Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Vadivel Ganapathy | + 3048 word(s) | 3048 | 2022-02-14 09:33:07 | | | |
| 2 | Conner Chen | -12 word(s) | 3036 | 2022-03-02 01:53:46 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ganapathy, V. Amino Acid Transporters. Encyclopedia. Available online: https://encyclopedia.pub/entry/20058 (accessed on 07 February 2026).
Ganapathy V. Amino Acid Transporters. Encyclopedia. Available at: https://encyclopedia.pub/entry/20058. Accessed February 07, 2026.
Ganapathy, Vadivel. "Amino Acid Transporters" Encyclopedia, https://encyclopedia.pub/entry/20058 (accessed February 07, 2026).
Ganapathy, V. (2022, March 01). Amino Acid Transporters. In Encyclopedia. https://encyclopedia.pub/entry/20058
Ganapathy, Vadivel. "Amino Acid Transporters." Encyclopedia. Web. 01 March, 2022.
Copy Citation
The conventional function of amino acid transporters in mammalian cells is in the maintenance of amino acid homeostasis. Every cell has a need for amino acids from extracellular sources, particularly for the essential amino acids that mammalian cells are not able to synthesize. This need cannot be met without the participation of specific transporters in the plasma membrane because of the hydrophilic nature of these amino acids, a feature that prevents their simple diffusion across the hydrophobic lipid bilayer.
amino acid transporters
1. Introduction
The conventional function of amino acid transporters in mammalian cells is in the maintenance of amino acid homeostasis. Every cell has a need for amino acids from extracellular sources, particularly for the essential amino acids that mammalian cells are not able to synthesize. This need cannot be met without the participation of specific transporters in the plasma membrane because of the hydrophilic nature of these amino acids, a feature that prevents their simple diffusion across the hydrophobic lipid bilayer. The transport process mediated by these amino acid transporters is either uniport (i.e., amino acid transfer in one specific direction) or obligatory exchange (i.e., transfer of one amino acid substrate in one direction which is obligatorily coupled to transfer of another amino acid substrate in the opposite direction). Some are simple facilitative transporters with no involvement of any co-transported ion, whereas others are coupled transporters with involvement of one or more ions (Na+, H+, K+ or Cl−). Depending on the stoichiometry of the amino acid substrates and co-transported ions, the transport process could be electroneutral or electrogenic. Therefore, the direction of the amino acid transfer mediated by a given amino acid transporter is dictated by multiple factors: concentration gradients for amino acid substrates and co-transported ions as well as membrane potential. These amino acid transporters are not expressed exclusively in the plasma membrane [1][2][3][4]; they are also found in intracellular membranes, particularly in the lysosomal [5][6] and mitochondrial membranes [7]. This makes sense because amino acid needs of cells are met not only by uptake from extracellular sources but also by transfer from lysosomes following proteolysis in conjunction with autophagy, pinocytosis and macropinocytosis. In mitochondria, many of the biochemical pathways that take place in the matrix involve amino acids (e.g., urea cycle, malate–aspartate shuttle), which requires the transporter-mediated transfer of amino acids in both directions across the inner-mitochondrial membrane.
There are numerous amino acid transporters in mammalian cells, and they differ in substrate selectivity, transport mechanism, driving forces and tissue expression pattern. In terms of the Human Genome Nomenclature, these transporters belong to ten different SLC (solute carrier) gene families (1, 6, 7, 16, 17, 25, 36, 38, 43 and 66) along with four non-transporter proteins (SLC3A1/rBAT, SLC3A2/4F2hc/CD98, ACE2 and collectrin) that serve as chaperones for some of these transporters [1][2]. Depending on their cell-type specific expression, they function not only in the cellular uptake of amino acids but also in transcellular transfer of amino acids across the barrier structures such as the blood–brain barrier, blood–retinal barrier, maternal–fetal barrier and in the absorption of dietary amino acids (intestine) and re-absorption-filtered amino acids (kidney). Loss-of-function mutations in many of these transporters cause specific genetic diseases (e.g., Hartnup disease, cystinuria) [8][9][10][11].
