
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Wangbo Jiao | + 3786 word(s) | 3786 | 2022-01-20 07:39:38 | | | |
| 2 | Lindsay Dong | Meta information modification | 3786 | 2022-03-02 06:23:52 | | |
Video Upload Options
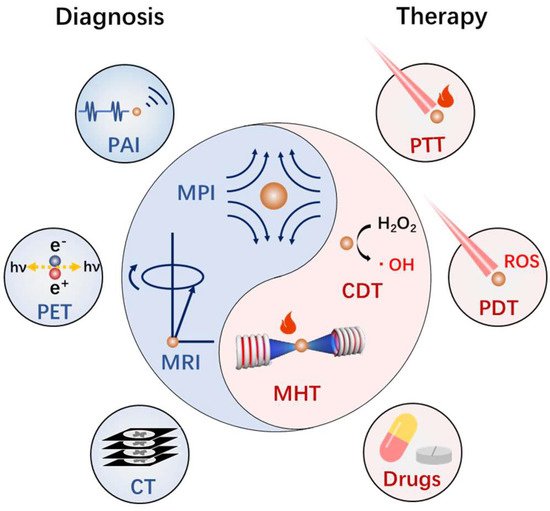
Cancer is the top cause of death globally. Developing smart nanomedicines that are capable of diagnosis and therapy (theranostics) in one–nanoparticle systems are highly desirable for improving cancer treatment outcomes. The magnetic nanoplatforms are the ideal system for cancer theranostics, because of their diverse physiochemical properties and biological effects. In particular, a biocompatible iron oxide nanoparticle based magnetic nanoplatform can exhibit multiple magnetic–responsive behaviors under an external magnetic field and realize the integration of diagnosis (magnetic resonance imaging, ultrasonic imaging, photoacoustic imaging, etc.) and therapy (magnetic hyperthermia, photothermal therapy, controlled drug delivery and release, etc.) in vivo. Furthermore, due to considerable variation among tumors and individual patients, it is a requirement to design iron oxide nanoplatforms by the coordination of diverse functionalities for efficient and individualized theranostics.
1. Introduction
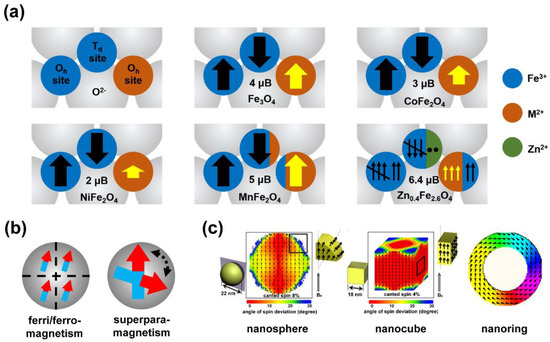
2. Controlled Synthesis of Magnetic Nanoplatforms
3. Basis of Magnetic Nanomaterials Mediated Diagnosis and Therapy of Cancer
3.1. Biosafety of Magnetic Nanoplatforms
3.2. Magnetic Resonance Imaging
3.3. Other Diagnosis Applications
3.4. Magnetic Hyperthermia
3.5. Chemodynamic Therapy
4. Implementation of Magnetotheranostic Based on Magnetic Nanoplatforms
4.1. Magnetotheranostics Based on Magnetic Nanoplatforms Only
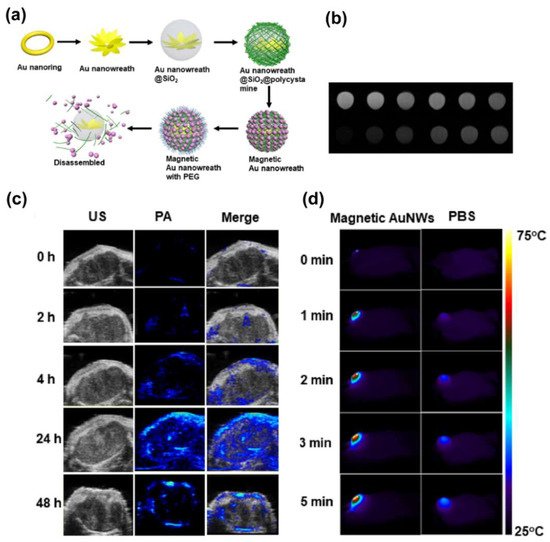
4.2. Integration of Magnetic Nanoplatforms with Phototheransotics
4.3. Integration of Magnetic Nanoplatforms with Fluorescence Imaging
Fluorescence imaging (FI) relies heavily on the penetration of light in tissue, which limits its application in the field of in vivo diagnosis and therapy. However, because fluorescent molecules can be designed to achieve highly specific responsiveness, fluorescence imaging is often used as an auxiliary imaging method for the magnetotheranostics nanoplatform to monitor its response behavior in the body. Zhou et al. [69] designed a nanoplatform capable of detecting tumor hypoxic environment, composed of ultrasmall SPIONs and assembly–responsive fluorescent dyes (NBD), and used nitroimidazole derivatives as hypoxia–sensitive detectors.
