Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Vishnu D. Rajput | + 2781 word(s) | 2781 | 2022-03-01 07:25:08 | | | |
| 2 | Camila Xu | -1 word(s) | 2780 | 2022-03-01 08:54:41 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Rajput, V.D. Nanobioremediation-Based Removal of Pollutants. Encyclopedia. Available online: https://encyclopedia.pub/entry/20033 (accessed on 07 February 2026).
Rajput VD. Nanobioremediation-Based Removal of Pollutants. Encyclopedia. Available at: https://encyclopedia.pub/entry/20033. Accessed February 07, 2026.
Rajput, Vishnu D.. "Nanobioremediation-Based Removal of Pollutants" Encyclopedia, https://encyclopedia.pub/entry/20033 (accessed February 07, 2026).
Rajput, V.D. (2022, March 01). Nanobioremediation-Based Removal of Pollutants. In Encyclopedia. https://encyclopedia.pub/entry/20033
Rajput, Vishnu D.. "Nanobioremediation-Based Removal of Pollutants." Encyclopedia. Web. 01 March, 2022.
Copy Citation
Nanobioremediation is a cost-effective technique of utilizing plants and microbes for the breakdown of pollutant compounds, ultimately improving soil quality and reducing pollution. By breaking down contaminants in the soil, the process may be able to eradicate, retain, or reduce the amount of pollutants present.
pollution
heavy metals and metalloids
phytoremediation potential
1. Introduction
Rapidly increasing anthropogenic/technogenic activities are adding potentially toxic metals, agrochemicals, and an excess of nutrients to the soil [1]. Soil is a basis of crop production as it supports plants to uptake nutrients [2]. In fact, agriculture sustains and defines human lives; however, it is often disruptive of natural ecosystems. Humans’ voracious appetites for getting the benefits from natural resources grow in tandem with population growth. The conflict between the benefits and the sustainable management of agricultural land and its conservation has been reported in the literature for a long time [3][4]. Land pollution is a threat to livelihoods, quality of life, and sustainable development [5]. Thus, conserving soil is the utmost requirement for the current era owing to the pressures of increasing population and the shrinking of arable lands by technogenic activities.
The advancements in nanotechnology open a window globally to remediate or restore polluted soil in an effective way [6][7]. It has been claimed that nanotechnology has great potential as an environmentally cleaner technology, including by alleviation of the toxicities of various metals/metalloids [8][9]. Besides, nanotechnology has been recognized as a potential method for the remediation of pollutants in a variety of environmental matrices, including soils [6]. In this context, soil remediation is one of the main domains where nanotechnological approaches have been widely used. The uses of NPs have been explored lately to remove contaminants in a variety of ways, including by adsorption, redox reactions, precipitation, and co-precipitation, all of which are aided by their enormous specific surface area [9].
With the help of NPs, hyperaccumulators and indigenous soil microbes could enhance biodegradation processes, thereby increasing the potential extent of remediation. This could be called nano-phytoremediation and microbial-mediated nano-remediation. The use of NPs with bioremediation approaches may result in a lot of benefits as these particles are small (1–100 nm) with a larger surface area and reactivity [10]. The broad range of studies indicated that the foliar use of NPs alleviates metal-pollutant toxicity and enhances plant growth, resulting in a high accumulation of elemental toxic content in plant tissues [11][12][13]. The degree of contamination, the bioavailability, and the accumulation of metals by the plants are decisive for the efficiency of nano-phytoremediation as a way of removing heavy metals (HMs) from contaminated sites [14][15].
The utilization of NPs based on a metal–organic framework (MOF) has received a lot of important attention lately, but it is primarily employed for drinking water and wastewater treatment [16][17][18]. Thus, there is a massive opportunity for scientists to envisage potential uses of MOF in soil remediation. However, concerns related to the safe use of NPs to remediate polluted soils, and their release into ecosystems, are still less explored, and this becomes a matter of concern [19][20].