2. Unconventional Functions of Amino Acid Transporters
Amino acids represent an important class of nutrients in cellular metabolism, serving as building blocks for protein synthesis and functioning in multiple metabolic pathways such as the urea cycle, heme biosynthesis, one-carbon metabolism, glutaminolysis and neurotransmission, to name just a few. Therefore, the cellular need for amino acids is functionally coupled to the expression levels of specific amino acid transporters in the plasma membrane to coordinate the two events, namely the availability and the utilization of amino acids. This coupling involves specific signaling pathways with direct participation of the involved transporter proteins in the functional crosstalk. This led to the coinage of the term “Transceptor” to highlight the non-traditional role of a protein functioning both as a transporter and as a receptor [12]. In mammalian cells, this novel aspect of an amino acid transporter was first noticed for the classical “system A” [13], a Na+-coupled transport system for short-chain amino acids and glutamine belonging to the SLC38 family [14]. System A consists of three specific transporters, SLC38A1 [15][16], SLC38A2 [17] and SLC38A4 [18]; among these three, the novel “transceptor” feature has been ascribed only to SLC38A2 [12][13][19][20]. This feature is responsible for the increase in the plasma membrane density of the transporter protein when cells are deficient in amino acids and conversely for the decrease in transporter density in the plasma membrane when cells are sated with amino acids. More recently, another member of the SLC38 family, namely SLC38A9, expressed in the lysosomal membrane, has been shown to link amino acids in the lysosomes and mTORC1 activity [21][22]. A similar feature has also been found for certain members of the SLC36 family of amino acid transporters [23][24]. In all these cases, amino acid transporters not only mediate amino acid transport but also function as amino acid sensors, thus coupling amino acid status within the cells to amino acid transporter density in the plasma membrane to modulate amino acid entry into cells and amino acid-dependent metabolic pathways. This ensures control of amino acid entry into cells in a manner that is appropriate for the amino acid status (deficient or excess) within the cells and also modulation of metabolic pathways that utilize amino acids such that the rates of these pathways are in tune with the magnitude of amino acid delivery into cells.
Another notable non-traditional function of amino acid transporters is their involvement as the cell surface receptors in retroviruses. Interestingly, this function is independent of the role of these transporters in amino acid transfer. This is in contrast to their “transceptor” function where amino acid transport is coupled to cellular signaling. Retroviruses hijack specific amino acid transporters to gain entry into their target cells [25]. In this process, the viruses bind to the external surface of specific transporters, and then the bound complex undergoes endocytosis, consequently delivering the viruses into the cells. Since the amino acid transporters are expressed in a cell type-specific manner, this also provides the molecular basis for target cell selectivity for these viruses. Furthermore, the variable amino acid sequences of a given amino acid transporter among different species dictates the specificity of virus interaction for the species-specific tropism of a given virus.
To date, three amino acid transporters and one transporter chaperone have been shown to serve as cell surface receptors for specific retroviruses (Table 1). The transporters are murine cationic amino acid transporter 1 (Slc7a1) for ecotropic murine leukemia virus (E-MLV) and bovine leukemia virus (BLV) [26][27], human alanine–serine–cysteine transporter 1 (ASCT1 or SLC1A4) for the feline infectious endogenous retrovirus RD-114, baboon endogenous retrovirus (BaEV), and human endogenous retrovirus HERV-W [28] and Alanine-Serine-Cysteine Transporter 2 (ASCT2 or SLC1A5) for baboon endogenous retrovirus (BaEV) and human endogenous retrovirus HERV-W [29][30]. Even though the transport function has nothing to do with the binding of the virus to the transporter protein, it is likely that the interaction impacts the transport function. Since the virus entry via the transporter involves endocytosis, it is possible that the binding of the virus to the transporter results in decreased density of the transporter protein in the plasma membrane, thus negatively affecting the transport function. This is also true with ACE2, the chaperone for the intestinal amino acid transporters SLC6A19 and SLC6A20 [31]; this chaperone protein serves as the primary receptor for the COVID-19 virus SARS-CoV-2 [32][33]. Deletion of Ace2 in mice led to a drastic decrease in the density of the two transporters in the apical membrane of the epithelial cells of the small intestine [34]. Therefore, it is probable that binding of SARS-CoV-2 to ACE2 in the intestine decreases the trafficking of SLC6A19 and SLC6A20 to the apical membrane, consequently decreasing the absorption of amino acids in the small intestine.