4.4. Integration of Magnetic Nanoplatforms with CT&PET/SPECT
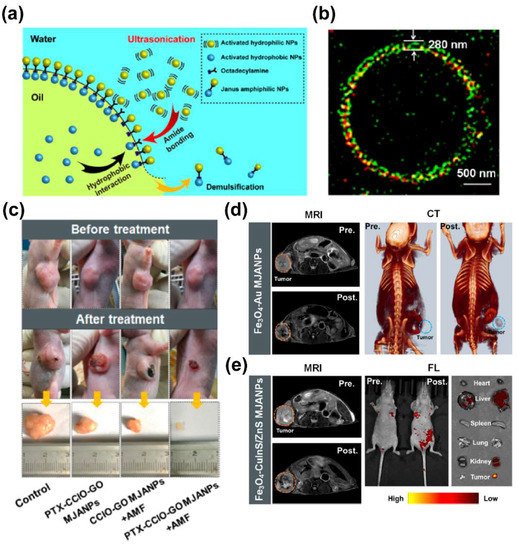
4.5. Magnetic Nanoplatforms Carrier Based Drug Delivery
The magnetotheranostic nanoplatform has become a representative delivery system due to its adjustable size, easy image tracking, and clear metabolic pathway. The delivery mechanism of MIONs can be divided into passive targeting and active targeting. Passive targeting mainly depends on the enhanced permeability and retention (EPR) effect of MIONs. Although the mechanism still needs to be further investigated [72][73], the EPR effect can indeed enhance the enrichment of nanoparticles (not just MIONs) in the tumor area [5]. In addition, the ligands of tumor characteristic markers can be employed to functionalize MIONs in order to give them the ability to actively target tumors [6], and for more efficient drug delivery. In a recent work, paclitaxel (PTX) and cisplatin (CDDP) have been loaded into the carboxymethyl dextran coating of the clinical iron supplement FMX, and actively target gliomas through HMC, which was an organic anion transport polypeptide targeting agent with near–infrared fluorescence. This system was used for MRI/FI visualized drug delivery of glioblastoma multiforme (GBM) [74]. Liu et al. [75] designed a delivery system with a Yolk–shell structure. The vesicles were composed of PEG–PPS–SS–PEG and loaded with ultrasmall SPIONs and DOX, which were encapsulated together by a polyacrylic acid coating. In tumor cells, vesicles were disintegrated under the influence of GSH. The complex of ultrasmall SPIONs and DOX was then separated, so that the drug release process could be monitored by an enhancement in T1 MRI. The complex microenvironment of the tumor tissue could limit the penetration of the nanoplatforms [76][77].
References
- Chen, H.; Zhang, W.; Zhu, G.; Xie, J.; Chen, X. Rethinking cancer nanotheranostics. Nat. Rev. Mater. 2017, 2, 17024.
- McCormack, P.L. Ferumoxytol In Iron Deficiency Anaemia in Adults with Chronic Kidney Disease. Drugs 2012, 72, 2013–2022.