2. An Appraisal of Nanobioremediation-Based Removal of Pollutants; Special Emphasis on Microbe-Mediated Remediation
Soil rich in vital nutrients and micronutrients is believed to best support the optimum health of the plant and its growth [21]. Human-made activities have currently polluted the soil with a variety of persistent organic compounds, viz., polyaromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs), volatile organic compounds (VOCs), HMs (Hg and Pb), agrochemicals (pesticides, fungicides, and fertilizers), and also sometimes with excess nutrients [22]. At the same time, urbanization and industrialization have also added solid wastes, varieties of chemicals, and solvents to the environment and agricultural soil [23].
Nanobioremediation is a cost-effective technique of utilizing plants and microbes for the breakdown of pollutant compounds, ultimately improving soil quality and reducing pollution. By breaking down contaminants in the soil, the process may be able to eradicate, retain, or reduce the amount of pollutants present [24][25]. The efficiency of bioremediation has been studied and enhanced in the past using chemical additives or biotechnology [26] but nanotechnology further improved the process with a newer aspect [27]. A summary of different NP-mediated removal of pollutants from contaminated media is elaborated in Table 1.
Table 1. Summary of different nanoparticles-mediated removal of different pollutants from contaminated media.
| Nanoparticles | Remediated Contaminant(s) | Operational Conditions and Removal Efficiency | References |
|---|---|---|---|
| Polyvinylpyrrolidone (PVP) coated iron oxide nanoparticles | Cd and Pb | NPs applications were integrated with the process of bioremediation mediated by Halomonas sp. In the removal setup of Cd and Pb, Halomonas sp. was inoculated for 48 h at 180 rpm, 28 °C. The 100% removal was recorded after 24 h, while for Cd, it was observed after 48 h. |
[28] |
| Zero-valent iron (nZVI) commercial suspension at two doses (1% and 10%) | As | pH was set at 12.2 ± 0.1 of the nZVI suspension. To avoid the aggregation of nZVI in the suspension, polyacrylic acid was used as a stabilizer. Maximal immobilization of As in brownfield soil was recorded at 10% of nZVI. |
[29] |
| Graphene oxide nanoparticles (nGOx) and nZVI | Metals, viz., Cd, Pb, Zn, Cu, and As in the As-Metals polluted soil | Applications of nZVI and nGOx to the polluted soils considerably impacted the availability of As and metals. Cu, Pb, and Cd were immobilized by nGOx, while mobilized As and P. In a turn of nZVI, it immobilized the effectively As and Pb, and poorly Cd but enhanced availability of Cu. This study revealed that both NPs applications might be act as strategies for the immobilization and stabilization that can later be utilized for phytoremediation. |
[30] |
| Titanium oxide nanoparticles-bonded-chitosan nanolayer (NTiO2-NCh) | Cd and Cu | The pH was set at 7.0 during the experimentation. The removal was assisted by 60–70 s heating by using microwave–enforced sorption approach. Application of NTiO2-NCh was found to eliminate Cu and Cd by 88.01% and 70.67%, respectively. |
[31] |
| Palladium (Pd), Pd NPs | Cr | The use Pd NPs as bionanocatalyst has been explored. It was found that Pd NPs completely reduced Cr6+ in 12 h. 6.3 mg of PdNPs was used to reduce 5.0 µmol of Cr6+. |
[32] |
| Magnetic iron oxide nanoparticles (Fe3O4 NPs) treated with Staphylococcus aureus, and surface encapsulated with phthalic acid (n-Fe3O4-Phth-S aureus) | Cu, Ni, Pb | n-Fe3O4-Phth-S was found to remediate 83.0–89.5%, for Cu2+, 99.4–100%, for Pb2+, and 92.6–7.5% for Ni2+. The study also identified n-Fe3O4-Phth-S. aureus as an excellent biosorbent for the removal of divalent ions from an aqueous medium. |
[33] |
| ZnO NPs | Cu, Cd, Cr, and Pb | The applications of ZnO-NPs at 5 mg L−1 with Bacillus cereus and Lysinibacillus macroides showed the maximal removal of Cr, Cu, and Pb which was 60%, 70%, and 85%, respectively. The optimal pH for efficient removal was 8.0. The removal was less in the case of bacteria-mediated remediation which was found to be 83 and 70% in B. cereus and 60 and 65% in L. macroides. |
[34] |
Nanobioremediation implies both nanotechnology and bioremediation together, where the process is executed at the nanoscale. The target pollutants are adsorbed, degraded, or modified owing to the unique physicochemical properties of the NPs, which also act as catalysts and help to reduce the activation energy required for breaking down the compounds [35]. The nanobioremediation process has been explored and studied, and the most exploited NPs are carbon- and metal-based [36][37]. Polymeric NPs in the form of nanocapsules or nanospheres are also exceptional in the elimination of persistent pesticide compounds and long-chain hydrocarbons [38]. However, in the case of HMs, the challenge is entirely different as they are non-biodegradable, as well as very prone to entering biological systems and food chains [39].