Table 1. Amino acid transporters moonlighting as cell surface receptors for cellular entry of specific viruses.
| Transporter | Species | Virus |
|---|---|---|
| Cat 1 (Slc7a1) | Mouse | Ecotropic murine leukemia virus; Bovine leukemia virus |
| ASCT 1 (SLC1A4) | Human | Feline endogenous retrovirus RD-114; Baboon endogenous retrovirus |
| ASCT 2 (SLC1A5) | Human | Baboon endogenous retrovirus; Human endogenous retrovirus HERV-W |
| ACE 2 (chaperone for intestinal amino acid transporters SLC6A19 and SLC6A20) | Human | COVID-19 virus SARS-CoV-2 |
There are several other members of the SLC family that are utilized as cell surface receptors for retroviruses [25], but these are not amino acid transporters. This includes the facilitative glucose transporter GLUT1 (SLC2A1), Na+/H+ exchanger NHE1 (SLC9A1), two phosphate transporters (SLC20A1 and SLC20A2), two vitamin transporters (SLC19A1 and SLC19A2) and the heme transporter SLC49A1.
More recently, two new unconventional functions of amino acid transporters have been brought to light. This includes potentiation of macropinocytosis and regulation of diet-induced obesity (potentiation or protection depending on the transporter involved).
3. Macropinocytosis and SLC38A5/SLC38A3
Macropinocytosis is a mechanism for a non-specific fluid-phase uptake in cells, which is distinct from other similar processes such as pinocytosis and receptor-mediated endocytosis [35][36]. This pathway plays a significant role in maintenance of amino acid nutrition in cells because of the entry of extracellular proteins followed by proteolysis in lysosomes with the delivery of the resultant amino acids to the cytoplasm. In some ways, this is akin to autophagy, which uses cellular proteins to maintain amino acid nutrition under specific conditions, again involving lysosomal proteolysis for generation of free amino acids. Interestingly, for some unknown reasons, primary emphasis is placed on macropinocytosis in amino acid nutrition, but this endocytic process cannot be limited to the uptake of extracellular proteins because of its involvement in the non-specific uptake of all components present in extracellular fluid. There is convincing evidence indicating the participation of macropinocytosis in the uptake of native as well as oxidized low-density lipoproteins (LDL) [37][38]. Therefore, macropinocytosis is also likely to provide lipid nutrients such as cholesterol, triglycerides and phospholipids to cells. Understandably, macropinocytosis and autophagy are critical for cancer cells under conditions of nutrient deprivation [39][40][41]. The importance of these alternative modes of amino acid delivery to cancer cells is underscored by the increased demand for amino acids and other nutrients to support the rapid proliferation in these cells. This is in addition to the marked upregulation of selective transporters for amino acids, peptides and other nutrients in cancer cells to satisfy the nutritional needs by the traditional pathway, namely transporter-mediated delivery [42][43][44][45][46].
Macropinocytosis is activated by various oncogenes such the EGF receptor and activating mutations in KRAS [47][48]. Even though macropinocytosis is independent of clathrin and caveolin, the process still requires remodeling of the cytoskeletal protein actin [49]. One of the major factors that positively influences this remodeling, which is necessary for the initiation of invagination of the plasma membrane for macropinocytosis, is the alkalinization of the pH on the cytoplasmic side of the plasma membrane. The activation of macropinocytosis by the EGF receptor involves the induction of the Na+/H+ exchanger subtype NHE1 (SLC9A1) that mediates the efflux of H+ from cells in exchange for the influx of Na+ [50]. Alternative signaling mechanisms may also participate in the induction of macropinocytosis by EGF [51]. In the case of KRAS mutations, it is the recruitment of vacuolar ATPase to the plasma membrane that accomplishes H+ efflux from the cells [52]. In both cases, the end result is the alkalinization of the cytoplasmic domain of the plasma membrane, which then promotes actin remodeling to initiate macropinocytosis.
