Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Amani Alharthi | + 3913 word(s) | 3913 | 2022-02-12 07:11:03 | | | |
| 2 | Jessie Wu | Meta information modification | 3913 | 2022-03-02 03:13:22 | | | | |
| 3 | Jessie Wu | -14 word(s) | 3899 | 2022-03-02 03:19:47 | | | | |
| 4 | Jessie Wu | Meta information modification | 3899 | 2022-03-02 03:41:09 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Alharthi, A.; Alhazmi, S. The Human Gut Microbiome in Autism Spectrum Disorder. Encyclopedia. Available online: https://encyclopedia.pub/entry/20045 (accessed on 08 February 2026).
Alharthi A, Alhazmi S. The Human Gut Microbiome in Autism Spectrum Disorder. Encyclopedia. Available at: https://encyclopedia.pub/entry/20045. Accessed February 08, 2026.
Alharthi, Amani, Safiah Alhazmi. "The Human Gut Microbiome in Autism Spectrum Disorder" Encyclopedia, https://encyclopedia.pub/entry/20045 (accessed February 08, 2026).
Alharthi, A., & Alhazmi, S. (2022, March 01). The Human Gut Microbiome in Autism Spectrum Disorder. In Encyclopedia. https://encyclopedia.pub/entry/20045
Alharthi, Amani and Safiah Alhazmi. "The Human Gut Microbiome in Autism Spectrum Disorder." Encyclopedia. Web. 01 March, 2022.
Copy Citation
Autism spectrum disorder (ASD) is a complicated neurodevelopmental disorder characterized by decreased verbal and social interactions, limited interests and activities, and repetitive behaviors. Along with these significant conditions, ASD regularly co-occurs with other clinical symptoms, including gastrointestinal disturbances (up to 70%), motor deficits (79%), sleep problems (50–80%), and intellectual disability (45%). The high prevalence of gastrointestinal (GI) disorders among autism spectrum disorder (ASD) patients has prompted scientists to look into the gut microbiota as a putative trigger in ASD pathogenesis.
autism spectrum disorder
gut microbiome
probiotics
1. Introduction
Autism prevalence has risen dramatically worldwide in the last few years, reaching 1 in 132, and with a remarkable increase in occurrence in boys compared with girls [1][2]. In the United States, the prevalence of autism spectrum disorder (ASD) rose from 1 in 150 children in 2000 to 1 in 54 in 2016 [3]. The dramatic increase in ASD reduces parental productivity and increases the financial burden on families, with central expenditures being linked with special schooling [4].
For many years, a high number of studies have been conducted worldwide focusing on the potential etiology of ASD; however, its precise etiology has not been clearly identified. Gene and chromosomal abnormalities, such as fragile X syndrome (FXS); tuberous sclerosis (TSC); and potential defects in chromosomes 2q, 7q, 15q, and 16p, are shown in 35 to 40% of ASD cases. Furthermore, the ASD rate was found to be higher in monozygotic twins than in dizygotic twins and was found to be 50-fold higher among siblings who belong to families that already have ASD children [5]. Additionally, the multigenic disorder of autism has been related to epigenetic effects [6]; nevertheless, no specific gene has been identified as being associated with all cases of ASD. Recently, 100 to 800 genes or genomic regions have been implicated in ASD etiologies [7][8].
60 to 65% of autism occurrence could be explained by prenatal, natal, and postnatal environmental risk factors (Figure 1). Prenatal risk factors involve maternal infection, maternal physical health, the health condition of pregnant women, folate and iron deficiency, and drug use in pregnancy. Natal risk factors include fetal complications, umbilical cord complications, hypoxia (lack of oxygen), cesarean delivery, abnormal presentation of the fetus, and abnormal gestational age (preterm or post-term). Postnatal risk factors include breastfeeding, air contamination, antibiotic intake, and nutrition factors [8][9][10][11][12]. Environmental risk factors can directly influence the neuronal activities of the growing brain of the fetus [11]. These environmental risk factors are largely found to shape the intestinal microbiota [13].
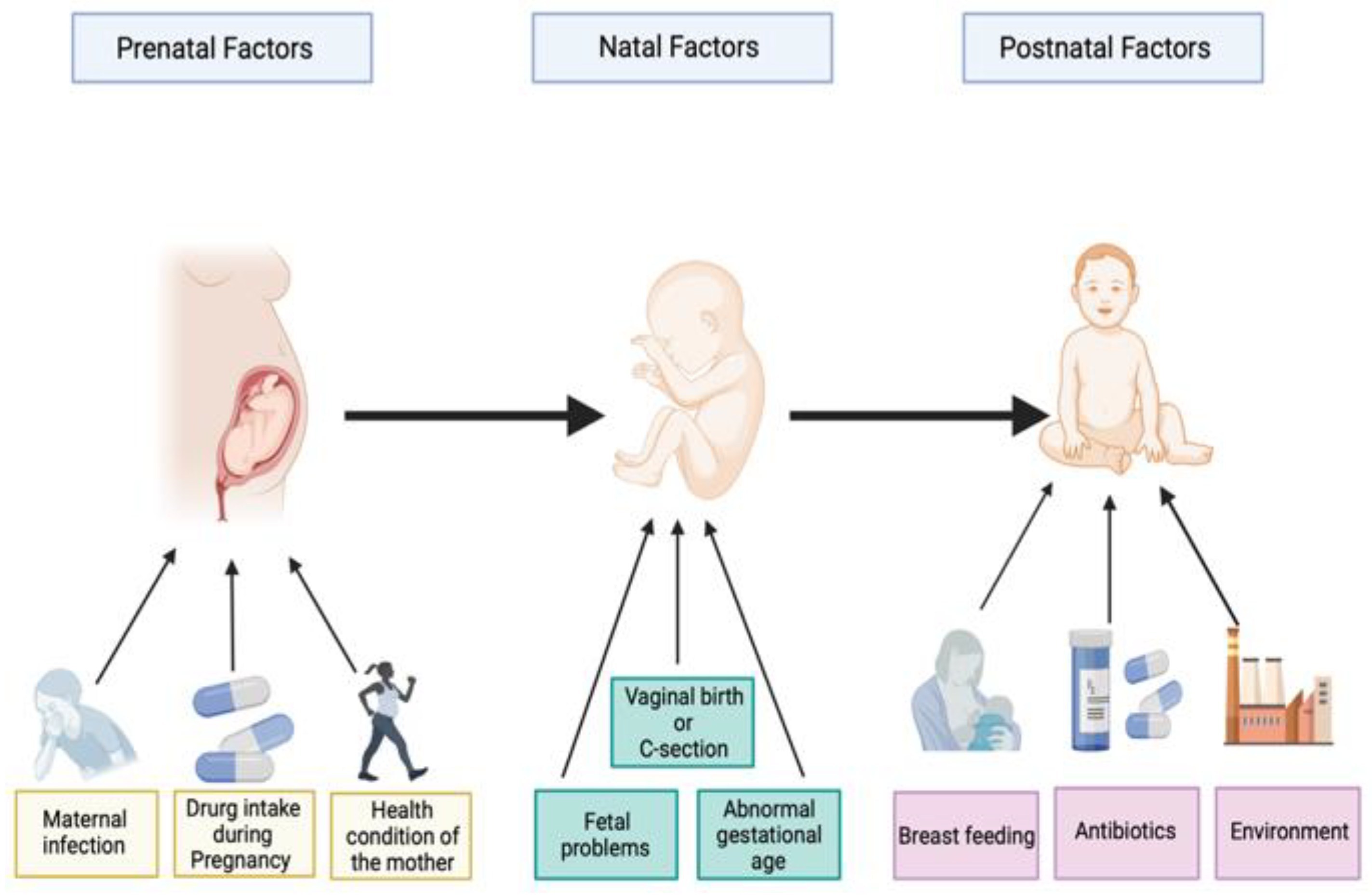
Figure 1. Illustration of some prenatal, perinatal, and postnatal factors associated with autism spectrum disorders. Created with BioRender.com.