Biosorption and bioaccumulation using plants and microbes are traditional methods to remove HMs from polluted soils. However, recent pieces of evidence have reported the use of NPs in HM remediation with remarkable outcomes [40]. Nanoparticles are reported to have been applied in combination simultaneously or sequentially with specific microbes and the results have been convincing [41]. They could help to speed up the elimination of HMs by acting as nanocarriers of microbes or microbial biosorbents [42]. A pictorial diagram that represents the process of nanobioremediation, especially for biogenic NPs, is depicted in the Figure 1.
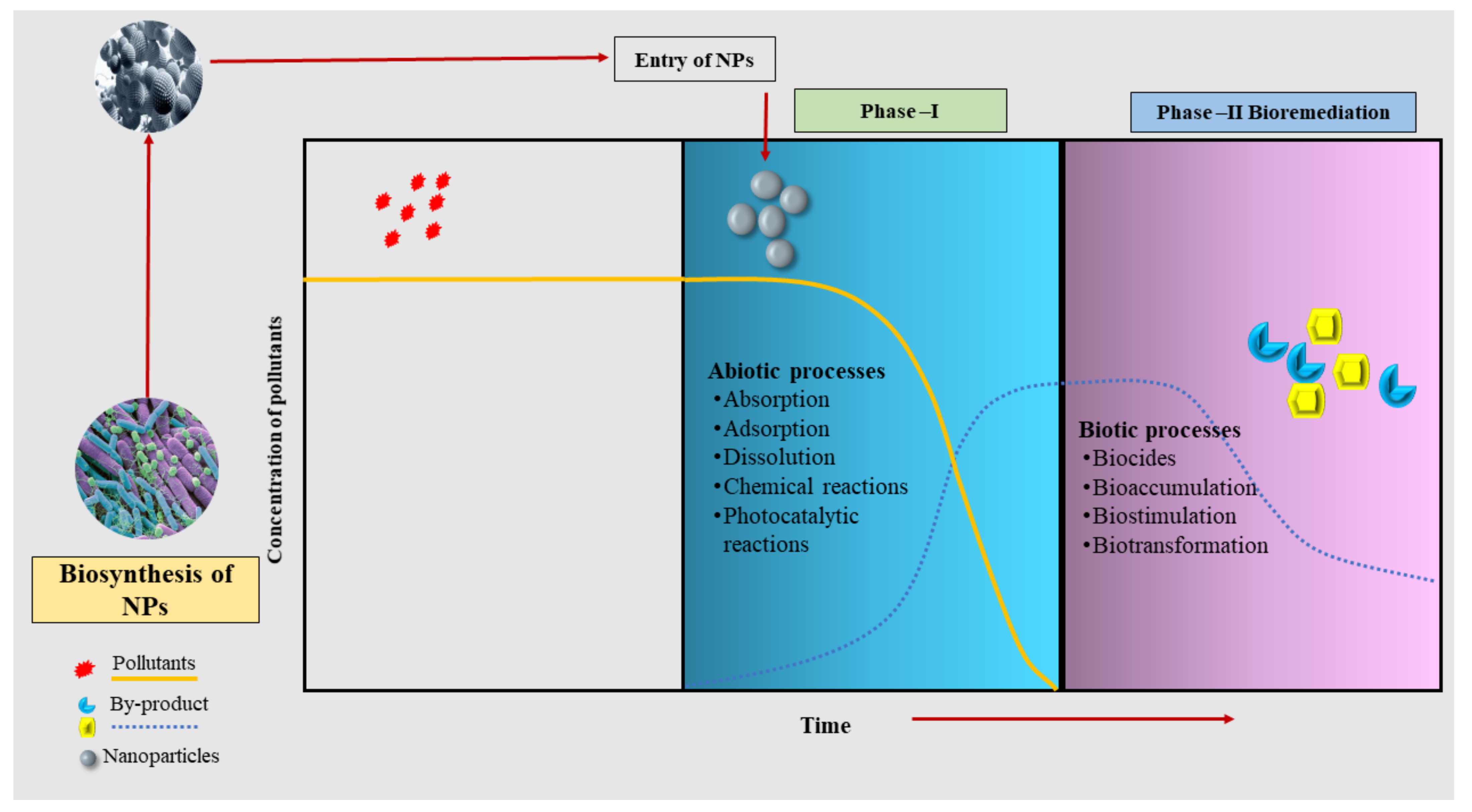 Figure 1. An overview of the processes of nanobioremediation using biogenic nanoparticles.
