
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Andrea Ehrmann | + 1339 word(s) | 1339 | 2020-08-25 08:08:53 | | | |
| 2 | Peter Tang | -1 word(s) | 1338 | 2020-09-12 10:20:17 | | |
Video Upload Options
PbS (lead sulfide) colloidal quantum dots consist of crystallites with diameters in the nanometer range with organic molecules on their surfaces, partly with additional metal complexes as ligands. These surface molecules are responsible for solubility and prevent aggregation, but the interface between semiconductor quantum dots and ligands also influences the electronic structure. PbS quantum dots are especially interesting for optoelectronic applications and spectroscopic techniques, including photoluminescence, photodiodes and solar cells.
1. Introduction
Usually, material properties are defined by the atomic or molecular composition of a material tested at the macro scale. This rule no longer holds when structures become very small, in the range of nanometers, where scaling effects lead to significantly different optical [1][2][3], chemical [4][5][6], magnetic [7][8][9] or other properties of nanoparticles or quantum dots. An interesting method to combine the bottom-up assembly of devices from nanoparticles with the mechanical flexibility of the device is now accessible, with the use of relatively simple chemical procedures based on colloidal semiconductor quantum dots [10]. A general reason for semiconducting quantum dots research and development is that they are universal technologically tunable systems, with adjustable energy levels for lighting and sensing applications.
The core of colloidal semiconductor quantum dots often consists of nanocrystals from some hundred to a thousand atoms of II-VI, III-V or IV-VI semiconductors [11]. On their surface, they bear organic molecules or metal complexes as ligands, as visible in Figure 1 [12].
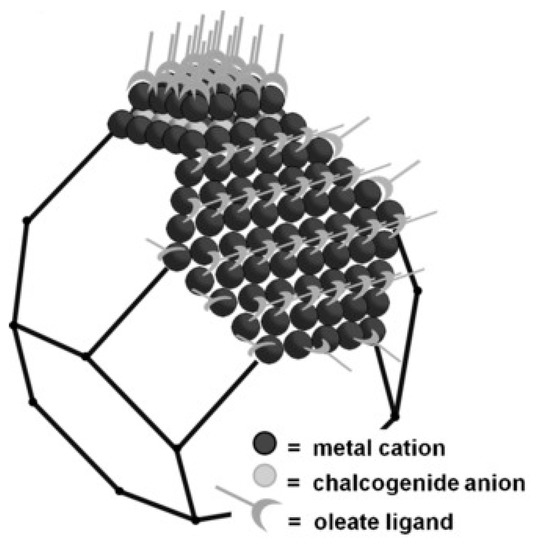
Figure 1. Pb-terminated truncated octahedral PbS quantum dot, depicting Pb cations, S anions and organic ligands. From [12], reprinted with permission. Copyright © Wiley 2016.
These ligands build the surface layer between the semiconductor quantum dot and the outside, in this way both influencing and being influenced by an inner and an outer interface. The inorganic/organic surface between quantum dots and ligands thus impacts the electronic structure of the quantum dot [13], as well as interactions with neighboring quantum dots [14].
A highly interesting material to produce such quantum dots is PbS. This IV-VI semiconductor typically has a sodium chloride crystal structure [15] and a temperature-dependent direct band gap between approx. 0.29 eV and 0.45 eV, making it useful for detecting infrared and visible light [16]. Besides macroscopic crystals and epitaxial thin films, PbS is often prepared in the form of nanocrystallites or quantum dots by diverse methods, from chemical routes [17] to electrodeposition [18] and microorganism-based synthesis [19]. One of the main areas of research and development in which PbS nanocrystallites—typically as colloidal PbS quantum dots—are used is photovoltaic applications. Similar to PbSe, PbS has high photosensitivity in the near-infrared spectrum [20], and allows for the tuning of the optical band gap to a broad range of around 0.7–2.1 eV [21][22], as well as the electronic structure of the quantum dot films and significantly shifting the band edges without large modifications of the band gap [23][24]. PbS colloidal quantum dots also show multiple exciton generation [25][26]. All these properties make PbS quantum dot solar cells belong to the quantum dot solar cells with the highest performance [27][28][29], and thus making PbS a highly interesting material for solar cells.
