
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | In-Sung Yeo | + 1336 word(s) | 1336 | 2019-12-31 10:39:45 | | | |
| 2 | In-Sung Yeo | + 1336 word(s) | 1336 | 2020-01-02 03:38:03 | | | | |
| 3 | Nicole Yin | -4 word(s) | 1332 | 2020-01-02 06:36:33 | | | | |
| 4 | Nicole Yin | -4 word(s) | 1332 | 2020-01-02 06:50:39 | | | | |
| 5 | Nicole Yin | Meta information modification | 1332 | 2020-07-31 05:30:14 | | | | |
| 6 | Nicole Yin | -8 word(s) | 1324 | 2020-10-23 03:27:53 | | |
Video Upload Options
Bone healing process at the interface between bone and implant surface includes haemostasis, inflammation, proliferation, and remodeling. The modifications of titanium dental implant surface that are globally marketed focus on early bone response to switch more quickly from inflammation to proliferation by roughening the surface at the micro-scale. Microstructural modifications change cell behavior around the modified surface, successfully enhancing osseointegration, but they have their own limits. For example, such a modified surface cannot avoid implant failure resulting from shear force because of the occlusal load on the bone-implant interface. This type of failure is able to be bypassed by providing the implant macrodesign with threads, which convert shear force into compressive force that the interface is more resistant to. Dental clinicians and researchers should consider both the implant macrostructure and microstructure to better understand clinical bone response to the dental implant, although this topic is based on the surface microstructural modification.
1. Sandblasted, Large-Grit, Acid-Etched (SLA) Surface
The computer numerical controlled milling of cp-Ti manufactures screw-shaped endosseous dental implants. The surface machined by this milling procedure, which is now called a turned Ti surface, shows many parallel grooves in scanning electron microscopy (SEM). The turned surface experiences no modification process, which has frequently served as a control to evaluate the biocompatibility of modified surfaces. When an implant is inserted into the bone and the implant surface becomes juxtaposed to the bone, bone healing (or osseointegration) on the surface is known to be fulfilled by two mechanisms: distance and contact osteogenesis[1][2]. In distance osteogenesis, new bone starts to be formed on the surfaces of bone. The direction of bone growth is from the bone towards the implant surface (Figure 1A). In contact osteogenesis, or de novo bone formation, new bone formation begins on the implant surface. The direction of bone growth is from the implant towards the bone, opposite to that for distance osteogenesis (Figure 1B). When an endosseous implant with a turned surface is placed into the jawbone, only distance osteogenesis occurs, which implies that more time is needed for sufficient osseointegration to withstand masticatory forces[1][3]. The necessity of reduction in the patient’s edentulous period has led the modification of an implant surface to accelerate bone healing.
The traditional approach to the surface modification of a Ti implant has been roughening at the micro-level. One of the most successful surfaces in clinical dentistry is the sandblasted, large-grit, and acid-etched (or SLA) surface. An SLA Ti surface is made by sandblasting the turned Ti surface with large-grit particles, the sizes of which range from 250 μm to 500 μm in general, and by acid-etching the blasted surface. The acids for etching are usually strong acids including hydrochloric, sulfuric, and nitric acids. SEM shows topographically changed irregularities on the SLA surface, with large dips, small micropits, sharp edges, and pointed tips. Sa, one of the surface parameters defined as the arithmetic mean height of the surface, is approximately 1.5 μm to 2 μm. Osteogenic cells migrate to the roughened Ti surface through the fibrin clot that is formed at the peri-implant site after bone drilling for implant insertion, and these cells appear to recognize the irregularities of the SLA surface as lacunae to be filled with bone materials[1][4]. Contact osteogenesis occurs as the osteogenic cells secrete a bone matrix. The occurrence of both contact and distance osteogenesis accelerates the osseointegration on the SLA surface compared to the turned surface.
The Ti surface of a dental implant is originally hydrophobic[5]. Water (H2O) is considered to have initial contact with the implant surface when the implant is inserted into the bone[6]. Therefore, there have been attempts to add hydrophilicity to an SLA surface, since hydrophilicity is expected to help accelerate the bone healing process[5][7]. A dental implant with a hydrophilic SLA surface, commercially called SLActive (Institute Straumann AG, Basel, Switzerland), is made with a water rinse of the original SLA implant in a nitrogen chamber and a packaging technique of storing the implant in an isotonic sodium chloride solution with no atmospheric contact, and this hydrophilic implant is being clinically used in the global market[8].