Figure 1. An overview of the processes of nanobioremediation using biogenic nanoparticles.The synergy of NPs and bacterial degradation has also gained attention; however, the availability of a handful of published papers would not permit the making of a categorical review. Many authors have given their efforts to nanobioremediation, and yet it is still too insignificant for making any conclusive decision [10].
Integration of NPs with microbes for bioremediation is a two-phasic process that involves overlapping abiotic and biotic processes (Figure 1) [43]. In the first phase, after the entry of NPs into the system, pollutants undergo varieties of physicochemical processes and modifications depicting abiotic processes such as absorption, adsorption, dissolution, and chemical catalysis of photocatalytic reactions [44]. The second phase includes biotic processes such as biocides, bioaccumulation, biostimulation, and biotransformation [45][46]. These biotic processes play a crucial role in the removal of pollutants from the system.
2.1. Nanobioremediation of Heavy Metals
The existence of HMs in the environment is largely due to increased anthropogenic activities. However, disturbed biogeochemical cycles are also responsible for their release into the environment as pollutants. Elements like As, Cd, Cr, Hg, and Pb have no biological functions to perform in the biological system. Heavy metals comprise major inorganic pollutants as they exhibit substantial toxic impacts on biota even at the lowest concentrations [47][48]. The toxicity of HMs also rests on their bioavailability and absorption [49]. Acidic environments instigate the toxicity of HMs, especially if the soil structure is poor and has low nutrients (e.g., mining areas) [50].
Heavy metals primarily affect the plants and lower soil organisms by inducing the generation of reactive oxygen species (ROS), which further results in the damage of macromolecules such as proteins and nucleic acids [48]. The existence of HMs in the soil affects crops and vegetation, their nutritional quality, and the ecological aspects associated with them. The effect of HMs on crops varies depending on the crop species, soil physicochemical characteristics, and HM type [51]. The general mechanism of toxicity exerted by HMs on crop plants includes ROS generation, which affects the cell organelles, macromolecules such as proteins and nucleic acids, and other components of the plant’s structure and function [51][52]. It has also been reported to affect respiration and photosynthesis, reduce enzyme activities, elevate oxidative stress, reduce biomass, diminish crop yield, and affect the abundance, activity, diversity, and genetic makeups of useful soil microflora [53][54].
One of the key methods for the elimination of HMs includes site stabilization that immobilizes them at a specific site to decreases mobility and availability in the soil, and stops them from leaching across the sites [55]. The use of various NPs, including biogenic, has been gaining a lot of attention for the removal of HMs [56]. Biogenic NPs are those that are synthesized using biological organisms. The commonly known biogenic NPs, such as Ag NPs, are formed by Morganella psychrotolerans [57][58].