2. Signaling Pathways Based on the Gut Microbiome Composition in Autism Spectrum Disorder Patients
2.1. Gut Permeability Pathway
The microbiota and its metabolite products modulate the function and integrity of the gut epithelium barrier. Therefore, a change in gut microbial diversity can influence the gut barrier integrity, potentially resulting in the “leaky gut” condition [14]. Indeed, an impaired gut barrier can increase the levels of gut microbial components (e.g., lipopolysaccharide (LPS)) in the blood; trigger the hypothalamic–pituitary–adrenal (HPA) axis; and stimulate immune responses, producing cytokines such as interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and IL-4. These immune cytokines can circulate and cross the blood–brain barrier (BBB), inducing systemic and CNS inflammation [15][16]. The serum level of LPS was found to be significantly increased in ASD individuals compared to healthy controls. This may be linked to a worse social communication score, which has been noticed in ASD patients [17]. In physiological states, LPS can enter the brain, possibly through a lipoprotein transport mechanism [18], and elicit neural impairment, behavioral alteration, and neuroinflammation by triggering the Nuclear Factor Kappa B (NF-kB) signaling pathway, which is related to microglia stimulation and neuronal cell loss [19]. The everyday injection of pregnant rats with lipopolysaccharide (LPS) resulted in ASD-like behavior in offspring, involving hyperlocomotion and social defects [20].
Multiple findings have suggested that ASD patients have abnormal intestinal permeabilities ranging from 43% to 76%, both with and without gastrointestinal symptoms [21]. Moreover, intestinal permeability was reported in 9 out of 21 autistic children, but not in 40 non-autistic children [22]. De Magistris and colleagues found that ASD individuals and their first-degree relatives had 36.7% and 21.2% altered gut permeabilities, respectively, while ordinary people had only 4.8% [23]. In accordance with previous studies, a significant decrease in the mRNA levels of occludin and zonulin was observed in male BTBR mice (a mouse model of idiopathic autism). Occludin and zonulin are intestinal permeability-modulating proteins that are associated with the maintenance of intestinal permeability [24][25]. Interestingly, intestinal permeability was found to be considerably reduced in autism patients who were on a gluten-free, casein-free diet [23].
In comparison with the above-mentioned studies, others have shown no changes in gut permeability in autistic children, demonstrating that the disruption of the intestinal barrier is not always a symptom of autism, but this primarily occurred in ASD children with intestinal abnormality [26][27]. Thus, additional studies with an increased sample size of ASD patients with and without intestinal abnormality are necessary to confirm and understand the connection between gut permeability and increased symptoms of autism.
2.2. Immune System Pathway
Immunological pathways have a vital function in the bidirectional connection between the microbiota, gut, and brain, allowing the gut and brain to influence each other. Gut microbial composition is an essential part of regulating immune hemostasis, since gut mucosal surfaces are constantly exposed to beneficial and pathogenic microorganisms and can trigger an immunological response [28][29]. In addition, the mucosal surface layers of the gut contain different types of immune cells involving gut-associated lymphoid tissue (GALT) [30]. GALT utilizes lymphocytes to produce immunoglobulins (IgA) [31]. IgA can modify the innate immune response once microbial cells come into contact with dendrites in the ENS. In some studies, a high level of IgA was recognized in ASD patients [27].
Different inflammatory signs have been found in ASD individuals. For example, elevated levels of tumor necrosis factor (TNF) and pro-inflammatory cytokines such as interferon (IFN), IL-1b, IL-6, IL-8, and IL-12p4 were found in the brains of ASD children compared to controls [32][33]. Moreover, the brains of ASD patients revealed a pattern of triggering immunological responses involving the activation of microglial cells, which are responsible for eliminating pathogens [34].
The defect in the immune system in autistic patients has been connected with the alteration of the gut microbial composition. For example, germ-free mice show a higher microglia density in various brain areas than mice grown in a specific pathogen-free (SPF) environment. Additionally, atypical social avoidance behavior and low immune response against virus infection were noticed in these GF mice. Both microglia defects and ASD-linked symptoms were improved following the supplementation of germ-free mice with microbial SCFAs [35]. This research proposed that the gut microbiota can indirectly affect the innate immune system, which can modify the circulating levels of pro-inflammatory and anti-inflammatory cytokines that directly impact microglia homeostasis.
Moreover, in the Hsiao et al. study, an increased level of IL-6 was detected in the adult offspring of a maternal immune activation (MIA) mouse model. Interestingly, the supplementation of MIA offspring with Bacteroides fragilis NCTC 9343 restored microbiota composition, IL-6 levels, and the integrity of the intestinal permeability [36]. Several cytokines, including IL-6, were found to adjust the tight junction transcription level and intestinal barrier integrity by modulating the levels of CLDN 8 and 15. Therefore, the research proposes that the B. fragilis-mediated restoration of IL-6 levels might underpin the role of IL-6 in gut permeability [36].
2.3. The Metabolic Pathway
The gut microbiota generates various metabolites that can travel across the systemic circulation and contact the host immune cells, impact the metabolism, and/or influence the ENS and afferent signaling pathways of the vagus nerve that send signals directly to the CNS [37]. The metabolites that are derived from the microbiota include multiple products, such as short-chain fatty acids (SCFAs), phenolic compounds, and free amino acids (FAAs) [38]. Butyric acid (BA), propionic acid (PAA), and acetic acid (AA) are all types of short-chain fatty acids that result from the anaerobic fermentation of indigestible carbohydrates [39]. SCFAs play a vital function in the body such as in the homeostasis of energy, in the enhancement of glucose metabolism, in lowering body weight, and in reducing the chance of colon cancer [40]. Additionally, SCFA is implicated in the regulation of the immune response by modulating the secretion of T-cell cytokines [41].
