2. Stimulation of Prostaglandylinositol Cyclic Phosphate (cyclic PIP) Synthesis by Metformin
All biguanides tested (metformin, buformin and phenformin) activated cyclic PIP synthase
[8]. Metformin increased it by 1.9 to 2-fold; buformin 3.0 to 3.2-fold and phenformin 3.5 to 4.2-fold (
Table 1). The most likely action of the biguanides is the activation of the insulin receptor tyrosine kinase
[9], which then activates cyclic PIP synthase by tyrosine phosphorylation
[10], leading to elevated cyclic PIP synthesis. Interestingly, this activation is additive to the activation of cyclic PIP synthase by fluoride
[11][10], which was suggested to activate G proteins comparably to the activation of adenylate cyclase via G-proteins. Fluoride may also act as a protein tyrosine phosphatase inhibitor. In this case, fluoride inhibits the dephosphorylation of the, by tyrosine phosphorylation, activated cyclic PIP synthase
[10]. However, the activation of basal cyclic PIP synthase by fluoride in the absence of ATP can best be explained by the action of a G protein (
Table 1), suggesting that fluoride may exert two independent effects on cyclic PIP synthase.
Table 1. Activation of particular cyclic PIP synthase by various chemicals and drugs.
| |
Cyclic PIP Synthase Activity |
|
| Addition |
Activation (x-fold) |
Inhibition (%) |
| Experiment 1 |
|
|
| No Additions (basal activity) |
1.0 |
|
| Fluoride (10mM) |
3.2 |
|
| ATP (10mM) |
10.6 |
|
| ATP + Fluoride |
14.4 |
|
| Experiment 2 |
|
|
| Metformin (0.5mM) |
2.1 |
|
| Buformin (1.0 mM) |
3.05 |
|
| Phenformin (1.0 mM) |
4.2 |
|
| Glibenclamide (0.1 mM) |
|
66 |
| (1.0 mM) |
|
99 |
| Chloropropamide (1.0 mM) |
|
95 |
| Tolbutamide (1.0 mM) |
|
97 |
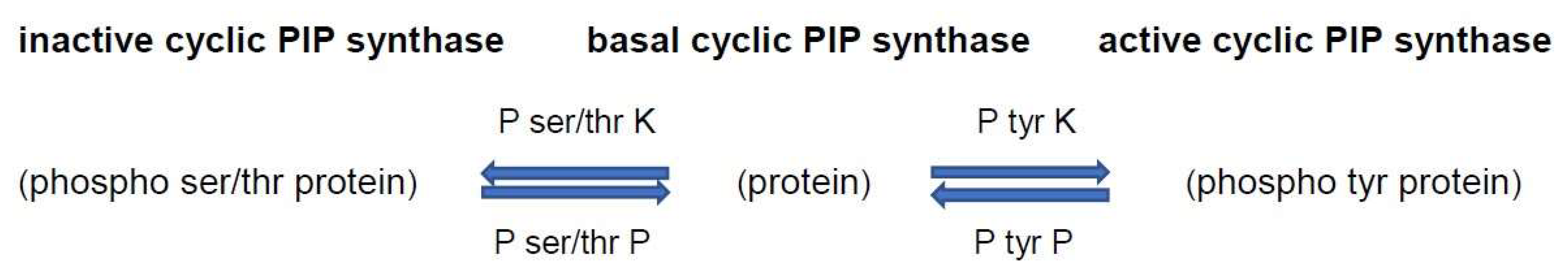
The activation of cyclic PIP synthase by tyrosine phosphorylation
[10] predicts that it is inactivated by dephosphorylation (
Figure 1). Consequently, a further way to keep cyclic PIP synthase active is to inhibit this protein tyrosine phosphatase. The antihypertensive drug captopril was shown to inhibit protein tyrosine phosphatase
[12] and it was shown that captopril and other antihypertensive drugs increase the synthesis of cyclic PIP
[8]. This suggests that a more efficient treatment of type 2 diabetics should be to treat these patients with a combination of these 2 drugs or their alternatives.
Figure 1. Regulation of the activity of cyclic PIP synthase by protein tyrosine and protein ser-ine/threonine phosphorylation and dephosphorylation. On insulin stimulation, the insulin receptor protein tyrosine kinase is activated and activates by tyrosine phosphorylation cyclic PIP synthase. Increased cyclic AMP levels activate P ser/thr kinases, which then inhibit cyclic PIP synthase. Abbreviations: P tyr K, protein tyrosine kinase; P tyr P, protein tyrosine phosphatase; P ser/thr K, protein serine/threonine kinase; P ser/thr P, protein serine/threonine phosphatase.