Nanoparticles of FeO coated with polyvinylpyrrolidone (PVP) have been successfully used for improving the bioremediation of the soil contaminated with Pb and Cd by a Gram-negative bacteria, Halomonas sp. This approach has significantly removed nearly 100% of Pb after 24 h, and Cd after 48 h, as compared to removal by bacteria or only NPs [59]. A biosorbent of magnetic Fe3O4 NPs treated with S. aureus, with a surface encapsulated with phthalic acid (as a n-Fe3O4-Phth-S complex), was used for the removal of Cu, Ni, and Pb, and the adsorptive removal of 795, 1355, and 985 µmol g−1 for Cu, Pb, and Ni was achieved, respectively. In terms of percentage, the recovery rates of 83.0–89.5% for Cu2+, 99.4–100% for Pb2+, and 92.6–7.5% for Ni2+ were observed. The comparative study with dried S. aureus and n-Fe3O4-Phth-S for HM removal inferred that the n-Fe3O4-Phth-S core of the NPs, as well as the functional groups present on the microbial surface, played a key role in the removal of HMs [33]. Thus, it revealed that the core of the NPs, as well as functional groups present on the microbial surface, had an important impact on the elimination of the contaminants.
A recent study on the removal of Cu, Cd, Cr, and Pb using HM-resistant bacteria such as B. cereus (PMBL-3) and L. macroides (PMBL-7) evidently confirmed that ZnO NPs at 5 mg L−1 synergistically removes the Cr by 60%, the Cu by 70%, and the Pb by 85%, as compared to B. cereus (80 and 60%) and L. macroides (55 and 50%) at neutral pH, respectively [30]. At neutral pH the surface of ZnO NPs exhibit negative charges that promote electrostatic interactions with metal cations; however, at lower pH, the HMs get precipitated as hydroxides and then hydrogen ions compete for binding with adsorbents [60]. The strain XMCr-6 of B. cereus has also been reported to reduce the Cr6+ through an enzyme-mediated process. The reduced Cr3+ was observed to have a binding affinity to cells using coordination bonds with the functional group present on the surface of the bacterial cell wall. The formation of Cr2O3 NPs was found on the cell surface as a by-product [61].
The use of probiotic bacteria (L. casei and L. fermentum) to absorb Cd from water in association with Se5+ and Se NPs was also investigated. The higher absorption of Cd by L. casei with Se4+ ions (65%), compared to Se NPs (55.90%), was discovered in this study, and it was correlated to the higher solubility of Se5+ compared to Se NPs. When comparing L. fermentum and L. casei, the efficiency of Cd absorption was significantly higher in L. fermentum (50.87%) than L. casei (43.78%). The percentage of Cd adsorption by L. casei when used in conjunction with Se NPs shows no significant change. However, with increased Se NPs ratio percentages, Cd absorption was slightly increased from 5.49 to 16.54 in the presence of L. casei with Se NPs, compared to L. casei [62].
A threefold approach is now gaining popularity as the HM pollutants can be used by selective microbes to synthesize biogenic NPs (resource recovery), thereby removing them from the environment (remediation) and yielding value for the waste (effective waste utilization). A study using Enterococcus faecalis for biorecovery of Pd as Pd NPs reported the synthesis of intra- and extra-cellular (membrane-bound). The range of Pd NPs was observed as 10 nm by transmission electron microscopy; however, the size of the Pd NPs was dependent on environmental conditions such as temperature, pH, and biomass. The obtained Pd NPs have great use as a bionanocatalyst that shows good catalytic efficiency (6.3 mg Pd NPs completely reduced 5.0 µmol Cr6+ in 12 h) and the application is potentially useful to treat industrial effluents [28].
A similar study produced Te NPs from anaerobic sludge based upon supplementation with riboflavin [63]. It formed insoluble elemental tellurium (Te0 NPs) using pollutant tellurite Te4+ oxyanions present in the wastewater. It has been reported that 2-Hydroxy-1,4-naphthoquinone promotes the reduction of Te4+ and the quantity of Te0NPs synthesis [64]. The process is supported by Rhodobacter capsulatus, where malate is the electron-donating substrate [65], and riboflavin speeds up the rate of Te4+ reduction by anaerobic methanogenic granular sludge [66].