Despite the data being slightly inconsistent, acetate and propionate have been found to be upregulated in individuals with ASD, whereas butyrate was shown to be significantly decreased [42][43]. PAA can act as a neurotoxin that affects the electron transport chain by inhibiting the formation of nicotinamide adenine dinucleotide (NADH), the primary substrate of the electron transport chain [44]. PAA can also trigger the immune response and change gene expression [12][45]. Increased levels of PAA have been related to increased severity of ASD. For example, in experimental trials, rats treated for eight days with PAA displayed hyperactivity and stereotypy movement. Additionally, PAA-treated rats exhibited significant changes in the composition of brain and plasma phospholipid molecular species. Alterations in brain plasma phospholipid composition, especially throughout development, can theoretically have severe effects on CNS function [46]. GI symptoms and modified blood phospholipid profiles have been detected in individuals with ASD. Thus, since phospholipids are the main structural components of many cellular and neuronal membranes [47], ASD, as a neurodevelopmental disorder, might be related to functional deficits or imbalances in fatty acid metabolism [48].
On the other hand, butyrate was observed to have a positive influence on ASD-related behavior [39]. In addition, butyrate can protect cells from oxidative stress and improve mitochondrial function during physiological stress [49]. Interestingly, butyrate was found to restore the ASD deficiencies introduced by PAA, likely by enhancing the BBB permeability [50]. GF mice colonized with Clostridium tyrobutyricum (butyrate-producing bacteria) or acetate and propionate-producing Bacteroides thetaiotaomicron can improve the expression of occludins, which were found to be associated with the reduced permeability of the BBB [51].
Moreover, p-Cresol and its conjugated derivatives were observed at an elevated rate in the urinary samples of children with ASD [52]. P-Cresol can aggravate ASD severity and gut function because it plays a role in many metabolic processes in the human body [42]. In addition, P-Cresol has been linked with nervous system abnormalities, including raising brain lipid peroxidation, reducing Na(+)-K+ ATPase function, and inhibiting noradrenaline formation [53]. Clostridium difficile is one of the most typical representative microbes and is known for forming p-Cresol. C. difficile can induce the p-hydroxyphenylacetate (p-HPA) enzyme and therefore stimulate the fermentation of tyrosine for the production of p-Cresol [54]. Notably, mice given p-Cresol in drinking water for four weeks exhibited an altered gut microbiota composition and social-behavioral defects [55]. The p-Cresol intervention also decreases the excitability of dopamine neurons in the ventral tegmental area (VTA) of these mice, a circuit implicated in the social reward system [56]. The influence of p-Cresol on behavior was associated with the gut microbial composition, as microbial transplantation from p-Cresol-treated mice to control mice can stimulate behavioral defects. However, microbial transplantation from normal mice to p-Cresol-treated mice was found to restore normal social behaviors [55]. A microbial metabolite such as p-Cresol could provoke ASD-like behavior in mice.
Collectively, all these previous studies are consistent with the emerging theory of disruption of excitatory/inhibitory neuronal function in ASD [57].
2.4. Neuronal Signaling Pathway
The microbiota of the gut can produce molecules such as serotonin (5-hydroxytryptamine, 5-HT), γ-aminobutyric acid (GABA), and acetylcholine, which can act as typical neurotransmitters influencing ENS and CNS activity [58]. Serotonin is one of the essential brain neurotransmitters that have a crucial function in regulating mood and GI activity [59]. About 95% of total serotonin in the human body is formed by enterochromaffin cells (Ecs) in the GI tract, while around 5% of the remaining serotonin is found in the brain [60]. Interestingly, gut microbes such as Escherichia spp., Enterococcus spp., Streptococcus spp., and Candida spp. have been shown to be engaged in the production of serotonin [61]. The production and secretion of 5-HT by Ecs have been suggested to be affected by the gut microbial composition [62]. For example, the depletion of the gut microbiota by antibiotics in mice was found to be associated with impaired learning and elevated depression-like behaviors. This occurred with changes in the levels of CNS 5-HT concentration, as well as with alterations in the mRNA levels of corticotrophin-releasing hormone receptor 1 and the glucocorticoid receptor [63]. Moreover, a positive relationship was detected between the level of 5-HT in the blood and the severity of gastrointestinal symptoms [64].
On other hand, serotonin can also be formed from the essential amino acid tryptophan (Trp) [65]. Clostridia spp. stimulates the transformation of tryptophan to 5-HT by raising the mRNA levels of tryptophan hydroxylase 1 in Ecs [60]. Reducing tryptophan in the diet indeed seems to increase autistic behavior. Consequently, these studies show that the gut microbiota can have a crucial role in the production and homeostasis of the 5-HT [66].
GABA is an amino acid that functions as the main inhibitory neurotransmitter in the brain. An altered pattern of GABA has been detected as a key feature of the neurophysiology of ASD patients [67]. If the inhibitory GABAergic transmission is altered in individuals with ASD, it can end in an irregular balance of excitation/inhibition in the brain and changes in neural communication, the handling of instructions, and responding performance [68]. Indeed, Bifidobacterium spp. and Lactobacillus spp. have the ability to produce GABA [69]; for example, the colonization of mice with Lactobacillus rhamnosus JB-1 increases the level of GABA receptors in the vagus nerve and decreases stress and depressive behavior [70].
Together, these outcomes emphasize the essential function of the gut microbiota in the communication pathways between the gut microbiota and the brain, suggesting that bacteria may prove to be a beneficial treatment.
2.5. Neuroendocrine Signaling Pathway
The hypothalamic–pituitary–adrenal (HPA) axis is another pathway by which the brain can control the activity of intestine effector cells, gut permeability, motility, mucus, and immunity, causing the translocation of gut microbial constituents. Under stress conditions, corticotrophin-releasing hormone (CRH) is released from the hypothalamus and causes the pituitary gland to secrete adrenocorticotropic hormone (ACTH). ACTH then regulates the adrenal glands to produce and secrete hormones, such as cortisol and glucocorticoids, into the blood, which affect many bodily organs including the brain [15][58]. This initial research demonstrated that the gut microbiota can directly affect the host HPA axis. GF mice that have been exposed to restraint stress showed an increased serum concentration of the two commonly associated stress hormones ACTH and CRH. However, the colonization of young mice with Bifidobacterium infantis reversed hormonal abnormalities [71]. In the same study, the expression of brain-derived neurotrophic factor (BDNF) and N-methyl-D-aspartate (NMDA) receptor was also reduced in the cerebral cortex and hippocampus of GF mice, influencing the expression and release of CRH and thereby altering the HPA axis function [71]. Several studies, particularly those carried out in individuals with ASD, have found altered levels of mRNA in the glucocorticoid receptor and CRH receptor 1 [72], which basically indicates the modification of this pathway.
3. Role of Epigenetics in Autism Spectrum Disorder
In the last few decades, the rapid rise in the rate of ASD has demonstrated that autism cannot be caused only by genetics. Therefore, scientists have examined the relationships between genetics and the environment, especially studying the role of epigenetics in causing ASD [6]. Epigenetics investigates the ways in which environmental and lifestyle factors influence DNA expression without changing the DNA sequence, which can be transmitted from one generation to another via germline cells. These epigenetic modifications can control when, or even if, a specific gene turns on and off in a cell or organism [73][74].