2. Solar Cells with PbS Quantum Dots—The Principle Function
With regards to solar cells, the most commercially important technology is based on silicon. There are, however, other technologies, such as those based on perovskites [30], which typically use an active layer from a perovskite material, i.e., a material with the crystal structure ABX3, with A denoting a monovalent cation, B a metal cation and X a halide anion [31]. This active layer is usually embedded between two electrodes, one of which has to be transparent to allow photons to reach the active layer, a hole transport layer on the one side and a hole-blocking layer on the opposite side, in this way defining the orientation of the current flow.
Organic solar cells, also named polymer solar cells, also embed the active parts between two electrodes. Usually, the hole transport layer is coated on the anode and the electron transport layer on the cathode. The active layer between them contains electron donors and acceptors [32].
Finally, dye-sensitized solar cells (DSSCs) are of growing interest. While the efficiencies reachable by them are still low, especially when using non-toxic and low-cost materials [33][34][35], they can be expected to be highly useful in large-scale applications where overall efficiency is less important than the cost-benefit ratio [36]. As the aforementioned types of solar cells, DSSCs contain two conductive electrodes surrounding the other layers. Usually, a semiconductor like TiO2 or ZnO is applied on the top electrode and dyed with a natural dye, or a more efficient—usually toxic—one. A catalyst is applied on the counter electrode, supporting electrons from an external circuit reaching the active layers of the DSSC. Between a dyed semiconductor and catalyst, an electrolyte fills the gap, usually based on iodine/triiodide or similar redox couples [37].
Generally, quantum dot solar cells enable multi-exciton generation, making such solar cells in principle more efficient than common thin-film or crystal-based ones [38][39][40].
3. PbS Quantum Dot Schottky Solar Cells
One recent idea to increase Schottky quantum dot solar cells' (QDSCs) power conversion efficiency is to combine a p-type PbS quantum dot layer with an n-type metal oxide layer for the front junction [41], or to use inverted Schottky QDSCs with a low-work-function, abstaining from metal oxides [42]. Mai et al. investigated different interfacial electrolytes to fit the electrolyte chemistry to the cell performance [43]. They capped PbS quantum dots (QDs) with oleic acid [42], and passivated them afterwards with Cl-. The transparent conductive oxide (TCO) front electrode's work function was lowered by spin-coating the TCO electrode with a few nanometers of polyethylenimine (PEI) or poly [(9,9-bis(3′-(N,N-dimethylamino)propyl)-2,7-fluorene)-alt-2,7-(9,9–dioctylfluorene)] (PFN), respectively. After adding layers of PbS quantum dots, and MoOx and Au/Ag as a back electrode, the cell was finished. For the different polymer electrolytes used to reduce the work function, the electrical characteristics of the inverted Schottky QDSC changed clearly. In this way, it was possible to reach an efficiency of 4.5% in an oxide-free QDSC [43].
4. PbS Quantum Dot Sensitized Solar Cells
Light harvesting properties of metal oxides like TiO2 or ZnO, which are typically applied as semiconducting layers in DSSCs, can be enhanced by decorating them with low bandgap metal sulfides. One of the techniques used for this purpose is the so-called pseudo-successive ionic layer absorption and reaction (p-SILAR). Ali et al. described in their most recent study how PbS and CdS quantum dots were deposited on TiO2 nanoparticles, performing a H2S treatment of the TiO2 nanoparticles in a rotary reactor. Using rhodamine B degradation as a measure, they showed the positive influence of the H2S treatment for the p-SILAR process [44].
5. PbS Quantum Dot Heterojunction Solar Cells
Plasmonic metal nanoparticles can be used to concentrate incident sunlight by plasmon resonance, in this way increasing the overall absorption of the incident light in a solar cell without increasing the quantum dot film thickness over the carrier diffusion length. Hong et al. used Au and Ag nanoparticles to introduce localized surface plasmon resonance, as well as the scattering of the incident light. By placing the small Au nanoparticles (diameter ~ 10 nm) at the ZnO coated front layer, followed by the PbS active layer and the larger Ag nanoparticles (diameter ~ 50 nm), and then the gold contact back electrode, the latter serves mostly as a far-field scatterer, while the Au nanoparticles enable near-field coupling, in this way increasing the efficiency by approx. 25% [45].