If efflux of H+ from the cells either via the Na+/H+ exchanger or v-ATPase promotes macropinocytosis, could amino acid transporters that mediate H+ from the cells as a part of their transport mechanism have a similar effect? This question led to the investigations of the amino acid transporter SLC38A5 regarding its potential connection to macropinocytosis [53]. SLC38A5, also called System N2 (SN2) or sodium-coupled neutral amino acid transporter 5 (SNAT5), is a Na+-coupled transporter for the amino acids glutamine, histidine, asparagine, glycine, serine and methionine, and its transport process is coupled to a simultaneous release of H+ from the cells (Figure 1). The Na+:H+ stoichiometry is 1:1, which makes the transport process electroneutral because all of its amino acid substrates are zwitterionic with no net charge. As such, SLC38A5 is an amino acid-dependent Na+/H+ exchanger [54][55]. Cellular uptake of amino acids via this transporter in the presence of extracellular Na+ does lead to intracellular alkalinization resulting from H+ efflux [55]. This transporter is highly upregulated in breast cancer, particularly in triple-negative breast cancer [56]. Since the substrate selectivity of SLC38A5 includes glycine, serine and methionine, the amino acids essential for one-carbon metabolism, and also glutamine, the amino acid obligatory for the cancer cell-specific metabolic pathway known as glutaminolysis, the increased expression and activity of this transporter in cancer cells fuels oncogenic metabolism and supports tumor growth [56]. Since triple-negative breast cancer cells express high levels of SLC38A5, the connection of this transporter to macropinocytosis was investigated in these cells [53]. These studies established convincingly that amino acid uptake into cells via this transporter is coupled to activation of macropinocytosis as monitored by the cellular uptake of TMR (tetramethylrhodamine)-dextran, a fluorescent marker which detects macropinocytosis. Interestingly, SLC38A5-stimulated macropinocytosis is inhibitable by the amiloride derivatives such as ethylisopropylamiloride [53]. This raised the question as to whether this amino acid transporter, which functions as a Na+/H+ exchanger in the presence of amino acids, is inhibitable by EIPA and other amiloride derivatives which are known for their activity as inhibitors of the classical Na+/H+ exchangers. Subsequent experiments showed that it is indeed the case; SLC38A5 is directly inhibited by amilorides [53].
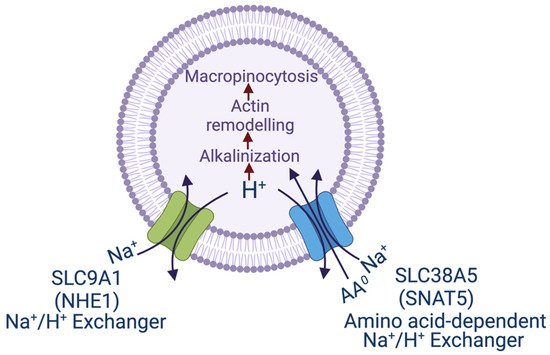
Figure 1. Transport pathways for NHE1 and SNAT5 and their relevance to macropinocytosis. The scheme shows the similarity between the two transporters in the efflux of H+, one being just the Na+/H+ exchanger (NHE1) and the other being an amino acid-dependent Na+/H+ exchanger. In both cases, the transport process leads to alkalinization in the cytoplasmic subdomain underneath the plasma membrane, which initiates remodeling of actin filaments and consequently promotes macropinocytosis. SNAT5, sodium-coupled neutral amino acid transporter 5, another name for SLC38A5.
Until recently, four amino acid transporters received most of the attention for their role in amino acid nutrition in cancer cells; these are SLC7A5, SLC1A5, SLC7A11 and SLC6A14 [42][43][44][45]. None of these has been shown to be associated with macropinocytosis. This makes SLC38A5 unique. This transporter is also upregulated in specific cancers, and its tumor-promoting functions are not restricted to the supply of selective amino acids to cancer cells. Its function is also coupled to regulation of intracellular pH because of the involvement of H+ as one of the cotransported ions. Influx of amino acid substrates via SLC38A5 is associated with removal of H+ from the cells, thus providing a novel mechanism for the maintenance of cellular pH. This process is critical for cancer cells because they generate large amounts of lactic acid via aerobic glycolysis and hence need effective pathways to remove H+ from cells [57]. SLC38A5 provides one such mechanism. The new findings that this amino acid transporter also promotes macropinocytosis underscores the importance of this transporter to tumor growth because macropinocytosis is an efficient pathway for the provision of amino acid and other nutrients to cancer cells. Analysis of data available at the Cancer Genome Atlas (TGCA) reveals that SLC38A5 is upregulated not only in triple-negative breast cancer but also in pancreatic cancer. In fact, the levels of SLC38A5 expression correlate reciprocally with the survival of the patients with pancreatic cancer. It is important to note that macropinocytosis has received the utmost attention in pancreatic cancer because of the widespread occurrence of activating mutations in KRAS and their role in the potentiation of macropinocytosis as a novel mechanism to ensure optimal nutrition in cancer cells. Therefore, SLC38A5, as not only the provider of amino acids but also as an activator of macropinocytosis, assumes a unique place among the amino acid transporters that are known to be upregulated in cancer.