DNA methylation, post-transcriptional histone modifications, and gene expression regulation by non-coding RNAs are some examples of epigenetic regulation [75]. DNA methylation has been related to the etiology of nervous disorders, including ASD [76]. For example, a methylome analysis study of the human placenta exhibited a significantly higher level of a methyl group in patients with ASD through the use of pyrosequencing [77].
Several compelling pieces of evidence suggesting that the gut microbial community is directly responsible for initiating epigenetic modifications [78]. Exchange talk between microbic metabolites and external effectors such as antibiotics, nutrition, and other environmental factors can shape the epigenome (temperature, oxygen, and pH) [79]. Commensal bacteria in the gut can synthesize folate, vitamin B12, and choline, all of which are fundamental in the production of a methyl group donor (6-methyltetrahydrofolate) and the formation of S-adenosylmethionine (SAM), which is the main methyl donor in the DNA methylation process [80]. For example, Bifidobacteria and Lactobacillus species are known for folate synthesis [81]. Another critical microbial metabolite that affects epigenetics is butyric acid, a potent inhibitor of histone deacetylases [82], which removes the acetyl group from histone proteins, letting the proteins re-associate with DNA and preventing DNA transcription. Moreover, the latest suggestion shows that some endosymbiotic bacteria make small non-coding RNAs that influence host processes [83].
Based on the above-mentioned findings regarding the involvement of epigenetics in ASD, one can assume that dysbiosis in the gut microbiota composition, particularly in the early periods of development, could directly switch a specific gene on or off. In this situation, the excessive use of antibiotics may affect microbial diversity and turn on a particular gene related to autism.
4. The Potential Therapeutic Perspectives of Autism Spectrum Disorder Targeting Gut Microbiota
There is no current reliable therapy for treating patients with ASD. However, because of the increasing amount of data regarding the role of gut microbial dysbiosis in ASD, researchers are currently focusing on strategies for treating such a disease by modulating the gut microbial community as a potential therapeutic approach. This approach involves oral prebiotic, probiotic, dietary, and/or fecal microbiota transplantation (FMT) as well as microbiota transfer therapy.
Mounting evidence from human and animal studies suggests that gut microbial-targeting therapy may be beneficial as a new and safe method for treating ASD patients. Antibiotics have been used as a possible treatment for ASD patients, but antibiotics influence gut homeostasis by targeting pathogens and commensal bacteria. Thus, antibiotics are not a possible option for long-term therapy for ASD. Probiotics can colonize the gut and restore the composition of bacterial populations, which, in turn, has been found to reduce autism-related symptoms. Though probiotics are commonly safe to use, a study by Rondanelli et al. [84] advises that individuals with serious underlying medical illnesses or weakened immune systems should not take probiotics, since some individuals with these circumstances were found to have bacterial or fungal infections as a consequence of probiotic intake [84]. Prebiotics serve as food for commensal bacteria, so they stimulate an increase in beneficial bacteria that are found naturally in the body and improve digestive health. Studies on ASD patients using prebiotics are limited, and there is a lack of available solid data [43]. Multiple studies have shown that ketogenic diets (KD), gluten-free and casein-free (GFCF) diets, and supplementation with omega-3-fatty acids have beneficial effects on the health of children with ASD, but the evidence available is limited and weak. Marí-Bauset et al. found some possible side effects of the GFCF diet, such as calcium deficiency and a lack of essential amino acids, resulting in decreased bone density and frequent bone fractures. Moreover, ASD patients who followed the GFCF diet needed more supplementation with vitamin D [85]. Fecal microbiota transplantation (FMT) can modify the gut microbiota composition by transplanting fecal microbiota from healthy donors to ASD patients [86]. FMT has emerged as a safe and promising therapeutic approach and can restore metabolites and immune function. However, FMT could have future unexpected health effects, since there are many microbes in the gut that have not been determined yet which may introduce pathogenic bacteria into the host’s intestinal system. Therefore, to obtain the most benefit from fecal transplantation, further strict donor screening is needed to minimize the risk of FMT. Microbiota transfer therapy (MTT) was found to reduce gut and ASD-like symptoms and regulate the gut microbiota of autistic individuals [87].
4.1. Probiotics
Probiotics are a group of living microorganisms that are well known for improving health conditions by re-establishing the gut microbial composition [86]. Although the mechanism involved is yet to be identified, it has been reported that probiotics may lower gut inflammation by decreasing the intestinal barrier permeability and reducing the inflammation produced by cytokines and other immunomodulatory effects [88]. Grossi and others introduced a case study in which ASD patients with serous cognitive impairment were treated for four weeks with the supplementation of VSL#3 (a combined mixture of live cells of 10 different probiotics). The treatment markedly alleviated autistic symptoms and relieved the severity of gastrointestinal symptoms. Furthermore, four months of daily supplementation with three probiotics containing Lactobacillus strains, two Bifidobacterium strains, and a Streptococcus strain normalized the ratio of Bacteroidetes/Firmicutes and decreased the abundance of Bifidobacterium sp. and Desulfovibrio spp. in the feces of autistic children [89]. Additionally, probiotic supplementation significantly reduced levels of TNFα. The research suggests that probiotic supplementation alters the gut microbial composition in ASD children [90]. Another study reported lower amounts of D-arabinitol in the urine of ASD children who received oral supplementation with an L. acidophilus strain, and it enhanced their ability to follow instructions [91]. These studies assumed that the appropriate use of probiotics could reduce autism-related symptoms, but additional studies are needed.
4.2. Prebiotics
Prebiotics are non-digestible oligosaccharides that stimulate an increase in beneficial bacteria found naturally in the body, especially lactobacilli and bifidobacteria. In general, the bacterial fermentation of prebiotics produces SCFAs, which are linked to their health benefits [82][92]. In an in vitro study on a gut model, the analysis of feces samples from children with ASD and non-autistic children showed that the prebiotic Galacto-oligosaccharide (B-GOS) raises the abundance of Bifidobacterium spp. [43]. Although probiotic treatments have been shown to relieve GI symptoms and regulate the gut microbiota, studies on ASD patients using prebiotics are limited and there is a lack of available solid data [43][62].
4.3. Dietary
According to the findings of many studies, autistic children strongly prefer starchy foods, snacks, and processed foods. Additionally, they consume fewer fruits, vegetables, and proteins than typical non-autistic children [93]. In addition, it is recognized that most ASD children are underweight because they ingest lower daily levels of vitamins, dietary fibers, calcium, and potassium [94]. In both human and animal models, research has demonstrated that ketogenic diets (KD) have some potential positive effects on the performance and symptoms of autistic patients. KD with a high-fat content (65–90%) is commonly used to lower ASD symptoms [94]. Other than KD, vitamins, minerals, omega-3-fatty acids, and antioxidants are thought to have beneficial effects for ASD. For example, the treatment of ASD patients with omega-3 fatty acids for 12 weeks enhanced their social behavior dramatically [95]. Multiple studies have shown that a gluten-free and casein-free (GFCF) diet is beneficial for the health of children with ASD [94]. However, in 2015, a study found that a GFCF diet plan had side effects due to calcium deficiency and a lack of essential amino acids, resulting in decreased bone density and frequent bone fractures. Moreover, ASD patients who followed a GFCF diet were found to need more vitamin D supplementation [85].