6. Conclusion
As this excerpt of some of the most recent, highly interesting and promising research on PbS quantum dots for solar cells shows, this material offers a broad bandwidth of applications, from light harvesting to electron transport, and correspondingly a large amount of possible solar cell types in which it can be utilized—in particular, the possibility to tailor the semiconductor band gap by changing quantum dot dimensions or ligands makes PbS an interesting material for solar cells with diverse material combinations. On the other hand, controlling quantum dot and ligand properties is necessary to gain sufficient power conversion efficiencies if this material is used in the solar cells of different types.
References
- Storhoff, J.J.; Lazarides, A.A.; Mucic, R.C.; Mirkin, C.A.; Letsinger, R.L.; Schatz, G.C. What controls the optical properties of DNA-linked gold nanoparticle assemblies? J. Am. Chem. Soc. 2000, 122, 4640-4650.
- Lopez, R.; Feldman, L.C.; Haglund, Jr., R. F. Size-dependent optical properties of VO2 nanoparticle arrays. Phys. Rev. Lett. 2004, 93, 177403.
- Byers, C.P.; Zhang, H.; Swearer, D.F.; Yorulmaz, M; Hoener, B.S.; Huang, D.; Hoggard, A.; Chang, W.S.; Mulvaney, P.; Ringe, E.; Halas, N.J.; Nordlander, P.; Link, S.; Landes, C.F. From tunable core-shell nanoparticles to plasmonic drawbridges: Active control of nanoparticle optical properties. Sci. Adv. 2015, 1, e1500988.
- Pfeiffer, C.; Rehbock, C.; Hühn, D.; Carillo-Carrion, C.; Jimenez De Aberasturi, D.; Merk, V.M.; Barcikowski, S.; Parak, W. J. Interaction of colloidal nanoparticles with their local environment: the (ionic) nanoenvironment around nanoparticles is different from bulk and determines the physico-chemical properties of the nanoparticles. J. R. Soc. Interface 2014, 11, 20130931.
- Horie, M.; Fujita, K.; Kato, H.; Endoh, S.; Nishio, K.; Komaba, L.K.; Nakamura, A.; Miyauchi, A.; Kinugasa, S.; Hagihara, Y.; Niki, E; et al. Association of the physical and chemical properties and the cytotoxicity of metal oxide nanoparticles: metal ion release, adsorption ability and specific surface area. Metallomics 2012, 4, 350–360.
- Wang, X.; Hanson, J.C.; Liu, G.; Rodriguez, J.A.; Iglesias-Juez, A.; Fernández-García, M. The behavior of mixed-metal oxides: Physical and chemical properties of bulk Ce1−xTbxO2 and nanoparticles of Ce1−xTbxOy. J. Chem. Phys. 2004, 121, 5434–5444.
- Sudsom, D.; Juhász Junger, I.; Döpke, C.; Blachowicz, T.; Hahn, L.; Ehrmann, A. Micromagnetic simulation of vortex development in magnetic bi-material bow-tie structures. Condens. Matter 2020, 5, 5; doi: 10.3390/condmat5010005.
- Sturm, S.; Siglreitmeier, M.; Wolf, D.; Vogel, K.; Gratz, M.; Faivre, D.; Lubk, A.; Büchner, B.; Sturm (née Rosseeva), E.V.; Cölfen, H. Magnetic nanoparticle chains in gelatin ferrogels: bioinspiration from magnetotactic bacteria. Adv. Funct. Mater. 2019, 29, 1905996.
- Blachowicz, T.; Kosmalska, D.; Döpke, C.; Leiste, H; Hahn, L.; Ehrmann, A. Varying steps in hysteresis loops of Co square nano-frames. J. Magn. Magn. Mater. 2019, 491, 165619.
- Kagan, C.R.; Lifshitz, E.; Sargent, E.H.; Talapin, D.V. Building devices from colloidal quantum dots. Sci. 2016, 353, aac5523.
- Talapin, D.V.; Lee, J.S.; Kovalenko, M.V.; Shevchenko, E.V. Prospects of colloidal nanocrystals for electronic and optoelectronic applications. Chem. Rev. 2010, 110, 389–458.
- Grisorio, R.; Debellis, D.; Suranna, G.P.; Gigli, G.; Giansante, C. The dynamic organic/inorganic interface of colloidal PbS quantum dots. Angew. Chem. Int. Ed. Engl. 2016, 55, 6628.