- Lartigue, L.; Alloyeau, D.; Kolosnjaj–Tabi, J.; Javed, Y.; Guardia, P.; Riedinger, A.; Péchoux, C.; Pellegrino, T.; Wilhelm, C.; Gazeau, F. Biodegradation of Iron Oxide Nanocubes: High–Resolution In Situ Monitoring. ACS Nano 2013, 7, 3939–3952.
- Tong, S.; Zhu, H.; Bao, G. Magnetic iron oxide nanoparticles for disease detection and therapy. Mater. Today 2019, 31, 86–99.
- Fang, J.; Islam, W.; Maeda, H. Exploiting the dynamics of the EPR effect and strategies to improve the therapeutic effects of nanomedicines by using EPR effect enhancers. Adv. Drug Deliv. Rev. 2020, 157, 142–160.
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2020, 20, 101–124.
- Park, J.; Jin, C.; Lee, S.; Kim, J.; Choi, H. Magnetically Actuated Degradable Microrobots for Actively Controlled Drug Release and Hyperthermia Therapy. Adv. Health Mater. 2019, 8, e1900213.
- Cazares–Cortes, E.; Cabana, S.; Boitard, C.; Nehlig, E.; Griffete, N.; Fresnais, J.; Wilhelm, C.; Abou–Hassan, A.; Ménager, C. Recent insights in magnetic hyperthermia: From the “hot–spot” effect for local delivery to combined magneto–photo–thermia using magneto–plasmonic hybrids. Adv. Drug Deliv. Rev. 2018, 138, 233–246.
- Ni, D.; Bu, W.; Ehlerding, E.B.; Cai, W.; Shi, J. Engineering of inorganic nanoparticles as magnetic resonance imaging contrast agents. Chem. Soc. Rev. 2017, 46, 7438–7468.
- Dadfar, S.M.; Roemhild, K.; Drude, N.; von Stillfried, S.; Knüchel, R.; Kiessling, F.; Lammers, T. Iron oxide nanoparticles: Diagnostic, therapeutic and theranostic applications. Adv. Drug Deliv. Rev. 2019, 138, 302–325.
- Lu, C.; Han, L.; Wang, J.; Wan, J.; Song, G.; Rao, J. Engineering of magnetic nanoparticles as magnetic particle imaging tracers. Chem. Soc. Rev. 2021, 50, 8102–8146.
- John, R.; Rezaeipoor, R.; Adie, S.G.; Chaney, E.J.; Oldenburg, A.L.; Marjanovic, M.; Haldar, J.P.; Sutton, B.P.; Boppart, S.A. In vivo magnetomotive optical molecular imaging using targeted magnetic nanoprobes. Proc. Natl. Acad. Sci. USA 2010, 107, 8085–8090.
- Teijeiro–Valiño, C.; Gómez, M.A.G.; Yañez–Villar, S.; García–Acevedo, P.; Arnosa–Prieto, A.; Belderbos, S.; Gsell, W.; Himmelreich, U.; Piñeiro, Y.; Rivas, J. Biocompatible magnetic gelatin nanoparticles with enhanced MRI contrast performance prepared by single–step desolvation method. Nano Express 2021, 2, 020011.
- Zhou, Y.; Fan, S.; Feng, L.; Huang, X.; Chen, X. Manipulating Intratumoral Fenton Chemistry for Enhanced Chemodynamic and Chemodynamic-Synergized Multimodal Therapy. Adv. Mater. 2021, 33, 2104223.
- Tromsdorf, U.I.; Bruns, O.; Salmen, S.C.; Beisiegel, U.; Weller, H. A Highly Effective, Nontoxic T1 MR Contrast Agent Based on Ultrasmall PEGylated Iron Oxide Nanoparticles. Nano Lett. 2009, 9, 4434–4440.
- Harisinghani, M.G.; Barentsz, J.; Hahn, P.F.; Deserno, W.M.; Tabatabaei, S.; Van De Kaa, C.H.; De La Rosette, J.; Weissleder, R. Noninvasive Detection of Clinically Occult Lymph–Node Metastases in Prostate Cancer. N. Engl. J. Med. 2003, 348, 2491–2499.