Past experience has shown that metformin loses its efficacy to help type 2 diabetics within five to ten years of treatment. A more recent observation is that metformin helps diabetics for a longer time
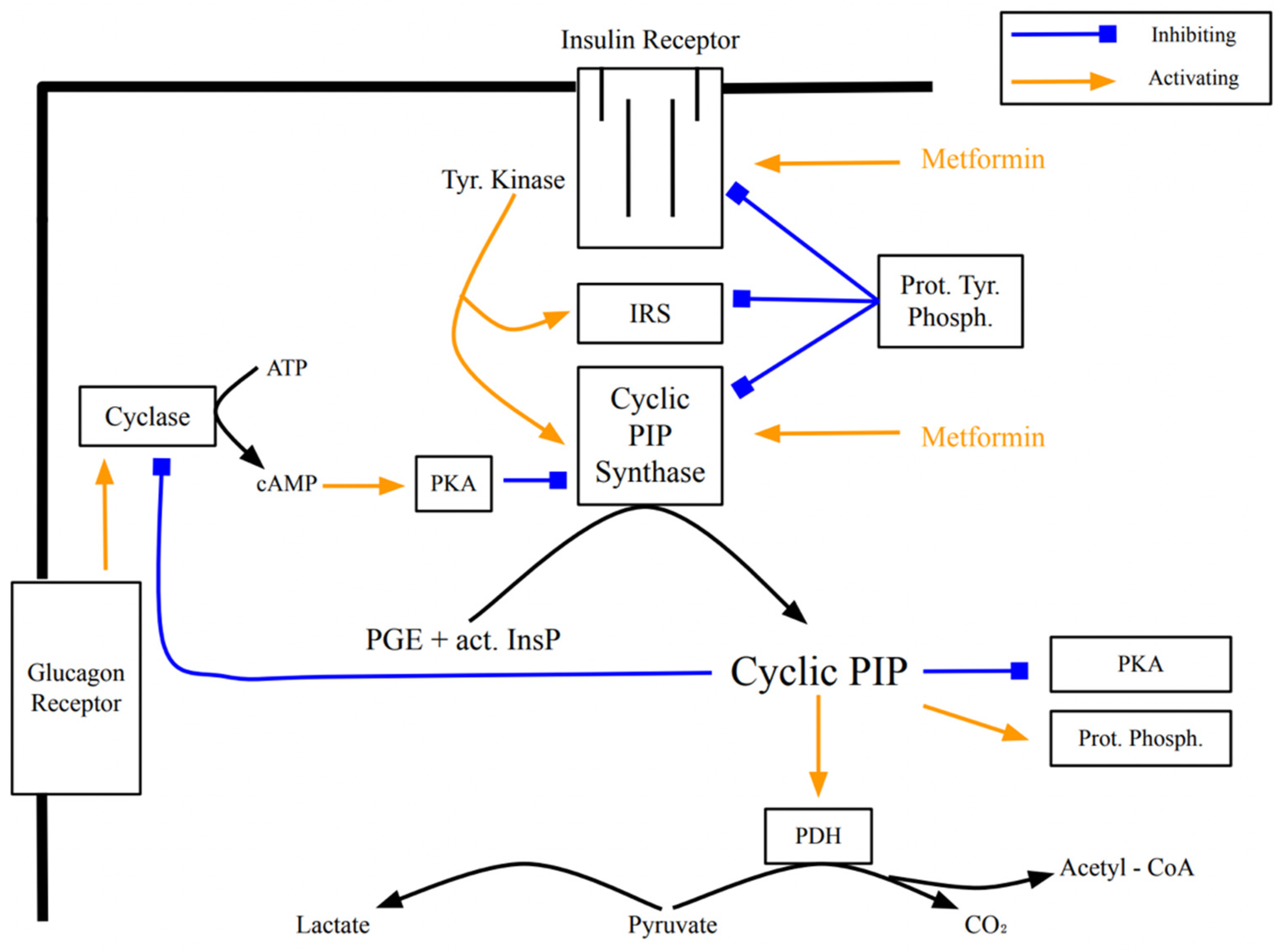
[13]. Metformin appears to act intracellularly on the insulin receptor (
Figure 2), activating down-stream signaling
[14]. The progression of insulin resistance is connected to a decreasing receptor density
[15][16]. Thus, the primary site of action of metformin and the location where effects of insulin resistance begin are closely located. It is of interest to ask whether the efficacy of metformin is affected by the decreasing receptor density. The two regulators, cyclic AMP and cyclic PIP, inhibit the synthesis and action of each other
[17]. There are at least two ways by which cyclic PIP synthesis is inhibited. Metformin activates cyclic PIP synthesis very well, but it is not yet known whether it shuts off the inhibitory mechanisms, such as (a) inactivation of cyclic PIP synthase by protein tyrosine dephosphorylation
[10] and (b) inhibition by protein serine/threonine phosphorylation, which is triggered by cyclic AMP
[8]. Better understanding of the interplay of the inhibitory mechanisms on cyclic PIP synthase will further improve the treatment of diabetics.