- Giansante, C.; Infante, I.; Fabiano, E.; Grisorio, R.; Suranna, G. P.; Gigli, G. “Darker-than-Black” PbS Quantum Dots: Enhancing Optical Absorption of Colloidal Semiconductor Nanocrystals via Short Conjugated Ligands. J. Am. Chem. Soc. 2015, 137, 1875–1886.
- Li, R.; Bian, K.; Hanrath, T.; Bassett, W.A.; Wang, Z. Decoding the Superlattice and Interface Structure of Truncate PbS Nanocrystal-Assembled Supercrystal and Associated Interaction Forces. J. Am. Chem. Soc. 2014, 136, 12047–12055.
- Kittel, C. Introduction to Solid State Physics, 3rd edition, John Wiley: New York, N.Y., USA, 1966.
- Dalven, R. A Review of the semiconductor properties of PbTe, PbSe, PbS and PbO. Infrared Phys. 1969, 9, 141-184.
- Wang, C.; Zhang, W.X.; Qian, X.F.; Zhang, X.M.; Xie, Y.; Qian, Y.T. A room temperature chemical route to nanocrystalline PbS semiconductor. Mater. Lett. 1999, 40, 255–258.
- Nanda, K.K.; Sahu, S.N. One-dimensional quantum confinement in electrodeposited PbS nanocrystalline semiconductors. Adv. Mater. 2001, 13, 280–283.
- Kowshik, M.; Vogel, W.; Urban, J.; Kulkarni, S.K.; Paknikar, K.M. Microbial synthesis of semiconductor PbS nanocrystallites. Adv. Mater. 2002, 14, 815–818.
- Li, Y.; Zhu, J.; Huang, Y.; Wei, J.; Liu, F.; Shao, Z.; Hu, L.; Chen, S.; Yang, S.; Tang, J.; Yao, J. X.; Dai, S. Efficient inorganic solid solar cell composed of perovskite and PbS quantum dots. Nanoscale 2015, 7, 9902–9907.
- Gao, J.; Luther, J.M.; Semonin, O.E.; Ellingson, R.J.; Nozik,A.J.; Beard, M.C. Quantum dot size dependent J-V characteristics in heterojunction ZnO/PbS quantum dot solar cells. Nano Lett. 2011, 11, 1002–1008.
- Talapin, D.V.; Murray, C.B. PbSe Nanocrystal solids for N- and P-channel thin film field-effect Transistors. Sci. 2005, 310, 86–89.
- Yuan, M.J.; Liu, M.X.; Sargent, E.H. Colloidal quantum dot solids for solution-processed solar cells. Nat. Energy 2016, 1, 16016.
- Brown, P.R.; Kim, D.H.; Lunt, R.R.; Zhao, N.; Bawendi, M.G.; Grossman, J.C.; Bulovic, V. Energy level modification in lead sulfide quantum dot thin films through ligand exchange. ACS Nano 2014, 8, 5863–5872.
- Scholes, G.D.; Rumbles, G. Excitons in nanoscale systems. Nat. Mater. 2006, 5, 683–696
- Sambur, J.B.; Novet, T.; Parkinson, B.A. Multiple exciton collection in a sensitized photovoltaic system. Sci. 2010, 330, 63–66.
- Hanna, M.C.; Ellingson, R.J.; Beard, M.; Yu, P.; Micic, O.I.; Nozik, A.J. Quantum dot solar cells: high efficiency through multiple exciton generation. In the Proceedings of the 2004 DOE Solar Energy Technologies, Denver, Colorado, 25–28 October 2004.
- Zhao, N.; Osedach, T.P.; Chang, L.Y.; Geyer, S.M.; Wanger, D.; Binda, M.T.; Arango, A.C.; Bawendi, M.G.; Bulovic, V. Colloidal PbS quantum dot solar cells with high fill factor. ACS Nano 2010, 4, 3743–3752.
- Ip, A.H.; Thon, S.M.; Hoogland, S.; Voznyy, O.; Zhitomirsky, D.; Debnath, R.; Levina, L.; Rollny, L.R.; Carey, G.H.; Fischer, A.; et al. Hybrid passivated colloidal quantum dot solids. Nat. Nanotechnol. 2012, 7, 577–582.