- Tang, L.; Casas, J.; Venkataramasubramani, M. Magnetic Nanoparticle Mediated Enhancement of Localized Surface Plasmon Resonance for Ultrasensitive Bioanalytical Assay in Human Blood Plasma. Anal. Chem. 2013, 85, 1431–1439.
- Li, B.; Gong, T.; Xu, N.; Cui, F.; Yuan, B.; Yuan, Q.; Sun, H.; Wang, L.; Liu, J. Improved Stability and Photothermal Performance of Polydopamine-Modified Fe3O4 Nanocomposites for Highly Efficient Magnetic Resonance Imaging-Guided Photothermal Therapy. Small 2020, 16, e2003969.
- Wang, P.; Shi, Y.; Zhang, S.; Huang, X.; Zhang, J.; Zhang, Y.; Si, W.; Dong, X. Hydrogen Peroxide Responsive Iron–Based Nanoplatform for Multimodal Imaging–Guided Cancer Therapy. Small 2018, 15, e1803791.
- Sodipo, B.K.; Aziz, A.A. Recent advances in synthesis and surface modification of superparamagnetic iron oxide nanoparticles with silica. J. Magn. Magn. Mater. 2016, 416, 275–291.
- Wu, W.; Wu, Z.; Yu, T.; Jiang, C.; Kim, W.-S. Recent progress on magnetic iron oxide nanoparticles: Synthesis, surface functional strategies and biomedical applications. Sci. Technol. Adv. Mater. 2015, 16, 023501.
- Pereira, C.; Pereira, A.M.; Fernandes, C.; Rocha, M.; Mendes, R.; Fernández–García, M.P.; Guedes, A.; Tavares, P.B.; Grenèche, J.-M.; Araújo, J.P.; et al. Superparamagnetic MFe2O4 (M = Fe, Co, Mn) Nanoparticles: Tuning the Particle Size and Magnetic Properties through a Novel One–Step Coprecipitation Route. Chem. Mater. 2012, 24, 1496–1504.
- Massart, R. Preparation of aqueous magnetic liquids in alkaline and acidic media. IEEE Trans. Magn. 1981, 17, 1247–1248.
- Park, J.; An, K.; Hwang, Y.; Park, J.G.; Noh, H.J.; Kim, J.Y.; Park, J.H.; Hwang, N.M.; Hyeon, T. Ultra–large–scale syntheses of mono disperse nanocrystals. Nat Mater 2004, 3, 891–895.
- Deng, H.; Li, X.; Peng, Q.; Wang, X.; Chen, J.; Li, Y. Monodisperse magnetic single–crystal ferrite microspheres. Angew. Chem. 2005, 117, 2842–2845.
- Hu, P.; Yu, L.; Zuo, A.; Guo, C.; Yuan, F. Fabrication of Monodisperse Magnetite Hollow Spheres. J. Phys. Chem. C 2008, 113, 900–906.
- Niederberger, M. Nonaqueous Sol–Gel Routes to Metal Oxide Nanoparticles. Accounts Chem. Res. 2007, 40, 793–800.
- Lee, Y.; Lee, J.; Bae, C.J.; Park, J.-G.; Noh, H.-J.; Hyeon, T. Large–Scale Synthesis of Uniform and Crystalline Magnetite Nanoparticles Using Reverse Micelles as Nanoreactors under Reflux Conditions. Adv. Funct. Mater. 2005, 15, 503–509.
- Lee, J.-H.; Huh, Y.-M.; Jun, Y.-W.; Seo, J.-W.; Jang, J.-T.; Song, H.-T.; Kim, S.; Cho, E.-J.; Yoon, H.-G.Y.; Suh, J.-S.; et al. Artificially engineered magnetic nanoparticles for ultra–sensitive molecular imaging. Nat. Med. 2006, 13, 95–99.