SLC38A3, also known as SN1 or SNAT3, represents another subtype of the amino acid transport system N [14]. The mechanism of transport function is identical for SLC38A5 and SLC38A3 in that the latter is also an amino acid-dependent Na+/H+ exchanger. It can be predicted that SLC38A3 is also capable of activating macropinocytosis in an amino acid-coupled manner. SLC38A3 plays an obligatory role in the kidney in the acid–base balance. During metabolic acidosis, the kidney has to eliminate the excess H+, and this requires a source of ammonia, which combines with H+, and the resultant NH4+ is eliminated across the apical membrane into urine. Glutamine constitutes this ammonia source, and SLC38A3 is the primary provider of this glutamine. Accordingly, SLC38A3 is induced in the basolateral membrane of the epithelial cells in the kidney during metabolic acidosis to provide this glutamine via Na+-coupled uptake from the circulation [58]. Interestingly, this increased expression is specific to SLC38A3; the expression of SLC38A5 in the kidney is not influenced by metabolic acidosis [58]. A loss-of-function mutation in Slc38a3 causing Slc38a3 deficiency in a mouse model confirmed the critical role of this transporter in metabolic acidosis [59]. it can be speculated that macropinocytosis might be activated in kidney tubular cells during metabolic acidosis and that cellular uptake of plasma proteins and lipoproteins might increase as a result. What this means in terms of tubular cell physiology and pathology needs to be investigated. At present, this line of thinking is only speculative, and needs experimental testing and validation.
SLC38A3 and SLC38A5 are also expressed in the brain, primarily in astrocytes where they are considered to play a role in the glutamine–glutamate cycle in which the transporters mediate the release of glutamine from astrocytes [60][61]. SLC38A5 is also expressed specifically at markedly high levels in endothelial cells of the blood–brain barrier and blood–retinal barrier [62] (https://www.proteinatlas.org/ENSG00000017483-SLC38A5/single+cell+type/eye, accessed on 1 January 2022). It would be interesting to ascertain in future studies if these transporters promote macropinocytosis in these cell types and if they do, what the physiological and pathological consequences are from the resultant entry of extracellular components into these cells.
References
- Broer, S. Amino acid transport across mammalian intestinal and renal epithelia. Physiol. Rev. 2008, 88, 249–286.
- Thwaites, D.T.; Anderson, C.M.H. The SLC36 family of proton-coupled amino acid transporters and their potential role in drug transport. Br. J. Pharmacol. 2011, 164, 1802–1816.
- Pochini, L.; Scalise, M.; Galluccio, M.; Indiveri, C. Membrane transporters for the special amino acid glutamine: Structure/function relationships and relevance to human health. Front. Chem. 2014, 2, 61.
- Papalazarou, V.; Maddocks, O.D.K. Supply and demand: Cellular nutrient uptake and exchange in cancer. Mol. Cell 2021, 81, 3731–3748.
- Wyant, G.A.; Abu-Remaileh, M.; Wolfson, R.L.; Chen, W.W.; Freinkman, E.; Danai, L.V.; Vander Heiden, M.G.; Sabatini, D.M. mTORC1 activator SLC38A9 is required to efflux essential amino acids from lysosomes and use protein as a nutrient. Cell 2017, 171, 642–654.e12.
- Huizing, M.; Gahl, W.A. Inherited disorders of lysosomal membrane transporters. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183336.
- Kunji, E.R.S.; King, M.S.; Ruprecht, J.J.; Thangaratnarajah, C. The SLC25 carrier family: Important transport proteins in mitochondrial physiology and pathology. Physiology 2020, 35, 302–327.
- Broer, A.; Cavanaugh, J.A.; Rasko, J.E.J.; Broer, S. The molecular basis of neutral aminoacidurias. Pflug. Arch. 2006, 451, 511–517.
- Broer, S. Diseases associated with general amino acid transporters of the solute carrier 6 family (SLC6). Curr. Mol. Pharmacol. 2013, 6, 74–87.
- Broer, S.; Fairweather, S.J. Amino acid transport across the mammalian intestine. Compr. Physiol. 2018, 9, 343–373.
- Yahyaoui, R.; Perez-Frias, J. Amino acid transport defects in human inherited metabolic disorders. Int. J. Mol. Sci. 2019, 21, 119.
- Taylor, P.M. Role of amino acid transporters in amino acid sensing. Am. J. Clin. Nutr. 2014, 99, 223S–230S.
- Pinilla, J.; Aledo, J.C.; Cwiklinski, E.; Hyde, R.; Taylor, P.M.; Hundal, H.S. SNAT2 transceptor signalling via mTOR: A role in cell growth and proliferation? Front. Biosci. 2011, 3, 1289–1299.