2.2. Degradation of Persistent Organic Pollutants
The pollution posed by POPs has been shown to have a negative influence on both the environment and human health, as certain POPs have been found to bioaccumulate in adipose tissue and to have the potential to act as carcinogens. Therefore, their remediation is a major challenge and is obligatory. A Gram-negative bacterial strain (NM05 of Sphingomonas) was earlier reported to degrade the pesticide hexachlorocyclohexane (HCH) [67] upon treatment with Pd/Fe0 bimetallic NPs (CMC-Pd/nFe0), showing the synergistic effect on the degradation of HCH that was enhanced by nearly 1.7–2.1-fold compared to the controls that had the Sphingomonas sp. strain NM05 or CMC-Pd/nFe0 alone [60]. The degradation process was found to be affected by experimental conditions (pH, temperature, HCH concentrations, etc.) [68].
The perovskite (LaFeO3) NPs and biochar from water caltrop (Trapa natans) shells studied on marine sediment reported enhanced degradation of PAHs. The study used lignocellulosic fiber-reinforced biodegradable composites (LFBC) at 0.75 g L−1 and pH 6.0 to activate the peroxymonosulfate (3 × 10−4 M) that helped in the oxidation of oxidizing PAHs in the sediments.
Up to 90% of total degradation was achieved; however, individually 2-ring PAHs 52%, 3-ring PAHs 61%, 4-ring PAHs 66%, 5-ring PAHs 56%, and 6-ring PAHs 29% were observed [69]. The process also reported improved microbial diversity of sediment and the major phylum Proteobacteria was observed initially, but after the process, Hyphomonas was predominantly observed [70]. In a continuous-flow experiments system for the degradation of naphthalene in the groundwater, 400 mg L−1 of synthesized CaO2 NPs degraded the naphthalene of optimum concentration 20 mg L−1. This study highlights complete remediation of naphthalene in the presence of CaO2 NPs and microbes (an abundance of Coccobacilli) from column effluent within 50 days [71].
In the case of soil, improving the microbial community by application of NPs is another way to reduce/remove the toxic pollutant loads from it. Si NPs have been reported to improve microbial colonization and biomass, including the rhizospheric microbes that are helpful for improving soil health [72][73]. However, prolonged exposure and accumulation of these NPs in soil may affect the nutrient and organic matter content.
References
- Midhat, L.; Ouazzani, N.; Hejjaj, A.; Ouhammou, A.; Mandi, L. Accumulation of heavy metals in metallophytes from three mining sites (Southern Centre Morocco) and evaluation of their phytoremediation potential. Ecotoxicol. Environ. Saf. 2019, 169, 150–160.
- Parikh, S.J.; James, B.R. Soil: The Foundation of Agriculture. Nat. Educ. Knowl. 2012, 3, 2.
- Struik, P.C.; Kuyper, T.W. Sustainable intensification in agriculture: The richer shade of green. A review. Agron. Sustain. Dev. 2017, 37, 39.
- Pretty, J.; Bharucha, Z.P. Sustainable intensification in agricultural systems. Ann. Bot. 2014, 114, 1571–1596.
- Martin, J.-L.; Maris, V.; Simberloff, D.S. The need to respect nature and its limits challenges society and conservation science. Proc. Natl. Acad. Sci. USA 2016, 113, 6105.
- Rajput, V.; Minkina, T.; Kumari, A.; Shende, S.; Ranjan, A.; Faizan, M.; Barakvov, A.; Gromovik, A.; Gorbunova, N.; Rajput, P.; et al. A review on nanobioremediation approaches for restoration of contaminated soil. Eurasian J. Soil Sci. 2021, 11, 43–60.
- Medina-Pérez, G.; Fernández-Luqueño, F.; Vazquez-Nuñez, E.; López-Valdez, F.; Prieto-Mendez, J.; Madariaga-Navarrete, A.; Miranda-Arámbula, M. Remediating polluted soils using nanotechnologies: Environmental benefits and risks. Pol. J. Environ. Stud. 2019, 28, 1013–1030.
- Kumari, A.; Kumari, P.; Rajput, V.D.; Sushkova, S.N.; Minkina, T. Metal(loid) nanosorbents in restoration of polluted soils: Geochemical, ecotoxicological, and remediation perspectives. Environ. Geochem. Health 2021, 44, 235–246.