Regardless of whether an SLA surface is hydrophobic or hydrophilic, this dental implant surface has shown excellent long-term clinical results[9][10][11][12][13]. A previous 10-year retrospective study investigating more than 500 SLA Ti implants concluded that both the survival and success rates were 97% or higher[9]. The 10-year survival rate of SLA Ti implants was reported to be higher than 95%, even in periodontally compromised patients, although strict periodontal interventions were applied to these patients[11]. Similar results were found in 10-year prospective studies investigating the survival rates of dental implants with SLA surfaces[10][12][13]. This modified surface, roughened at the micro-scale, is one of the dental implant surfaces that has been most frequently tested in clinics for the longest period.
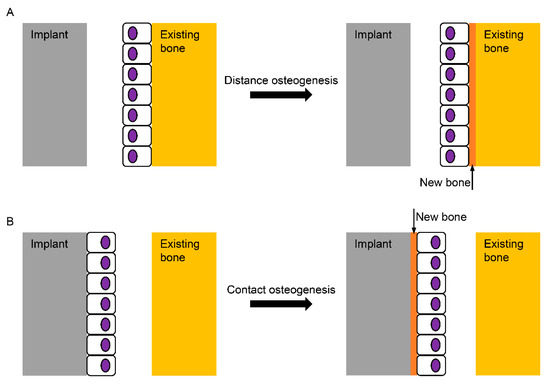
Figure 1. Schematic diagram for the healing mechanisms of the bone surrounding an implant. (A) In distance osteogenesis, the direction of bone formation is from the existing bone to the implant; (B) in contact osteogenesis, however, the direction is opposite, from the implant to the existing bone, which is known not to occur on the turned Ti (Titanium) surface without any modification.
2. Anodic Oxidation
The genuine biocompatible surface on the Ti dental implant is Ti oxide (TiO2), not Ti itself, which is spontaneously formed when the Ti surface is exposed to the atmosphere. However, this Ti oxide layer is very thin (a few nm in thickness) and is imperfect with defects[14]. Also, chemically unstable Ti3+ and Ti2+ are known to exist in the oxide layer[15]. Therefore, there have been several techniques developed to thicken and stabilize the Ti oxide layer, which is considered to increase the biocompatibility of the surface[16][17][18]. When Ti becomes the anode under an electric potential in an electrochemical cell, Ti is oxidized to be Ti4+, and the TiO2 layer is able to be thickened and roughened[6]. Topographically, the oxidized Ti surface for a dental implant has many volcano-like micropores with various sizes, which are observed in SEM. The surface characteristics of the anodized Ti surface depend on the applied potential, surface treatment time, concentrations, and types of electrolytes[6][18]. The arithmetic mean height of this surface, or Sa, is evaluated to be approximately 1 to 1.5 μm for dental use[19][20][21][22].
Osteogenic cells appear to recognize the topography of a dental implant surface although we do not yet know which surface topography is more proper in bone healing, or if the irregularities of the SLA surface are more effective for the osteogenic cell response than the microporous structure of the anodized surface[23]. To date, no in vivo model has found any significant differences in bone responses to the microtopographies of Ti dental implant surfaces[24][25]. What is definitely known about implant surface topography is that the cp-Ti surfaces topographically modified at the microscale accelerate osseointegration more than the turned surface, and these modified surfaces show superior results to the turned surface during in vitro, in vivo, and clinical studies.
The anodically oxidized Ti surface has shown superior results to the turned surface in various in vitro tests and in vivo histomorphometry[22][25][26][27]. A previous meta-analytic study reported lower failure rates of the oxidized Ti implants than those of the turned implants from the included 38 clinical investigations[28]. A prior retrospective and a 10-year prospective study concluded that that success rates were higher than 95% for the TiUnite surface (Brånemark System, Nobel Biocare, Göteborg, Sweden), which is a trade name for the oxidized Ti surface[29][30]. However, a recent 20-year randomized controlled clinical trial notably reported a similar marginal bone loss between micro-roughened and turned Ti implants[31]. This clinical study used an identical implant design with an implant-abutment connection structure and internal friction connection[32]. Identifying which of the two factors (surface characteristics and implant design) is a major contributor to the long-term clinical success of dental implants needs to be thoroughly investigated, although higher success or survival rates have been steadily published for Ti dental implants with modified surfaces at the micro-scale, compared to the turned implant[10][33][34].
References
- J E Davies; Mechanisms of endosseous integration.. The International Journal of Prosthodontics 1999, 11, 391–401.
- Osborn, J.; Newesely, H.; Dynamic aspects of the implant-bone interface. Dental implants 1980, 111, 123.
- P.-I. Brånemark; U. Breine; R. Adell; B. O. Hansson; J. Lindström; A. Ohlsson; Intra-Osseous Anchorage of Dental Prostheses: I. Experimental Studies. Scandinavian Journal of Plastic and Reconstructive Surgery 1969, 3, 81-100, 10.3109/02844316909036699.