4.4. Fecal Microbiota Transplantation (FMT)
Fecal microbiota transplantation (FMT) modifies the gut microbiota composition by fecal transplantation from healthy donors to the patient [96]. FMT was developed to treat inflammatory bowel disease (IBD) and irritable bowel syndrome (IBS) patients based on the theory that it could help with constipation symptoms [97][98]. As a result, researchers are keen to investigate the use of FMT to cure ASD children. However, because some adverse effects, such as diarrhea, abdominal pain, bloating, and transitory low-grade fever have been recorded, the safety of FMT should be considered further [99].
4.5. Microbiota Transfer Therapy (MTT)
Microbiota transfer therapy (MTT) is similar to FMT. Nevertheless, MTT involves two weeks of antibiotic treatment, a bowel cleanse, a stomach acid suppressant, and a fecal microbiota transplant with a high starting dose for 7–8 weeks. MTT has been found to reduce gut and ASD-related symptoms and regulate the gut microbiota of autistic individuals [87].
References
- Lasheras, I.; Seral, P.; Latorre, E.; Barroso, E.; Gracia-García, P.; Santabárbara, J. Microbiota and gut-brain axis dysfunction in autism spectrum disorder: Evidence for functional gastrointestinal disorders. Asian J. Psychiatry 2020, 47, 101874.
- Hossain, M.D.; Ahmed, H.U.; Uddin, M.M.J.; Chowdhury, W.A.; Iqbal, M.S.; Kabir, R.I.; Chowdhury, I.A.; Aftab, A.; Datta, P.G.; Rabbani, G.; et al. Autism Spectrum disorders (ASD) in South Asia: A systematic review. BMC Psychiatry 2017, 17, 281.
- National Center on Birth Defects and Developmental Disabilities; Centers for Disease Control and Prevention. Data & Statistics on Autism Spectrum Disorder. Available online: https://www.cdc.gov/ncbddd/autism/data.html (accessed on 11 November 2021).
- Christensen, D.L.; Braun, K.V.N.; Baio, J.; Bilder, D.; Charles, J.; Constantino, J.N.; Daniels, J.; Durkin, M.S.; Fitzgerald, R.T.; Kurzius-Spencer, M.; et al. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2012. MMWR Surveill. Summ. 2018, 65, 1–23.
- Park, H.R.; Lee, J.M.; Moon, H.E.; Lee, D.S.; Kim, B.-N.; Kim, J.; Kim, D.G.; Paek, S.H. A Short Review on the Current Understanding of Autism Spectrum Disorders. Exp. Neurobiol. 2016, 25, 1–13.
- Eshraghi, A.A.; Liu, G.; Kay, S.-I.S.; Eshraghi, R.S.; Mittal, J.; Moshiree, B.; Mittal, R. Epigenetics and Autism Spectrum Disorder: Is There a Correlation? Front. Cell. Neurosci. 2018, 12, 78.
- Lord, C.; Elsabbagh, M.; Baird, G.; Veenstra-Vanderweele, J. Autism spectrum disorder. Lancet 2018, 392, 508–520.
- Masini, E.; Loi, E.; Vega-Benedetti, A.F.; Carta, M.; Doneddu, G.; Fadda, R.; Zavattari, P. An Overview of the Main Genetic, Epigenetic and Environmental Factors Involved in Autism Spectrum Disorder Focusing on Synaptic Activity. Int. J. Mol. Sci. 2020, 21, 8290.
- Wang, C.; Geng, H.; Liu, W.; Zhang, G. Prenatal, perinatal, and postnatal factors associated with autism. Medicine 2017, 96, e6696.
- Ghozy, S.; Tran, L.; Naveed, S.; Quynh, T.T.H.; Zayan, A.H.; Waqas, A.; Sayed, A.K.H.; Karimzadeh, S.; Hirayama, K.; Huy, N.T. Association of breastfeeding status with risk of autism spectrum disorder: A systematic review, dose-response analysis and meta-analysis. Asian J. Psychiatry 2019, 48, 101916.
- Karahmadi, M.; Karimi, P.; Kamali, E.; Mousavi, S.M. Environmental factors influencing the risk of autism. J. Res. Med. Sci. 2017, 22, 27.
- Garcia-Gutierrez, E.; Narbad, A.; Rodríguez, J.M. Autism Spectrum Disorder Associated With Gut Microbiota at Immune, Metabolomic, and Neuroactive Level. Front. Neurosci. 2020, 14, 578666.
- Rothschild, D.; Weissbrod, O.; Barkan, E.; Kurilshikov, A.; Korem, T.; Zeevi, D.; Costea, P.I.; Godneva, A.; Kalka, I.N.; Bar, N.; et al. Environment dominates over host genetics in shaping human gut microbiota. Nature 2018, 555, 210–215.
- Coury, D.L.; Ashwood, P.; Fasano, A.; Fuchs, G.; Geraghty, M.; Kaul, A.; Mawe, G.; Patterson, P.; Jones, N.E. Gastrointestinal Conditions in Children With Autism Spectrum Disorder: Developing a Research Agenda. Pediatrics 2012, 130, S160–S168.
- Farzi, A.; Fröhlich, E.E.; Holzer, P. Gut Microbiota and the Neuroendocrine System. Neurotherapeutics 2018, 15, 5–22.
- Yarandi, S.S.; A Peterson, D.; Treisman, G.J.; Moran, T.H.; Pasricha, P.J. Modulatory Effects of Gut Microbiota on the Central Nervous System: How Gut Could Play a Role in Neuropsychiatric Health and Diseases. J. Neurogastroenterol. Motil. 2016, 22, 201–212.
- Emanuele, E.; Orsi, P.; Boso, M.; Broglia, D.; Brondino, N.; Barale, F.; di Nemi, S.U.; Politi, P. Low-grade endotoxemia in patients with severe autism. Neurosci. Lett. 2010, 471, 162–165.
- Vargas-Caraveo, A.; Sayd, A.; Maus, S.R.; Caso, J.; Madrigal, J.; García-Bueno, B.; Leza, J.C. Lipopolysaccharide enters the rat brain by a lipoprotein-mediated transport mechanism in physiological conditions. Sci. Rep. 2017, 7, 13113.
- Zhao, J.; Bi, W.; Xiao, S.; Lan, X.; Cheng, X.; Zhang, J.; Lu, D.; Wei, W.; Wang, Y.; Li, H.; et al. Neuroinflammation induced by lipopolysaccharide causes cognitive impairment in mice. Sci. Rep. 2019, 9, 5790.
- Foley, K.A.; MacFabe, D.F.; Kavaliers, M.; Ossenkopp, K.-P. Sexually dimorphic effects of prenatal exposure to lipopolysaccharide, and prenatal and postnatal exposure to propionic acid, on acoustic startle response and prepulse inhibition in adolescent rats: Relevance to autism spectrum disorders. Behav. Brain Res. 2015, 278, 244–256.