- Raffa, C.M.; Chiampo, F.; Shanthakumar, S. Remediation of metal/metalloid-polluted soils: A short review. Appl. Sci. 2021, 11, 4134.
- Alazaiza, M.Y.D.; Albahnasawi, A.; Ali, G.A.M.; Bashir, M.J.K.; Copty, N.K.; Amr, S.S.A.; Abushammala, M.F.M.; Al Maskari, T. Recent advances of nanoremediation technologies for soil and groundwater remediation: A review. Water 2021, 13, 2186.
- Ghani, M.I.; Saleem, S.; Rather, S.A.; Rehmani, M.S.; Alamri, S.; Rajput, V.D.; Kalaji, H.M.; Saleem, N.; Sial, T.A.; Liu, M. Foliar application of zinc oxide nanoparticles: An effective strategy to mitigate drought stress in cucumber seedling by modulating antioxidant defense system and osmolytes accumulation. Chemosphere 2022, 289, 133202.
- Faizan, M.; Sehar, S.; Rajput, V.D.; Faraz, A.; Afzal, S.; Minkina, T.; Sushkova, S.; Adil, M.F.; Yu, F.; Alatar, A.A.; et al. Modulation of cellular redox status and antioxidant defense system after synergistic application of zinc oxide nanoparticles and salicylic acid in rice (Oryza sativa) plant under arsenic stress. Plants 2021, 10, 2254.
- Faizan, M.; Rajput, V.D.; Al-Khuraif, A.A.; Arshad, M.; Minkina, T.; Sushkova, S.; Yu, F. Effect of foliar fertigation of chitosan nanoparticles on cadmium accumulation and toxicity in Solanum lycopersicum. Biology 2021, 10, 666.
- Rajput, V.; Minkina, T.; Semenkov, I.; Klink, G.; Tarigholizadeh, S.; Sushkova, S. Phylogenetic analysis of hyperaccumulator plant species for heavy metals and polycyclic aromatic hydrocarbons. Environ. Geochem. Health 2020, 16, 68–75.
- Ghazaryan, K.A.; Movsesyan, H.S.; Khachatryan, H.E.; Ghazaryan, N.P.; Minkina, T.M.; Sushkova, S.N.; Mandzhieva, S.S.; Rajput, V.D. Copper phytoextraction and phytostabilization potential of wild plant species growing in the mine polluted areas of Armenia. Environ. Geochem. Health 2018, 19, 155–163.
- Haldar, D.; Duarah, P.; Purkait, M.K. MOFs for the treatment of arsenic, fluoride and iron contaminated drinking water: A review. Chemosphere 2020, 251, 126388.
- Younis, S.A.; Serp, P.; Nassar, H.N. Photocatalytic and biocidal activities of ZnTiO2 oxynitride heterojunction with MOF-5 and g-C3N4: A case study for textile wastewater treatment under direct sunlight. J. Hazard. Mater. 2021, 410, 124562.
- Yang, G.; Zhang, D.; Zhu, G.; Zhou, T.; Song, M.; Qu, L.; Xiong, K.; Li, H. A Sm-MOF/GO nanocomposite membrane for efficient organic dye removal from wastewater. RSC Adv. 2020, 10, 8540–8547.
- Moameri, M.; Khalaki, M.A. Chapter 5–Toxicity/risk assessment of nanomaterials when used in soil treatment. In Nanomaterials for Soil Remediation; Amrane, A., Mohan, D., Nguyen, T.A., Assadi, A.A., Yasin, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 87–100.
- Rajput, V.; Minkina, T.; Mazarji, M.; Shende, S.; Sushkova, S.; Mandzhieva, S.; Burachevskaya, M.; Chaplygin, V.; Singh, A.; Jatav, H. Accumulation of nanoparticles in the soil-plant systems and their effects on human health. Ann. Agric. Sci. 2020, 65, 137–143.
- McGrath, J.M.; Spargo, J.; Penn, C.J. Soil Fertility and Plant Nutrition. In Encyclopedia of Agriculture and Food Systems; Van Alfen, N.K., Ed.; Academic Press: Oxford, UK, 2014; pp. 166–184.