- Broer, S. The SLC38 family of sodium-amino acid co-transporters. Pflug. Arch. 2014, 466, 155–172.
- Varoqui, H.; Zhu, H.; Yao, D.; Ming, H.; Erickson, J.D. Cloning and functional identification of a neuronal glutamine transporter. J. Biol. Chem. 2000, 275, 4049–4054.
- Wang, H.; Huang, W.; Sugawara, M.; Devoe, L.D.; Leibach, F.H.; Prasad, P.D.; Ganapathy, V. Cloning and functional expression of ATA1, a subtype of amino acid transporter A, from human placenta. Biochem. Biophys. Res. Commun. 2000, 273, 1175–1179.
- Sugawara, M.; Nakanishi, T.; Fei, Y.J.; Huang, W.; Ganapathy, M.E.; Leibach, F.H.; Ganapathy, V. Cloning of an amino acid transporter with functional characteristics and tissue expression pattern identical to that of system A. J. Biol. Chem. 2000, 275, 16473–16477.
- Sugawara, M.; Nakanishi, T.; Fei, Y.J.; Martindale, R.G.; Ganapathy, M.E.; Leibach, F.H.; Ganapathy, V. Structure and function of ATA3, a new subtype of amino acid transport system A, primarily expressed in the liver and skeletal muscle. Biochim. Biophys. Acta 2000, 1509, 7–13.
- Hyde, R.; Cwiklinski, E.L.; MacAulay, K.; Taylor, P.M.; Hundal, H.S. Distinct sensor pathways in the hierarchical control of SNAT2, a putative amino acid transporter, by amino acid availability. J. Biol. Chem. 2007, 282, 19788–19798.
- Ling, R.; Bridges, C.C.; Sugawara, M.; Fujita, T.; Leibach, F.H.; Prasad, P.D.; Ganapathy, V. Involvement of transporter recruitment as well as gene expression in the substrate-induced adaptive regulation of amino acid transport system A. Biochim. Biophys. Acta 2001, 1512, 15–21.
- Rebsamen, M.; Pochini, L.; Stasyk, T.; de Araujo, M.E.; Galluccio, M.; Kandasamy, R.K.; Snijder, B.; Fauster, A.; Rudashevskaya, E.L.; Bruckner, M.; et al. SLC38A9 is a component of the lysosomal amino acid sensing machinery that controls mTORC1. Nature 2015, 519, 477–481.
- Wang, S.; Tsun, Z.Y.; Wolfson, R.L.; Shen, K.; Wyant, G.A.; Plovanich, M.E.; Yuan, E.D.; Jones, T.D.; Chantranupong, L.; Comb, W.; et al. Lysosomal amino acid transporter SLC38A9 signals arginine sufficiency to mTORC1. Science 2015, 347, 188–194.
- Heublein, S.; Kazi, S.; Ogmundsdottir, M.H.; Attwood, E.V.; Kala, S.; Boyd, C.A.; Wilson, C.; Goberdhan, D.C. Proton-assisted amino-acid transporters are conserved regulators of proliferation and amino-acid-dependent mTORC1 activation. Oncogene 2010, 29, 4068–4079.
- Goberdhan, D.C.; Wilson, C.; Harris, A.L. Amino acid sensing by mTORC1: Intracellular transporters mark the spot. Cell Metab. 2016, 23, 580–589.
- Greenwood, A.D.; Ishida, Y.; O’Brien, S.P.; Roca, A.L.; Eiden, M.V. Transmission, evolution, and endogenization: Lessons learned from recent retroviral invasions. Microbiol. Mol. Biol. Rev. 2018, 82, e00044-17.
- Kim, J.W.; Closs, E.I.; Albritton, L.M.; Cunningham, J.M. Transport of cationic amino acids by the mouse ecotropic retrovirus receptor. Nature 1991, 352, 725–728.
- Wang, H.; Kavanaugh, M.P.; North, R.A.; Kabat, D. Cell-surface receptor for murine retroviruses is a basic amino-acid transporter. Nature 1991, 352, 729–731.
- Marin, M.; Tailor, C.S.; Nouri, A.; Kabat, D. Sodium-dependent neutral amino acid transporter type 1 is an auxiliary receptor for baboon endogenous retrovirus. J. Virol. 2000, 74, 8085–8093.