- Settanni, C.R.; Bibbò, S.; Ianiro, G.; Rinninella, E.; Cintoni, M.; Mele, M.C.; Cammarota, G.; Gasbarrini, A. Gastrointestinal involvement of autism spectrum disorder: Focus on gut microbiota. Expert Rev. Gastroenterol. Hepatol. 2021, 15, 599–622.
- Eshraghi, R.S.; Deth, R.C.; Mittal, R.; Aranke, M.; Kay, S.-I.S.; Moshiree, B.; Eshraghi, A.A. Early Disruption of the Microbiome Leading to Decreased Antioxidant Capacity and Epigenetic Changes: Implications for the Rise in Autism. Front. Cell. Neurosci. 2018, 12, 256.
- de Magistris, L.; Familiari, V.; Pascotto, A.; Sapone, A.; Frolli, A.; Iardino, P.; Carteni, M.; De Rosa, M.; Francavilla, R.; Riegler, G.; et al. Alterations of the Intestinal Barrier in Patients With Autism Spectrum Disorders and in Their First-degree Relatives. J. Pediatr. Gastroenterol. Nutr. 2010, 51, 418–424.
- Sturgeon, C.; Lan, J.; Fasano, A. Zonulin transgenic mice show altered gut permeability and increased morbidity/mortality in the DSS colitis model. Ann. N. Y. Acad. Sci. 2017, 1397, 130–142.
- Weber, C.R. Dynamic properties of the tight junction barrier. Ann. N. Y. Acad. Sci. 2012, 1257, 77–84.
- Dalton, N.; Chandler, S.; Turner, C.; Charman, T.; Pickles, A.; Loucas, T.; Simonoff, E.; Sullivan, P.; Baird, G. Gut Permeability in Autism Spectrum Disorders. Autism Res. 2014, 7, 305–313.
- Kushak, R.I.; Buie, T.M.; Murray, K.F.; Newburg, D.S.; Chen, C.; Nestoridi, E.; Winter, H.S. Evaluation of Intestinal Function in Children With Autism and Gastrointestinal Symptoms. J. Pediatr. Gastroenterol. Nutr. 2016, 62, 687–691.
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; S Bastiaanssen, T.F.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013.
- Margolis, K.G.; Cryan, J.F.; Mayer, E.A. The Microbiota-Gut-Brain Axis: From Motility to Mood. Gastroenterology 2021, 160, 1486–1501.
- O’Connor, R.M.; Grenham, S.; Dinan, T.G.; Cryan, J.F. microRNAs as novel antidepressant targets: Converging effects of ketamine and electroconvulsive shock therapy in the rat hippocampus. Int. J. Neuropsychopharmacol. 2013, 16, 1885–1892.
- Allaire, J.; Crowley, S.M.; Law, H.T.; Chang, S.-Y.; Ko, H.-J.; Vallance, B.A. The Intestinal Epithelium: Central Coordinator of Mucosal Immunity. Trends Immunol. 2018, 39, 677–696.
- Kim, J.W.; Hong, J.Y.; Bae, S.M. Microglia and autism spectrum disorder: Overview of current evidence and novel immuno-modulatory treatment options. Clin. Psychopharmacol. Neurosci. 2018, 16, 246–252.
- Machado, C.J.; Whitaker, A.M.; Smith, S.E.; Patterson, P.H.; Bauman, M.D. Maternal Immune Activation in Nonhuman Primates Alters Social Attention in Juvenile Offspring. Biol. Psychiatry 2015, 77, 823–832.
- Gupta, S.; Ellis, S.; Ashar, F.N.; Moes, A.; Bader, J.S.; Zhan, J.; West, A.B.; Arking, D.E. Transcriptome analysis reveals dysregulation of innate immune response genes and neuronal activity-dependent genes in autism. Nat. Commun. 2014, 5, 5748.
- Erny, D.; Hrabě de Angelis, A.L.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977.
- Hsiao, E.Y.; McBride, S.W.; Hsien, S.; Sharon, G.; Hyde, E.R.; McCue, T.; Codelli, J.A.; Chow, J.; Reisman, S.E.; Petrosino, J.F.; et al. Microbiota Modulate Behavioral and Physiological Abnormalities Associated with Neurodevelopmental Disorders. Cell 2013, 155, 1451–1463.
- Morais, L.H.; Schreiber, H.L., IV; Mazmanian, S.K. The gut microbiota–brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 2021, 19, 241–255.
- Rojo, D.; Méndez-García, C.; Raczkowska, B.A.; Bargiela, R.; Moya, A.; Ferrer, M.; Barbas, C. Exploring the human microbiome from multiple perspectives: Factors altering its composition and function. FEMS Microbiol. Rev. 2017, 41, 453–478.
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids from Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25.
- Chambers, E.S.; Viardot, A.; Psichas, A.; Morrison, D.J.; Murphy, K.G.; Zac-Varghese, S.E.K.; MacDougall, K.; Preston, T.; Tedford, C.; Finlayson, G.S.; et al. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut 2015, 64, 1744–1754.
- Rose, S.; Bennuri, S.C.; Murray, K.F.; Buie, T.; Winter, H.; Frye, R.E. Mitochondrial dysfunction in the gastrointestinal mucosa of children with autism: A blinded case-control study. PLoS ONE 2017, 12, e0186377.
- De Angelis, M.; Piccolo, M.; Vannini, L.; Siragusa, S.; De Giacomo, A.; Serrazzanetti, D.I.; Cristofori, F.; Guerzoni, M.E.; Gobbetti, M.; Francavilla, R. Fecal Microbiota and Metabolome of Children with Autism and Pervasive Developmental Disorder Not Otherwise Specified. PLoS ONE 2013, 8, e76993.
- Grimaldi, R.; Cela, D.; Swann, J.; Vulevic, J.; Gibson, G.R.; Tzortzis, G.; Costabile, A. In vitrofermentation of B-GOS: Impact on faecal bacterial populations and metabolic activity in autistic and non-autistic children. FEMS Microbiol. Ecol. 2017, 93, 93.
- Frye, R.E.; Rose, S.; Chacko, J.; Wynne, R.; Bennuri, S.C.; Slattery, J.C.; Tippett, M.; Delhey, L.; Melnyk, S.; Kahler, S.G.; et al. Modulation of mitochondrial function by the microbiome metabolite propionic acid in autism and control cell lines. Transl. Psychiat. 2016, 6, e927.
- Meeking, M.M.; MacFabe, D.F.; Mepham, J.R.; Foley, K.A.; Tichenoff, L.J.; Boon, F.H.; Kavaliers, M.; Ossenkopp, K.-P. Propionic acid induced behavioural effects of relevance to autism spectrum disorder evaluated in the hole board test with rats. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 97, 109794.
- Thomas, R.H.; Meeking, M.M.; Mepham, J.R.; Tichenoff, L.; Possmayer, F.; Liu, S.; Macfabe, D.F. The enteric bacterial metabolite propionic acid alters brain and plasma phospholipid molecular species: Further development of a rodent model of autism spectrum disorders. J. Neuroinflammation 2012, 9, 153.