- Noh, S.-H.; Na, W.; Jang, J.-T.; Lee, J.-H.; Lee, E.J.; Moon, S.H.; Lim, Y.; Shin, J.-S.; Cheon, J. Nanoscale Magnetism Control via Surface and Exchange Anisotropy for Optimized Ferrimagnetic Hysteresis. Nano Lett. 2012, 12, 3716–3721.
- Yang, Y.; Liu, X.-L.; Yi, J.-B.; Yang, Y.; Fan, H.-M.; Ding, J. Stable vortex magnetite nanorings colloid: Micromagnetic simulation and experimental demonstration. J. Appl. Phys. 2012, 111, 044303.
- Ling, D.; Lee, N.; Hyeon, T. Chemical Synthesis and Assembly of Uniformly Sized Iron Oxide Nanoparticles for Medical Applications. Accounts Chem. Res. 2015, 48, 1276–1285.
- Liu, X.L.; Fan, H.M. Innovative magnetic nanoparticle platform for magnetic resonance imaging and magnetic fluid hyperthermia applications. Curr. Opin. Chem. Eng. 2014, 4, 38–46.
- Liu, X.L.; Yang, Y.; Ng, C.T.; Zhao, L.Y.; Zhang, Y.; Bay, B.H.; Fan, H.M.; Ding, J. Magnetic Vortex Nanorings: A New Class of Hyperthermia Agent for Highly Efficient In Vivo Regression of Tumors. Adv. Mater. 2015, 27, 1939–1944.
- Cole, A.J.; David, A.E.; Wang, J.; Galbán, C.J.; Yang, V.C. Magnetic brain tumor targeting and biodistribution of long–circulating PEG–modified, cross–linked starch–coated iron oxide nanoparticles. Biomaterials 2011, 32, 6291–6301.
- Liu, G.; Xie, J.; Zhang, F.; Wang, Z.-Y.; Luo, K.; Zhu, L.; Quan, Q.-M.; Niu, G.; Lee, S.; Ai, H.; et al. N–Alkyl–PEI–functionalized iron oxide nanoclusters for efficient siRNA delivery. Small 2011, 7, 2742–2749.
- Kang, X.-J.; Dai, Y.-L.; Ma, P.-A.; Yang, D.-M.; Li, C.-X.; Hou, Z.-Y.; Cheng, Z.-Y.; Lin, J. Poly(acrylic acid)–Modified Fe3O4Microspheres for Magnetic–Targeted and pH–Triggered Anticancer Drug Delivery. Chem. Eur. J. 2012, 18, 15676–15682.
- Riedinger, A.; Leal, M.P.; Deka, S.R.; George, C.; Franchini, I.R.; Falqui, A.; Cingolani, R.; Pellegrino, T. “Nanohybrids” Based on pH–Responsive Hydrogels and Inorganic Nanoparticles for Drug Delivery and Sensor Applications. Nano Lett. 2011, 11, 3136–3141.
- KC, R.B.; Lee, S.M.; Yoo, E.S.; Choi, J.H.; Ghim, H.D. Glycoconjugated chitosan stabilized iron oxide nanoparticles as a multifunctional nanoprobe. Mater. Sci. Eng. C 2009, 29, 1668–1673.
- Kim, J.; Arifin, D.R.; Muja, N.; Kim, T.; Gilad, A.A.; Kim, H.; Arepally, A.; Hyeon, T.; Bulte, J.W.M. Multifunctional Capsule–in–Capsules for Immunoprotection and Trimodal Imaging. Angew. Chem. Int. Ed. 2011, 50, 2317–2321.
- Li, Y.; Liu, J.; Zhong, Y.; Zhang, D.; Wang, Z.; An, Y.-L.; Lin, M.; Gao, Z.; Zhang, J. Biocompatibility of Fe3O4@Au composite magnetic nanoparticles in vitro and in vivo. Int. J. Nanomed. 2011, 6, 2805–2819.
- Khan, M.I.; Mohammad, A.; Patil, G.; Naqvi, S.; Chauhan, L.; Ahmad, I. Induction of ROS, mitochondrial damage and autophagy in lung epithelial cancer cells by iron oxide nanoparticles. Biomaterials 2012, 33, 1477–1488.