- Tailor, C.S.; Nouri, A.; Zhao, Y.; Takeuchi, Y.; Kabat, D. A sodium-dependent neutral amino-acid transporter mediates infections of feline and baboon endogenous retroviruses and simian type D retroviruses. J. Virol. 1999, 73, 4470–4474.
- Rasco, J.E.; Battini, J.L.; Gottschalk, R.J.; Mazo, I.; Miller, A.D. The RD114/simian type D retrovirus receptor is a neutral amino acid transporter. Proc. Natl. Acad. Sci. USA 1999, 96, 2129–2134.
- Singer, D.; Camargo, S.M. Collectrin and ACE2 in renal and intestinal amino acid transport. Channels 2011, 5, 410–423.
- Li, W.; Moore, M.J.; Vasilieva, N.; Sui, J.; Wong, S.K.; Berne, M.A.; Somasundaran, M.; Sullivan, J.L.; Luzuriaga, K.; Greenough, T.C.; et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003, 426, 450–454.
- Scialo, F.; Daniele, A.; Amato, F.; Pastore, L.; Matera, M.G.; Cazzola, M.; Castaldo, G.; Bianco, A. ACE2: The major cell entry receptor for SARS-CoV-2. Lung 2020, 198, 867–877.
- Singer, D.; Camargo, S.M.; Ramadan, T.; Schafer, M.; Mariotta, L.; Herzog, B.; Huggel, K.; Wolfer, D.; Werner, S.; Penninger, J.M.; et al. Defective intestinal amino acid absorption in Ace2 null mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G686–G695.
- Lin, X.P.; Mintern, J.D.; Gleeson, P.A. Macropinocytosis in different cell types: Similarities and differences. Membranes 2020, 10, 177.
- Puccini, J.; Badgley, M.A.; Bar-Sagi, D. Exploiting cancer’s drinking problem: Regulation and therapeutic potential of macropinocytosis. Trends Cancer 2021, 8, 54–64.
- Csanyi, G.; Feck, D.M.; Ghoshal, P.; Singla, B.; Lin, H.; Nagarajan, S.; Meijles, D.N.; Ghouleh, I.A.; Cantu-Medellin, N.; Kelley, E.E.; et al. CD47 and Nox1 mediate dynamic fluid-phase macropinocytosis of native LDL. Antioxid. Redox Signal. 2017, 26, 886–901.
- Doodnauth, S.A.; Grinstein, S.; Maxson, M.E. Constitutive and stimulated macropinocytosis in macrophages: Roles in immunity and in the pathogenesis of atherosclerosis. Philos. Trans. R. Soc. B 2019, 374, 20180147.
- Commisso, C. The pervasiveness of macropinocytosis in oncological malignancies. Philos. Trans. R. Soc. B 2019, 374, 20180153.
- Stow, J.L.; Hung, Y.; Wall, A.A. Macropinocytosis: Insights from immunology and cancer. Curr. Opin. Cell Biol. 2020, 65, 131–140.
- Encarnacion-Rosado, J.; Kimmelman, A.C. Harnessing metabolic dependencies in pancreatic cancers. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 482–492.
- Ganapathy, V.; Thangaraju, M.; Prasad, P.D. Nutrient transporters in cancer: Relevance to Warburg hypothesis and beyond. Pharmacol. Ther. 2009, 121, 29–40.
- Bhutia, Y.D.; Babu, E.; Ramachandran, S.; Ganapathy, V. Amino acid transporters in cancer and their relevance to “glutamine addiction”: Novel targets for the design of a new class of anticancer drugs. Cancer Res. 2015, 75, 1782–1788.
- Bhutia, Y.D.; Ganapathy, V. Glutamine transporters in mammalian cells and their functions in physiology and cancer. Biochim. Biophys. Acta 2016, 1863, 2531–2539.
- Broer, S. Amino acid transporters as targets for cancer therapy: Why, where, when and how? Int. J. Mol. Sci. 2020, 21, 6156.
- Schniers, B.K.; Rajasekaran, D.; Korac, K.; Sniegowski, T.; Ganapathy, V.; Bhutia, Y.D. PEPT1 is essential for the growth of pancreatic cancer cells: A viable drug target. Biochem. J. 2021, 478, 3757–3774.
- Weerasekara, V.K.; Patra, K.C.; Bardeesy, N. EGFR pathway links amino acid levels and induction of macropinocytosis. Dev. Cell 2019, 50, 261–263.