- Käkelä, R.; Somerharju, P.; Tyynelä, J. Analysis of phospholipid molecular species in brains from patients with infantile and juvenile neuronal-ceroid lipofuscinosis using liquid chromatography-electrospray ionization mass spectrometry. J. Neurochem. 2003, 84, 1051–1065.
- Bu, B.; Ashwood, P.; Harvey, D.; King, I.; de Water, J.; Jin, L.-W. Fatty acid compositions of red blood cell phospholipids in children with autism. Prostaglandins, Leukot. Essent. Fat. Acids 2006, 74, 215–221.
- Rose, S.; Bennuri, S.C.; Davis, J.E.; Wynne, R.; Slattery, J.C.; Tippett, M.; Delhey, L.; Melnyk, S.; Kahler, S.G.; Macfabe, D.F.; et al. Butyrate enhances mitochondrial function during oxidative stress in cell lines from boys with autism. Transl. Psychiatry 2018, 8, 42.
- Downs, R.; Perna, J.; Vitelli, A.; Cook, D.; Dhurjati, P. Model-based hypothesis of gut microbe populations and gut/brain barrier permeabilities in the development of regressive autism. Med. Hypotheses 2014, 83, 649–655.
- Braniste, V.; Al-Asmakh, M.; Kowal, C.; Anuar, F.; Abbaspour, A.; Tóth, M.; Korecka, A.; Bakocevic, N.; Ng, L.G.; Kundu, P.; et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014, 6, 263ra158.
- Gabriele, S.; Sacco, R.; Cerullo, S.; Neri, C.; Urbani, A.; Tripi, G.; Malvy, J.; Barthelemy, C.; Bonnet-Brilhault, F.; Persico, A. Urinaryp-cresol is elevated in young French children with autism spectrum disorder: A replication study. Biomarkers 2014, 19, 463–470.
- Oh, D.; Cheon, K.-A. Alteration of Gut Microbiota in Autism Spectrum Disorder: An Overview. J. Korean Acad. Child Adolesc. Psychiatry 2020, 31, 131–145.
- Altieri, L.; Neri, C.; Sacco, R.; Curatolo, P.; Benvenuto, A.; Muratori, F.; Santocchi, E.; Bravaccio, C.; Lenti, C.; Saccani, M.; et al. Urinary p-cresol is elevated in small children with severe autism spectrum disorder. Biomarkers 2011, 16, 252–260.
- Bermudez-Martin, P.; Becker, J.A.J.; Caramello, N.; Fernandez, S.P.; Costa-Campos, R.; Canaguier, J.; Barbosa, S.; Martinez-Gili, L.; Myridakis, A.; Dumas, M.-E.; et al. The microbial metabolite p-Cresol induces autistic-like behaviors in mice by remodeling the gut microbiota. Microbiome 2021, 9, 157.
- Hung, L.W.; Neuner, S.; Polepalli, J.S.; Beier, K.T.; Wright, M.; Walsh, J.J.; Lewis, E.M.; Luo, L.; Deisseroth, K.; Dölen, G.; et al. Gating of social reward by oxytocin in the ventral tegmental area. Science 2017, 357, 1406–1411.
- Fukuchi, M.; Nii, T.; Ishimaru, N.; Minamino, A.; Hara, D.; Takasaki, I.; Tabuchi, A.; Tsuda, M. Valproic acid induces up- or down-regulation of gene expression responsible for the neuronal excitation and inhibition in rat cortical neurons through its epigenetic actions. Neurosci. Res. 2009, 65, 35–43.
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203–209.
- Chidambaram, S.B.; Tuladhar, S.; Bhat, A.; Mahalakshmi, A.M.; Ray, B.; Essa, M.M.; Bishir, M.; Bolla, S.R.; Nanjaiah, N.D.; Guillemin, G.J.; et al. Autism and Gut–Brain Axis: Role of Probiotics. Adv. Neurobiol. 2020, 24, 587–600.
- Reigstad, C.S.; Salmonson, C.E.; Rainey, J.F., III; Szurszewski, J.H.; Linden, D.R.; Sonnenburg, J.L.; Farrugia, G.; Kashyap, P.C. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 2015, 29, 1395–1403.
- Scriven, M.; Dinan, T.G.; Cryan, J.F.; Wall, M. Neuropsychiatric Disorders: Influence of Gut Microbe to Brain Signalling. Diseases 2018, 6, 78.
- Fattorusso, A.; Di Genova, L.; Dell’Isola, G.B.; Mencaroni, E.; Esposito, S. Autism Spectrum Disorders and the Gut Microbiota. Nutrients 2019, 11, 521.
- Hoban, A.; Moloney, R.; Golubeva, A.; Neufeld, K.M.; O’Sullivan, O.; Patterson, E.; Stanton, C.; Dinan, T.; Clarke, G.; Cryan, J. Behavioural and neurochemical consequences of chronic gut microbiota depletion during adulthood in the rat. Neuroscience 2016, 339, 463–477.
- Iglesias-Vázquez, L.; Riba, G.V.G.; Arija, V.; Canals, J. Composition of Gut Microbiota in Children with Autism Spectrum Disorder: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 792.
- Martin, C.R.; Osadchiy, V.; Kalani, A.; Mayer, E.A. The Brain-Gut-Microbiome Axis. Cell. Mol. Gastroenterol. Hepatol. 2018, 6, 133–148.
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous Bacteria from the Gut Microbiota Regulate Host Serotonin Biosynthesis. Cell 2015, 161, 264–276.
- Umesawa, Y.; Atsumi, T.; Chakrabarty, M.; Fukatsu, R.; Ide, M. GABA Concentration in the Left Ventral Premotor Cortex Associates With Sensory Hyper-Responsiveness in Autism Spectrum Disorders without Intellectual Disability. Front. Neurosci. 2020, 14, 482.
- Foss-Feig, J.H.; Adkinson, B.D.; Ji, J.L.; Yang, G.; Srihari, V.H.; McPartland, J.C.; Krystal, J.H.; Murray, J.D.; Anticevic, A. Searching for Cross-Diagnostic Convergence: Neural Mechanisms Governing Excitation and Inhibition Balance in Schizophrenia and Autism Spectrum Disorders. Biol. Psychiatry 2017, 81, 848–861.
- Ristori, M.V.; Quagliariello, A.; Reddel, S.; Ianiro, G.; Vicari, S.; Gasbarrini, A.; Putignani, L. Autism, Gastrointestinal Symptoms and Modulation of Gut Microbiota by Nutritional Interventions. Nutrients 2019, 11, 2812.
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055.
- Sudo, N.; Chida, Y.; Aiba, Y.; Sonoda, J.; Oyama, N.; Yu, X.-N.; Kubo, C.; Koga, Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol. 2004, 558, 263–275.