- Yang, W.S; Park, B.W.; Jung, E.H; Jeon, N.J.; Kim, Y.C.; Lee, D.U.; Shin, S.S.; Seo, J.; Kim, E.K.; et al. Iodide management in formamidinium-lead-halide-based perovskite layers for efficient solar cells. Sci. 2017, 356, 1376–1379.
- Wang, D.; Wright, M.; Elumalai, N.K.; Uddin, A. Stability of perovskite solar cells. Sol Energy Mater. Sol. Cells 2016, 147, 255–275.
- Li, Y.; Xu, G..; Cui, C..; Li, Y. Flexible and semitransparent organic solar cells. Adv. Energy Mater 2018, 8, 1701791.
- Kohn, S.; Wehlage, D.; Juhász Junger, I.; Ehrmann, A. Electrospinning a dye-sensitized solar cell. Catalysts 2019, 9, 975.
- Juhász Junger, I.; Udomrungkhajornchai, S.; Grimmelsmann, N.; Blachowicz, T.; Ehrmann, A. Effect of caffeine copigmentation of anthocyanin dyes on the DSSC efficiency. Mater. 2019, 12, 2692.
- Mamun, A.; Trabelsi, M.; Klöcker, M.; Sabantina, L.; Großerhode, C.; Blachowicz, T.; Grötsch, G.; Cornelißen, C.; Streitenberger, A.; Ehrmann, A. Electrospun nanofiber mats with embedded non-sintered TiO2 for dye-sensitized solar cells (DSSCs). Fibers 2019, 7, 60.
- Ehrmann, A.; Blachowicz, T. Recent coating materials for textile-based solar cells. AIMS Mater. Sci. 2019, 6, 234–251.
- Ye, M.; Wen, X.; Wang, M.; Iocozzia, J.; Zhang, N.; Lin, C.; Lin, Z. Recent advances in dye-sensitized solar cells: from photoanodes, sensitizers and electrolytes to counter electrodes. Mater Today 2015, 18, 155–162.
- Sikhovatkin, S.; Hinds, S.; Brzozowski, L.; Sargent, E.H. Colloidal quantum-dot photodetectors exploiting multiexciton generation. Sci. 2009, 324, 1542–1544.
- Pijpers, J.J.H.; Ulbricht, R.; Tielrooij, K.J.; Osherov, A.; Golan, Y.; Delerue, C.; Allan, G.; Bonn, M. Assessment of carrier-multiplication efficiency in bulk PbSe and PbS. Nat. Phys. 2009, 5, 811–814.
- Beard, M.C.; Knutsen, K.P.; Yu, P.; Luther, J.M.; Song, Q.; Metzger, W.K.; Ellingson, R.J.; Nozik, A.J. Multiple exciton generation in colloidal silicon nanocrystals. Nano Lett. 2007, 7, 2506–2512.
- Chuang, C.H.; Brown, P.R.; Bulovic, V.; Bawendi, M.G. Improved performance and stability in quantum dot solar cells through band alignment engineering. Nat. Mater. 2014, 13, 796–801.
- Mai, X.-D.; An, H.J.; Song, J.H.; Jang, J.; Kim, S.; Jeong, S. Inverted Schottky quantum dot solar cells with enhanced carrier extraction and air-stability. J. Mater. Chem. A 2014, 2, 20799–20805.
- Mai, V.-T.; Duong, N.-H.; Mai, X.-D. Boosting the current density in inverted Schottky PbS quantum dot solar cells with conjugated electrolyte. Mater. Lett. 2019, 249, 37–40.
- Ali, I.; Muhyuddin, M.; Mullani, N.; Kim, D.W.; Kim, D.H.; Basit, M.A.; Park, T.J. Modernized H2S-treatment of TiO2 nanoparticles: Improving quantum-dot deposition for enhanced photocatalytic performance. Curr. Appl. Phys. 2020, 20, 384–390.
- Hong, J.; Kim, B. S.; Hou, B.; Cho, Y.; Lee, S. H.; Pak, S.; Morris, S. M.; Sohn, J. I.; Cha, S. Plasmonic Effects of Dual-Metal Nanoparticle Layers for High-Performance Quantum Dot Solar Cells. Plasmon. 2020, doi: 10.1007/s11468-020-01120-y.