- Yoshida, S.; Pacitto, R.; Inoki, K.; Swanson, J. Macropinocytosis, mTORC1 and cellular growth control. Cell. Mol. Life Sci. 2018, 75, 1227–1239.
- Kay, R.R.; Williams, T.D.; Manton, J.D.; Traynor, D.; Paschke, P. Living on soup: Macropinocytic feeding in amoebae. Int. J. Dev. Biol. 2019, 63, 473–483.
- Sasabe, E.; Tomomura, A.; Liu, H.; Sento, S.; Kitamura, N.; Yamamoto, T. EGF/EGFR signaling blockage inhibits tumor cell-derived exosome uptake by oral squamous cell carcinoma through macropinocytosis. Cancer Sci. 2021, in press.
- Lee, S.W.; Zhang, Y.; Jung, M.; Cruz, N.; Alas, B.; Commisso, C. EGFR-Pak signaling selectively regulates glutamine deprivation-induced macropinocytosis. Dev. Cell 2019, 50, 381–392.
- Ramirez, C.; Hauser, A.D.; Vucic, E.A.; Bar-Sagi, D. Plasma membrane v-ATPase controls oncogenic Ras-induced macropinocytosis. Nature 2019, 576, 477–481.
- Ramachandran, S.; Sennoune, S.R.; Sharma, M.; Thangaraju, M.; Suresh, V.V.; Sniegowski, T.; Bhutia, Y.D.; Pruitt, K.; Ganapathy, V. Expression and function of SLC38A5, an amino acid-coupled Na+/H+ exchanger, in triple-negative breast cancer and its relevance to macropinocytosis. Biochem. J. 2021, 478, 3857–3976.
- Nakanishi, T.; Sugawara, M.; Huang, W.; Martindale, R.G.; Leibach, F.H.; Ganapathy, M.E.; Prasad, P.D.; Ganapathy, V. Structure, function, and tissue expression pattern of human SN2, a subtype of the amino acid transport system N. Biochem. Biophys. Res. Commun. 2001, 281, 1343–1348.
- Nakanishi, T.; Kekuda, R.; Fei, Y.J.; Hatanaka, T.; Sugawara, M.; Martindale, R.G.; Leibach, F.H.; Prasad, P.D.; Ganapathy, V. Cloning and functional characterization of a new subtype of the amino acid transport system N. Am. J. Physiol. Cell Physiol. 2001, 281, C1757–C1768.
- Sniegowski, T.; Korac, K.; Bhutia, Y.D.; Ganapathy, V. SLC6A14 and SLC38A5 drive the glutaminolysis and serine-glycine-one-carbon pathways in cancer. Pharmaceuticals 2021, 14, 216.
- Brown, T.P.; Ganapathy, V. Lactate/GPR81 signaling and proton motive fore in cancer: Role in angiogenesis, immune escape, nutrition, and Warburg phenomenon. Pharmacol. Ther. 2020, 206, 107451.
- Busque, S.M.; Wagner, C.A. Potassium restriction, high protein intake, and metabolic acidosis increase expression of the glutamine transporter SNAT3 (Slc38a3) in mouse kidney. Am. J. Physiol. Ren. Physiol. 2009, 297, F440–F450.
- Chan, K.; Busque, S.M.; Sailer, M.; Stoeger, C.; Broer, S.; Daniel, H.; Rubio-Aliaga, I.; Wagner, C.A. Loss of function mutation of the Slc38a3 glutamine transporter reveals its critical role for amino acid metabolism in the liver, brain, and kidney. Pflug. Arch. 2016, 468, 213–227.
- Rubio-Aliaga, R.; Wagner, C.A. Regulation and function of the SLC38A3 (SNAT3) glutamine transporter. Channels 2016, 10, 440–452.
- Umapathy, N.S.; Li, W.; Mysona, B.A.; Smith, S.B.; Ganapathy, V. Expression and function of glutamine transporters SN1 (SNAT3) and SN2 (SNAT5) in retinal Muller cells. Investig. Ophthalmol. Vis. Sci. 2005, 46, 3980–3987.
- Daneman, R.; Zhou, L.; Agalliu, D.; Cahoy, J.D.; Kaushal, A.; Barres, B.A. The mouse blood-brain barrier transcriptome: A new resource for understanding the development and function of brain endothelial cells. PLoS ONE 2010, 5, e13741.
More
Information
Subjects:
Cell Biology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.9K
Revisions:
2 times
(View History)
Update Date:
02 Mar 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