- Patel, N.; Crider, A.; Pandya, C.D.; Ahmed, A.O.; Pillai, A. Altered mRNA Levels of Glucocorticoid Receptor, Mineralocorticoid Receptor, and Co-Chaperones (FKBP5 and PTGES3) in the Middle Frontal Gyrus of Autism Spectrum Disorder Subjects. Mol. Neurobiol. 2015, 53, 2090–2099.
- Wątroba, M.; Dudek, I.; Skoda, M.; Stangret, A.; Rzodkiewicz, P.; Szukiewicz, D. Sirtuins, epigenetics and longevity. Ageing Res. Rev. 2017, 40, 11–19.
- Virzã, G.M.; Clementi, A.; Brocca, A.; De Cal, M.; Ronco, C. Epigenetics: A potential key mechanism involved in the pathogenesis of cardiorenal syndromes. J. Nephrol. 2017, 31, 333–341.
- Sharma, M.; Li, Y.; Stoll, M.L.; Tollefsbol, T.O. The Epigenetic Connection Between the Gut Microbiome in Obesity and Diabetes. Front. Genet. 2020, 10, 1329.
- Ladd-Acosta, C.; Hansen, K.; Briem, E.; Fallin, M.D.; Kaufmann, W.E.; Feinberg, A.P. Common DNA methylation alterations in multiple brain regions in autism. Mol. Psychiatry 2014, 19, 862–871.
- Schroeder, D.I.; Schmidt, R.J.; Crary-Dooley, F.K.; Walker, C.K.; Ozonoff, S.; Tancredi, D.J.; Hertz-Picciotto, I.; LaSalle, J.M. Placental methylome analysis from a prospective autism study. Mol. Autism 2016, 7, 51.
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450.
- Romano, K.A.; Vivas, E.I.; Amador-Noguez, D.; Rey, F.E. Intestinal Microbiota Composition Modulates Choline Bioavailability from Diet and Accumulation of the Proatherogenic Metabolite Trimethylamine- N-Oxide. mBio 2015, 6, e02481-14.
- Mahmoud, A.M.; Ali, M.M. Methyl Donor Micronutrients that Modify DNA Methylation and Cancer Outcome. Nutrients 2019, 11, 608.
- Degnan, P.H.; Barry, N.A.; Mok, K.; Taga, M.E.; Goodman, A.L. Human Gut Microbes Use Multiple Transporters to Distinguish Vitamin B12 Analogs and Compete in the Gut. Cell Host Microbe 2014, 15, 47–57.
- Rosenfeld, C.S. Microbiome Disturbances and Autism Spectrum Disorders. Drug Metab. Dispos. 2015, 43, 1557–1571.
- Mayoral, J.G.; Hussain, M.; Joubert, D.A.; Iturbe-Ormaetxe, I.; O’Neill, S.L.; Asgari, S. Wolbachiasmall noncoding RNAs and their role in cross-kingdom communications. Proc. Natl. Acad. Sci. USA 2014, 111, 18721–18726.
- Rondanelli, M.; Faliva, M.A.; Perna, S.; Giacosa, A.; Peroni, G.; Castellazzi, A.M. Using probiotics in clinical practice: Where are we now? A review of existing meta-analyses. Gut Microbes 2017, 8, 521–543.
- Bauset, S.M.; Llopis-González, A.; Zazpe, I.; Marí-Sanchis, A.; Suárez-Varela, M.M. Nutritional Impact of a Gluten-Free Casein-Free Diet in Children with Autism Spectrum Disorder. J. Autism Dev. Disord. 2016, 46, 673–684.
- Santocchi, E.; Guiducci, L.; Fulceri, F.; Billeci, L.; Buzzigoli, E.; Apicella, F.; Calderoni, S.; Grossi, E.; Morales, M.A.; Muratori, F. Gut to brain interaction in Autism Spectrum Disorders: A randomized controlled trial on the role of probiotics on clinical, biochemical and neurophysiological parameters. BMC Psychiatry 2016, 16, 183.
- Kang, D.-W.; Adams, J.B.; Gregory, A.C.; Borody, T.; Chittick, L.; Fasano, A.; Khoruts, A.; Geis, E.; Maldonado, J.; McDonough-Means, S.; et al. Microbiota Transfer Therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: An open-label study. Microbiome 2017, 5, 10.
- Jonkers, D.; Penders, J.; Masclee, A.; Pierik, M. Probiotics in the Management of Inflammatory Bowel Disease. Drugs 2012, 72, 803–823.
- Grossi, E.; Melli, S.; Dunca, D.; Terruzzi, V. Unexpected improvement in core autism spectrum disorder symptoms after long-term treatment with probiotics. SAGE Open Med. Case Rep. 2016, 4, 4.
- Tomova, A.; Husarova, V.; Lakatosova, S.; Bakos, J.; Vlkova, B.; Babinska, K.; Ostatnikova, D. Gastrointestinal microbiota in children with autism in Slovakia. Physiol. Behav. 2015, 138, 179–187.
- Kałużna-Czaplińska, J.; Błaszczyk, S. The level of arabinitol in autistic children after probiotic therapy. Nutrition 2012, 28, 124–126.
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92.
- Sharp, W.G.; Jaquess, D.L.; Lukens, C.T. Multi-method assessment of feeding problems among children with autism spectrum disorders. Res. Autism Spectr. Disord. 2013, 7, 56–65.
- Sivamaruthi, B.S.; Suganthy, N.; Kesika, P.; Chaiyasut, C. The Role of Microbiome, Dietary Supplements, and Probiotics in Autism Spectrum Disorder. Int. J. Environ. Res. Public Health 2020, 17, 2647.
- Ooi, Y.P.; Weng, S.-J.; Jang, L.Y.; Low, L.; Seah, J.; Teo, S.; Ang, R.; Lim, C.G.; Liew, A.; Fung, D.S.; et al. Omega-3 fatty acids in the management of autism spectrum disorders: Findings from an open-label pilot study in Singapore. Eur. J. Clin. Nutr. 2015, 69, 969–971.
- Vindigni, S.M.; Surawicz, C.M. Fecal Microbiota Transplantation. Gastroenterol. Clin. N. Am. 2017, 46, 171–185.
- Rossen, N.G.; Macdonald, J.K.; De Vries, E.M.; D’Haens, G.R.; De Vos, W.M.; Zoetendal, E.G.; Ponsioen, C.Y. Fecal microbiota transplantation as novel therapy in gastroenterology: A systematic review. World J. Gastroenterol. 2015, 21, 5359–5371.
- Aroniadis, O.C.; Brandt, L.J. Fecal microbiota transplantation. Curr. Opin. Gastroenterol. 2013, 29, 79–84.
- Kelly, C.R.; Kahn, S.; Kashyap, P.; Laine, L.; Rubin, D.; Atreja, A.; Moore, T.; Wu, G. Update on Fecal Microbiota Transplantation 2015: Indications, Methodologies, Mechanisms, and Outlook. Gastroenterology 2015, 149, 223–237.
More
Information
Subjects:
Gastroenterology & Hepatology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.3K
Revisions:
4 times
(View History)
Update Date:
02 Mar 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