- Natalie A. Sims; Jonathan H. Gooi; Bone remodeling: Multiple cellular interactions required for coupling of bone formation and resorption. Seminars in Cell & Developmental Biology 2008, 19, 444-451, 10.1016/j.semcdb.2008.07.016.
- F. Rupp; L. Scheideler; N. Olshanska; M. De Wild; M. Wieland; J. Geis-Gerstorfer; Enhancing surface free energy and hydrophilicity through chemical modification of microstructured titanium implant surfaces. Journal of Biomedical Materials Research Part A 2005, 76, 323-334, 10.1002/jbm.a.30518.
- Yeo, I.-S.. Bone Response to Dental Implant Materials; Woodhead Publishing: Cambridge, UK, 2017; pp. 43–64.
- F. Rupp; L. Scheideler; D. Rehbein; D. Axmann; J. Geis-Gerstorfer; Roughness induced dynamic changes of wettability of acid etched titanium implant modifications.. Biomaterials 2003, 25, 1429-1438, 10.1016/j.biomaterials.2003.08.015.
- Ivan Wall; Nikos Donos; Karin Carlqvist; Francis Jones; Peter Brett; Modified titanium surfaces promote accelerated osteogenic differentiation of mesenchymal stromal cells in vitro. Bone 2009, 45, 17-26, 10.1016/j.bone.2009.03.662.
- Daniel Buser; Simone F. M. Janner; Julia-Gabriela Wittneben; Urs Bragger; Christoph A. Ramseier; Giovanni E. Salvi; 10-Year Survival and Success Rates of 511 Titanium Implants with a Sandblasted and Acid-Etched Surface: A Retrospective Study in 303 Partially Edentulous Patients. Clinical Implant Dentistry and Related Research 2012, 14, 839-851, 10.1111/j.1708-8208.2012.00456.x.
- Nicolau, P.; Guerra, F.; Reis, R.; Krafft, T.; Benz, K.; Jackowski, J. 10-year outcomes with immediate and early loaded implants with a chemically modified SLA surface. Quintessence Int. 2018, 50, 2–12.
- Long-term results of a three arms prospective cohort study on implants in periodontally compromised patients: 10-year data around sandblasted and acid-etched (SLA) surface. British Dental Journal 2015, 218, 523-523, 10.1038/sj.bdj.2015.366.
- Fabio Rossi; Niklaus P. Lang; Emanuele Ricci; Lorenzo Ferraioli; Niccolò Baldi; Daniele Botticelli; Long-term follow-up of single crowns supported by short, moderately rough implants-A prospective 10-year cohort study. Clinical Oral Implants Research 2018, 29, 1212-1219, 10.1111/clr.13386.
- Frank J.J. Van Velzen; Ronen Ofec; Engelbert A.J.M. Schulten; Christiaan M. Ten Bruggenkate; 10-year survival rate and the incidence of peri-implant disease of 374 titanium dental implants with a SLA surface: a prospective cohort study in 177 fully and partially edentulous patients. Clinical Oral Implants Research 2014, 26, 1121-1128, 10.1111/clr.12499.
- Songmei Li; Wenhui Yao; Jianhua Liu; Mei Yu; Liang Wu; Kun Ma; Study on anodic oxidation process and property of composite film formed on Ti–10V–2Fe–3Al alloy in SiC nanoparticle suspension. Surface and Coatings Technology 2015, 277, 234-241, 10.1016/j.surfcoat.2015.07.050.
- T. Hanawa; Metal ion release from metal implants. Materials Science and Engineering: C 2004, 24, 745-752, 10.1016/j.msec.2004.08.018.
- T.M. Manhabosco; S.M. Tamborim; C.B. Dos Santos; I.L. Müller; Tribological, electrochemical and tribo-electrochemical characterization of bare and nitrided Ti6Al4V in simulated body fluid solution. Corrosion Science 2011, 53, 1786-1793, 10.1016/j.corsci.2011.01.057.
- Wang, J.W.; Ma, Y.; Guan, J.; Zhang, D.W. Characterizations of anodic oxide films formed on Ti6A14V in the silicate electrolyte with sodium polyacrylate as an additive. Surf. Coat Tech. 2018, 338, 14–21.
- Ling Zhang; Yanqing Duan; Rui Gao; Jianyun Yang; Keyi Wei; Danyu Tang; Tianlin Fu; The Effect of Potential on Surface Characteristic and Corrosion Resistance of Anodic Oxide Film Formed on Commercial Pure Titanium at the Potentiodynamic-Aging Mode. Materials 2019, 12, 370, 10.3390/ma12030370.