Figure 2. Signal transduction of insulin and the activation of cyclic PIP synthase by tyrosine phosphorylation, and regulatory effects of cyclic PIP. The points of action of metformin are indicated. (used abbreviations: Tyr Kinase is the insulin receptor tyrosine kinase; Prot Phosph the protein ser/thr phosphatase; act InsP the activated inositol cyclic phosphate; Cyclase the adenylate cyclase; Prot Tyr Phosph a protein tyr phosphatase; PDH the pyruvate dehydrogenase and PKA protein kinase A).
A rare but serious side effect of the biguanides is that they can cause lactic acidosis
[18][19]. A puzzling point is that the biguanides buformin and phenformin are more efficient in stimulating cyclic PIP synthase than metformin and one would expect that these biguanides would be more beneficiary for diabetics. It is rather peculiar that these more efficient biguanides are the ones with the greater risk to cause lactic acidosis. Could this problem of lactic acidosis be connected to overdosage of these drugs, especially as overdosing of insulin results in hypo-glycemia? The same problem is expected in cases of excess levels of cyclic PIP. Cyclic PIP stimulates the pyruvate dehydrogenase complex up to 5-fold
[17]. This means that the intracellularly produced pyruvate should not be converted to lactate but to acetyl-CoA, and the question remains why is pyruvate transformed in high amounts to lactate. One possibility is that in these patients the biguanide could not increase cyclic PIP synthesis. This could result, for instance, from consumption of easily accessible, non-steroidal-anti-inflammatory drugs, which inhibit prostaglandin synthesis preventing cyclic PIP synthesis
[20]. Furthermore, type 1 and type 2 diabetes have a tendency to develop lactic acidosis, and a large study showed an incidence rate of 3% for the patients with diabetes mellitus compared to an incidence rate of 0.1% in the non-diabetic control group
[21]. Other studies came to the conclusion that the use of metformin did not increase the risk of lactic acidosis
[22][23]. However, the well-recognized risk of lactic acidosis is feared because of its high mortality rate
[24]. Though risk-factors for the development of lactic acidosis, such as hypoxia, sepsis, dehydration, and worsening of renal and cardiac failure, are recognized, more studies are needed to fully understand the problem at a molecular level and to further reduce/prevent the occurrence of lactic acidosis in type 2 diabetic patients on treatment with metformin. A question that needs to be answered is: Is the development of lactic acidosis more the result of other risk factors in the diabetic patient or does it solely result from the treatment with biguanides? All biguanides stimulate the synthesis of cyclic PIP, which activates pyruvate dehydrogenase, preventing the overproduction of lactate. Thus, it should not be a biguanide that initiates overproduction of lactate leading to lactic acidosis.
The goal of research should be to ask for a more effective drug than metformin. One can remember phenformin, which activates the biosynthesis of cyclic PIP twice as effectively as metformin, though phenformin was taken from the market because of the lactic acidosis problem. If causes of lactic acidosis become better understood and found not to be the result of the action of the biguanides, a drug such as phenformin might be reconsidered.
The regulation of the activity of cyclic PIP synthase appears to be modulated by many drugs. For instance, antidiabetic drugs of the sulfonylurea group inhibit cyclic PIP synthase (
Table 1)
[8]. These drugs are known to increase insulin secretion and one may ask if this can be beneficiary, especially when one takes into consideration that insulin and cyclic PIP switch off the secretion of insulin
[17]. Further, it is known that in the early stage of type 2 diabetes patients have elevated serum insulin levels, most likely as a result of the reduced or lost switch off mechanism. Certainly, one can see the increase in insulin secretion as positive and helpful, but one needs to be aware that these drugs inhibit cyclic PIP synthase not only in the β-cells of the pancreas but in all cells of a body
[8] and this inhibition will decrease the efficacy of insulin.