- Huang, D.-M.; Hsiao, J.-K.; Chen, Y.-C.; Chien, L.-Y.; Yao, M.; Chen, Y.-K.; Ko, B.-S.; Hsu, S.-C.; Tai, L.-A.; Cheng, H.-Y.; et al. The promotion of human mesenchymal stem cell proliferation by superparamagnetic iron oxide nanoparticles. Biomaterials 2009, 30, 3645–3651.
- Brito, B.; Price, T.W.; Gallo, J.; Bañobre–López, M.; Stasiuk, G.J. Smart magnetic resonance imaging–based theranostics for cancer. Theranostics 2021, 11, 8706–8737.
- Wahsner, J.; Gale, E.M.; Rodríguez–Rodríguez, A.; Caravan, P. Chemistry of MRI Contrast Agents: Current Challenges and New Frontiers. Chem. Rev. 2018, 119, 957–1057.
- Wu, C.; Xu, Y.; Yang, L.; Wu, J.; Zhu, W.; Li, D.; Cheng, Z.; Xia, C.; Guo, Y.; Gong, Q.; et al. Negatively Charged Magnetite Nanoparticle Clusters as Efficient MRI Probes for Dendritic Cell Labeling and In Vivo Tracking. Adv. Funct. Mater. 2015, 25, 3581–3591.
- Karimian–Jazi, K.; Münch, P.; Alexander, A.; Fischer, M.; Pfleiderer, K.; Piechutta, M.; Karreman, M.A.; Solecki, G.M.; Berghoff, A.S.; Friedrich, M.; et al. Monitoring innate immune cell dynamics in the glioma microenvironment by magnetic resonance imaging and multiphoton microscopy (MR–MPM). Theranostics 2020, 10, 1873–1883.
- Marckmann, P.; Skov, L.; Rossen, K.; Dupont, A.; Damholt, M.B.; Heaf, J.G.; Thomsen, H.S. Nephrogenic systemic fibrosis: Suspected causative role of gadodiamide used for contrast–enhanced magnetic resonance imaging. J. Am. Soc. Nephrol. 2006, 17, 2359–2362.
- Sieber, M.A.; Lengsfeld, P.; Walter, J.; Schirmer, H.; Frenzel, T.; Siegmund, F.; Weinmann, H.-J.; Pietsch, H. Gadolinium–based contrast agents and their potential role in the pathogenesis of nephrogenic systemic fibrosis: The role of excess ligand. J. Magn. Reson. Imaging 2008, 27, 955–962.
- Zhang, H.; Li, L.; Liu, X.L.; Jiao, J.; Ng, C.-T.; Yi, J.; E Luo, Y.; Bay, B.-H.; Zhao, L.Y.; Peng, M.L.; et al. Ultrasmall Ferrite Nanoparticles Synthesized via Dynamic Simultaneous Thermal Decomposition for High–Performance and Multifunctional T1 Magnetic Resonance Imaging Contrast Agent. ACS Nano 2017, 11, 3614–3631.
- Kim, B.H.; Lee, N.; Kim, H.; An, K.; Park, Y.I.; Choi, Y.; Shin, K.; Lee, Y.; Kwon, S.G.; Bin Na, H.; et al. Large–Scale Synthesis of Uniform and Extremely Small–Sized Iron Oxide Nanoparticles for High–Resolution T1 Magnetic Resonance Imaging Contrast Agents. J. Am. Chem. Soc. 2011, 133, 12624–12631.
- Chandrasekharan, P.; Tay, Z.W.; Hensley, D.; Zhou, X.Y.; Fung, B.K.; Colson, C.; Lu, Y.; Fellows, B.D.; Huynh, Q.; Saayujya, C.; et al. Using magnetic particle imaging systems to localize and guide magnetic hyperthermia treatment: Tracers, hardware, and future medical applications. Theranostics 2020, 10, 2965–2981.