- Taek-Ka Kwon; Hyo-Jung Lee; Seung-Ki Min; In-Sung Yeo; Evaluation of Early Bone Response to Fluoride-Modified and Anodically Oxidized Titanium Implants Through Continuous Removal Torque Analysis. Implant Dentistry 2012, 21, 427-432, 10.1097/id.0b013e31826917f6.
- Hyo-Jung Lee; Il-Hyung Yang; Seong-Kyun Kim; In-Sung Yeo; Taek-Ka Kwon; In vivo comparison between the effects of chemically modified hydrophilic and anodically oxidized titanium surfaces on initial bone healing. Journal of Periodontal & Implant Science 2015, 45, 94-100, 10.5051/jpis.2015.45.3.94.
- Ann Wennerberg; Tomas Albrektsson; On implant surfaces: a review of current knowledge and opinions.. The International Journal of Oral & Maxillofacial Implants 2010, 25, , null.
- In-Sung Yeo; Jung-Suk Han; Jae-Ho Yang; Biomechanical and histomorphometric study of dental implants with different surface characteristics. Journal of Biomedical Materials Research Part B: Applied Biomaterials 2008, 87, 303-311, 10.1002/jbm.b.31104.
- Lyndon F. Cooper; A role for surface topography in creating and maintaining bone at titanium endosseous implants. The Journal of Prosthetic Dentistry 2000, 84, 522-534, 10.1067/mpr.2000.111966.
- Mauro Donati; Annika Ekestubbe; Jan Lindhe; Jan L Wennström; Marginal bone loss at implants with different surface characteristics - A 20-year follow-up of a randomized controlled clinical trial. Clinical Oral Implants Research 2018, 29, 480-487, 10.1111/clr.13145.
- Jung-Woo Koh; Young-Sung Kim; Jae-Ho Yang; In-Sung Yeo; Effects of a calcium phosphate-coated and anodized titanium surface on early bone response.. The International Journal of Oral & Maxillofacial Implants 2013, 28, 790-797, 10.11607/jomi.2783.
- In-Sung Yeo; Reality of Dental Implant Surface Modification: A Short Literature Review. The Open Biomedical Engineering Journal 2014, 8, 114-119, 10.2174/1874095201408010114.
- Kang, H.K.; Kim, O.B.; Min, S.K.; Jung, S.Y.; Jang, D.H.; Kwon, T.K.; Min, B.M.; Yeo, I.S. The effect of the DLTIDDSYWYRI motif of the human laminin alpha2 chain on implant osseointegration. Biomaterials 2013, 34, 4027–4037.
- Seung-Ki Min; Hyun Ki Kang; Da Hyun Jang; Sung Youn Jung; O. Bok Kim; Byung-Moo Min; In-Sung Yeo; Titanium Surface Coating with a Laminin-Derived Functional Peptide Promotes Bone Cell Adhesion. BioMed Research International 2013, 2013, 1-8, 10.1155/2013/638348.
- B. R. Chrcanovic; T. Albrektsson; A. Wennerberg; Turned versus anodised dental implants: a meta-analysis. Journal of Oral Rehabilitation 2016, 43, 716-728, 10.1111/joor.12415.
- Marco Degidi; Diego Nardi; Adriano Piattelli; 10-Year Follow-Up of Immediately Loaded Implants with TiUnite Porous Anodized Surface. Clinical Implant Dentistry and Related Research 2012, 14, 828-838, 10.1111/j.1708-8208.2012.00446.x.
- Yasuyuki Shibuya; Masaki Kobayashi; Junichiro Takeuchi; Tomoko Asai; Maho Murata; Masahiro Umeda; Takahide Komori; Analysis of 472 Brånemark system TiUnite implants:a retrospective study.. The Kobe journal of medical sciences 2010, 55, E73–E81.
- Yasuyuki Shibuya; Masaki Kobayashi; Junichiro Takeuchi; Tomoko Asai; Maho Murata; Masahiro Umeda; Takahide Komori; Analysis of 472 Brånemark system TiUnite implants:a retrospective study.. The Kobe journal of medical sciences 2010, 55, , null.
- R. Adell; U Lekholm; B. Rockler; P.-I. Brånemark; A 15-year study of osseointegrated implants in the treatment of the edentulous jaw. International Journal of Oral Surgery 1981, 10, 387-416, 10.1016/s0300-9785(81)80077-4.
- Antonio Rocci; Marta Rocci; Cecilia Rocci; Andrea Scoccia; Marco Gargari; Massimiliano Martignoni; Jan Gottlow; Lars Sennerby; Immediate loading of Brånemark system TiUnite and machined-surface implants in the posterior mandible, part II: a randomized open-ended 9-year follow-up clinical trial.. The International Journal of Oral & Maxillofacial Implants 2013, 28, 891-895, 10.11607/jomi.2397.




