Rond, V.; Hardwick, R.M.; Nieuwboer, A. Functional neuroimaging of human postural control: A systematic review with meta-analysis. Neurosci. Biobehav. Rev. 2020, 115, 351–362.
- Mori, S.; Matsui, T.; Kuze, B.; Asanome, M.; Nakajima, K.; Matsuyama, K. Stimulation of a restricted region in the midline cerebellar white matter evokes coordinated quadrupedal locomotion in the decerebrate cat. J. Neurophysiol. 1999, 82, 290–300.
- Mauritz, K.H.; Dichgans, J.; Hufschmidt, A. Quantitative-analysis of stance in late cortical cerebellar atrophy of the anterior lobe and other forms of cerebellar-ataxia. Brain 1979, 102, 461–482.
- Horak, F.B.; Diener, H.C. Cerebellar control of postural scaling and central set in stance. J. Neurophysiol. 1994, 72, 479–493.
- Timmann, D.; Horak, F.B. Perturbed step initiation in cerebellar subjects: 2. Modification of anticipatory postural adjustments. Exp. Brain Res. 2001, 141, 110–120.
- Jahn, K.; Deutschlader, A.; Stephan, T.; Kalla, R.; Wiesmann, M.; Strupp, M.; Brandt, T. Imaging human supraspinal locomotor centers in brainstem and cerebellum. Neuroimage 2008, 39, 786–792.
- Imagawa, M.; Graf, W.; Sato, H.; Suwa, H.; Isu, N.; Izumi, R.; Uchino, Y. Morphology of single afferents of the saccular macula in cats. Neurosci. Lett. 1998, 240, 127–130.
- Korte, G.E.; Mugnaini, E. Cerebellar projection of the vestibular nerve in the cat. J. Comp. Neurol. 1979, 184, 265–277.
- Uchino, Y.; Sato, H.; Suwa, H. Excitatory and inhibitory inputs from saccular afferents to single vestibular neurons in the cat. J. Neurophysiol. 1997, 78, 2186–2192.
- Bostan, A.C.; Dum, R.P.; Strick, P.L. The basal ganglia communicate with the cerebellum. Proc. Natl. Acad. Sci. USA 2010, 107, 8452–8456.
- Bostan, A.C.; Dum, R.P.; Strick, P.L. Cerebellar networks with the cerebral cortex and basal ganglia. Trends Cogn. Sci. 2013, 17, 241–254.
- Manto, M.; Argyropoulos, G.P.D.; Bocci, T.; Celnik, P.A.; Corben, L.A.; Guidetti, M.; Koch, G.; Priori, A.; Rothwell, J.C.; Sadnicka, A.; et al. Consensus Paper: Novel directions and next steps of non-invasive brain stimulation of the cerebellum in health and disease. Cerebellum 2021.
- Miterko, L.N.; Baker, K.B.; Beckinghausen, J.; Bradnam, L.V.; Cheng, M.Y.; Cooperrider, J.; DeLong, M.R.; Gornati, S.V.; Hallett, M.; Heck, D.H.; et al. Consensus Paper: Experimental neurostimulation of the cerebellum. Cerebellum 2019, 18, 1064–1097.
- Miterko, L.N.; Lin, T.; Zhou, J.; van der Heijden, M.E.; Beckinghausen, J.; White, J.J.; Sillitoe, R.V. Neuromodulation of the cerebellum rescues movement in a mouse model of ataxia. Nat. Commun. 2021, 12, 1295.
- Ponce, G.V.; Klaus, J.; Schutter, D. A brief history of cerebellar neurostimulation. Cerebellum 2021.
- Workman, C.D.; Fietsam, A.C.; Uc, E.Y.; Rudroff, T. Cerebellar transcranial direct current stimulation in people with Parkinson’s Disease: A pilot study. Brain Sci. 2020, 10, 96.
- Hazrati, L.N.; Parent, A. Projection from the deep cerebellar nuclei to the pedunculopontine nucleus in the squirrel-monkey. Brain Res. 1992, 585, 267–271.
- Raghu, A.L.B.; Parker, T.; Zand, A.P.D.; Payne, S.; Andersson, J.; Stein, J.; Aziz, T.Z.; Green, A.L. Tractography patterns of pedunculopontine nucleus deep brain stimulation. J. Neural Transm. 2021, 128, 659–670.
- Tubert, C.; Galtieri, D.; Surmeier, D.J. The pedunclopontine nucleus and Parkinson’s disease. Neurobiol. Dis. 2019, 128, 3–8.
- Takakusaki, K.; Chiba, R.; Nozu, T.; Okumura, T. Brainstem control of locomotion and muscle tone with special reference to the role of the mesopontine tegmentum and medullary reticulospinal systems. J. Neural Transm. 2016, 123, 695–729.
- Molina, R.; Hass, C.J.; Sowalsky, K.; Schmitt, A.C.; Opri, E.; Roper, J.A.; Martinez-Ramirez, D.; Hess, C.W.; Foote, K.D.; Okun, M.S.; et al. Neurophysiological correlates of gait in the human basal ganglia and the PPN region in Parkinson’s Disease. Front. Hum. Neurosci. 2020, 14, 194.
- Takakusaki, K. Functional neuroanatomy for posture and gait control. J. Mov. Disord. 2017, 10, 1–17.
- Noga, B.R.; Guest, J.D. Combined neuromodulatory approaches in the central nervous system for treatment of spinal cord injury. Curr. Opin. Neurol. 2021, 34, 804–811.
- Lai, H.; Tsumori, T.; Shiroyama, T.; Yokota, S.; Nakano, K.; Yasui, Y. Morphological evidence for a vestibulo-thalamo-striatal pathway via the parafascicular nucleus in the rat. Brain Res. 2000, 872, 208–214.
- Stiles, L.; Smith, P.F. The vestibular-basal ganglia connection: Balancing motor control. Brain Res. 2015, 1597, 180–188.
- Smith, P.F. The growing evidence for the importance of the otoliths in spatial memory. Front. Neural Circuits 2019, 13, 66.
- Valjent, E.; Gangarossa, G. The tail of the striatum: From anatomy to connectivity and function. Trends Neurosci. 2021, 44, 203–214.
- Cai, J.Y.; Lee, S.; Ba, F.; Garg, S.; Kim, L.J.; Liu, A.P.; Kim, D.; Wang, Z.J.; McKeown, M.J. Galvanic vestibular stimulation (GVS) augments deficient pedunculopontine nucleus (PPN) connectivity in mild Parkinson’s Disease: fMRI effects of different stimuli. Front. Neurosci. 2018, 12, 101.
- Hitier, M.; Zhang, Y.F.; Sato, G.; Besnard, S.; Zheng, Y.W.; Smith, P.F. Stratification of hippocampal electrophysiological activation evoked by selective electrical stimulation of different angular and linear acceleration sensors in the rat peripheral vestibular system. Hear. Res. 2021, 403, 108173.
- Le Gall, A.; Hilber, P.; Chesneau, C.; Bulla, J.; Toulouse, J.; Machado, M.L.; Philoxene, B.; Smith, P.F.; Besnard, S. The critical role of vestibular graviception during cognitive-motor development. Behav. Brain Res. 2019, 372, 112040.
More
Information
Subjects:
Otorhinolaryngology; Physiology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
844
Revisions:
3 times
(View History)
Update Date:
11 Mar 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