- Gloag, L.; Mehdipour, M.; Ulanova, M.; Mariandry, K.; Nichol, M.A.; Hernández–Castillo, D.J.; Gaudet, J.; Qiao, R.; Zhang, J.; Nelson, M.; et al. Zero valent iron core–iron oxide shell nanoparticles as small magnetic particle imaging tracers. Chem. Commun. 2020, 56, 3504–3507.
- Song, G.; Chen, M.; Zhang, Y.; Cui, L.; Qu, H.; Zheng, X.; Wintermark, M.; Liu, Z.; Rao, J. Janus Iron Oxides @ Semiconducting Polymer Nanoparticle Tracer for Cell Tracking by Magnetic Particle Imaging. Nano Lett. 2017, 18, 182–189.
- Oldenburg, A.L.; Crecea, V.; Rinne, S.A.; Boppart, S.A. Phase–resolved magnetomotive OCT for imaging nanomolar concentrations of magnetic nanoparticles in tissues. Opt. Express 2008, 16, 11525–11539.
- Huang, P.-C.; Chaney, E.J.; Aksamitiene, E.; Barkalifa, R.; Spillman, D.R.; Bogan, B.J.; Boppart, S.A. Biomechanical sensing of in vivo magnetic nanoparticle hyperthermia–treated melanoma using magnetomotive optical coherence elastography. Theranostics 2021, 11, 5620–5633.
- Xu, C.; Zheng, Y.; Gao, W.; Xu, J.; Zuo, G.; Chen, Y.; Zhao, M.; Li, J.; Song, J.; Zhang, N.; et al. Magnetic Hyperthermia Ablation of Tumors Using Injectable Fe3O4/Calcium Phosphate Cement. ACS Appl. Mater. Interfaces 2015, 7, 13866–13875.
- Yin, P.; Shah, S.; Pasquale, N.J.; Garbuzenko, O.B.; Minko, T.; Lee, K. –B. Stem cell–based gene therapy activated using magnetic hyperthermia to enhance the treatment of cancer. Biomaterials 2015, 81, 46–57.
- Moise, S.; Byrne, J.M.; El Haj, A.J.; Telling, N.D. The potential of magnetic hyperthermia for triggering the differentiation of cancer cells. Nanoscale 2018, 10, 20519–20525.
- Liu, X.L.; Ng, C.T.; Chandrasekharan, P.; Yang, H.T.; Zhao, L.Y.; Peng, E.; Lv, Y.B.; Xiao, W.; Fang, J.; Yi, J.; et al. Synthesis of Ferromagnetic Fe0.6Mn0.4O Nanoflowers as a New Class of Magnetic Theranostic Platform for In Vivo T1–T2Dual–Mode Magnetic Resonance Imaging and Magnetic Hyperthermia Therapy. Adv. Health Mater. 2016, 5, 2092–2104.
- Zhou, Z.; Song, J.; Tian, R.; Yang, Z.; Yu, G.; Lin, L.; Zhang, G.; Fan, W.; Zhang, F.; Niu, G.; et al. Activatable Singlet Oxygen Generation from Lipid Hydroperoxide Nanoparticles for Cancer Therapy. Angew. Chem. Int. Ed. 2017, 56, 6492–6496.
- Du, J.; Bao, J.; Fu, X.; Lu, C.; Kim, S.H. Mesoporous sulfur–modified iron oxide as an effective Fenton–like catalyst for degradation of bisphenol A. Appl. Catal. B Environ. 2016, 184, 132–141.
- Zhao, S.; Yu, X.; Qian, Y.; Chen, W.; Shen, J. Multifunctional magnetic iron oxide nanoparticles: An advanced platform for cancer theranostics. Theranostics 2020, 10, 6278–6309.
- Jang, J.-T.; Nah, H.; Lee, J.-H.; Moon, S.H.; Kim, M.G.; Cheon, J. Critical Enhancements of MRI Contrast and Hyperthermic Effects by Dopant–Controlled Magnetic Nanoparticles. Angew. Chem. Int. Ed. 2009, 48, 1234–1238.
- Liang, H.; Guo, J.; Shi, Y.; Zhao, G.; Sun, S.; Sun, X. Porous yolk–shell Fe/Fe3O4 nanoparticles with controlled exposure of highly active Fe(0) for cancer therapy. Biomaterials 2020, 268, 120530.
- Zhou, H.; Tang, J.; Li, J.; Li, W.; Liu, Y.; Chen, C. In vivo aggregation–induced transition between T1and T2relaxations of magnetic ultra–small iron oxide nanoparticles in tumor microenvironment. Nanoscale 2017, 9, 3040–3050.
- Xu, C.; Pu, K. Second near–infrared photothermal materials for combinational nanotheranostics. Chem. Soc. Rev. 2020, 50, 1111–1137.
- Liu, Y.; Yang, Z.; Huang, X.; Yu, G.; Wang, S.; Zhou, Z.; Shen, Z.; Fan, W.; Liu, Y.; Davission, M.; et al. Glutathione–Responsive Self–Assembled Magnetic Gold Nanowreath for Enhanced Tumor Imaging and Imaging–Guided Photothermal Therapy. ACS Nano 2018, 12, 8129–8137.
- Zhou, H.; Guo, M.; Li, J.; Qin, F.; Wang, Y.; Liu, T.; Liu, J.; Sabet, Z.F.; Wang, Y.; Liu, Y.; et al. Hypoxia–Triggered Self–Assembly of Ultrasmall Iron Oxide Nanoparticles to Amplify the Imaging Signal of a Tumor. J. Am. Chem. Soc. 2021, 143, 1846–1853.
- Ma, J.; Li, P.; Wang, W.; Wang, S.; Pan, X.; Zhang, F.; Li, S.; Liu, S.; Wang, H.; Gao, G.; et al. Biodegradable Poly(amino acid)–Gold–Magnetic Complex with Efficient Endocytosis for Multimodal Imaging–Guided Chemo–photothermal Therapy. ACS Nano 2018, 12, 9022–9032.
- Liu, X.; Peng, M.L.; Li, G.; Miao, Y.Q.; Luo, H.; Jing, G.; He, Y.; Zhang, C.; Zhang, F.; Fan, H. Ultrasonication–Triggered Ubiquitous Assembly of Magnetic Janus Amphiphilic Nanoparticles in Cancer Theranostic Applications. Nano Lett. 2019, 19, 4118–4125.
- Shi, Y.; Van Der Meel, R.; Chen, X.; Lammers, T. The EPR effect and beyond: Strategies to improve tumor targeting and cancer nanomedicine treatment efficacy. Theranostics 2020, 10, 7921–7924.
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014.
- Reichel, D.; Sagong, B.; Teh, J.; Zhang, Y.; Wagner, S.; Wang, H.; Chung, L.W.K.; Butte, P.; Black, K.L.; Yu, J.S.; et al. Near Infrared Fluorescent Nanoplatform for Targeted Intraoperative Resection and Chemotherapeutic Treatment of Glioblastoma. ACS Nano 2020, 14, 8392–8408.
- Liu, D.; Zhou, Z.; Wang, X.; Deng, H.; Sun, L.; Lin, H.; Kang, F.; Zhang, Y.; Wang, Z.; Yang, W.; et al. Yolk–shell nanovesicles endow glutathione–responsive concurrent drug release and T1 MRI activation for cancer theranostics. Biomaterials 2020, 244, 119979.
- Zhou, Q.; Shao, S.; Wang, J.; Xu, C.; Xiang, J.; Piao, Y.; Zhou, Z.; Yu, Q.; Tang, J.; Liu, X.; et al. Enzyme–activatable polymer–drug conjugate augments tumour penetration and treatment efficacy. Nat. Nanotechnol. 2019, 14, 799–809.
- Dai, Q.; Wilhelm, S.; Ding, D.; Syed, A.; Sindhwani, S.; Zhang, Y.; Chen, Y.Y.; MacMillan, P.; Chan, W.C.W. Quantifying the Ligand–Coated Nanoparticle Delivery to Cancer Cells in Solid Tumors. ACS Nano 2018, 12, 8423–8435.