- Gorovtsov, A.; Demin, K.; Sushkova, S.; Minkina, T.; Grigoryeva, T.; Dudnikova, T.; Barbashev, A.; Semenkov, I.; Romanova, V.; Laikov, A.; et al. The effect of combined pollution by PAHs and heavy metals on the topsoil microbial communities of Spolic Technosols of the lake Atamanskoe, Southern Russia. Environ. Geochem. Health 2021, 1–17.
- Rajput, V.D.; Yadav, A.N.; Jatav, H.S.; Singh, S.K.; Minkina, T. Sustainable Management and Utilization of Sewage Sludge; Springer: Cham, Switzerland, 2022.
- Ashraf, S.; Siddiqa, A.; Shahida, S.; Qaisar, S. Titanium-based nanocomposite materials for arsenic removal from water: A review. Heliyon 2019, 5, e01577.
- Chandra, R.; Kumar, V.; Tripathi, S.; Sharma, P. Phytoremediation of industrial pollutants and life cycle assessment. In Phytoremediation of Environmental Pollutants; CRC Press: Boca Raton, FL, USA, 2017; pp. 441–470.
- Gerhardt, K.E.; Gerwing, P.D.; Greenberg, B.M. Opinion: Taking phytoremediation from proven technology to accepted practice. Plant Sci. 2017, 256, 170–185.
- Gong, X.; Huang, D.; Liu, Y.; Peng, Z.; Zeng, G.; Xu, P.; Cheng, M.; Wang, R.; Wan, J. Remediation of contaminated soils by biotechnology with nanomaterials: Bio-behavior, applications, and perspectives. Crit. Rev. Biotechnol. 2018, 38, 455–468.
- Cao, X.; Alabresm, A.; Chen, Y.P.; Decho, A.W.; Lead, J. Improved metal remediation using a combined bacterial and nanoscience approach. Sci. Total Environ. 2020, 704, 135378.
- Gil-Díaz, M.; Diez-Pascual, S.; González, A.; Alonso, J.; Rodríguez-Valdés, E.; Gallego, J.R.; Lobo, M.C. A nanoremediation strategy for the recovery of an As-polluted soil. Chemosphere 2016, 149, 137–145.
- Baragaño, D.; Forján, R.; Welte, L.; Gallego, J.L.R. Nanoremediation of As and metals polluted soils by means of graphene oxide nanoparticles. Sci. Rep. 2020, 10, 1896.
- Mahmoud, M.E.; Abou Ali, S.A.A.; Elweshahy, S.M.T. Microwave functionalization of titanium oxide nanoparticles with chitosan nanolayer for instantaneous microwave sorption of Cu(II) and Cd(II) from water. Int. J. Biol. Macromol. 2018, 111, 393–399.
- Ha, C.; Zhu, N.; Shang, R.; Shi, C.; Cui, J.; Sohoo, I.; Wu, P.; Cao, Y. Biorecovery of palladium as nanoparticles by Enterococcus faecalis and its catalysis for chromate reduction. Chem. Eng. J. 2016, 288, 246–254.
- Mahmoud, M.E.; Abdou, A.E.H.; Mohamed, S.M.S.; Osman, M.M. Engineered staphylococcus aureus via immobilization on magnetic Fe3O4-phthalate nanoparticles for biosorption of divalent ions from aqueous solutions. J. Environ. Chem. Eng. 2016, 4, 3810–3824.
- Akhtar, N.; Khan, S.; Rehman, S.U.; Rehman, Z.U.; Khatoon, A.; Rha, E.S.; Jamil, M. Synergistic effects of zinc oxide nanoparticles and bacteria reduce heavy metals toxicity in rice (Oryza sativa L.) Plant. Toxics 2021, 9, 113.
- Mehndiratta, P.; Jain, A.; Srivastava, S.; Gupta, N. Environmental pollution and nanotechnology. Environ. Pollut. 2013, 2, 49.
- Gong, J.-L.; Wang, B.; Zeng, G.-M.; Yang, C.-P.; Niu, C.-G.; Niu, Q.-Y.; Zhou, W.-J.; Liang, Y. Removal of cationic dyes from aqueous solution using magnetic multi-wall carbon nanotube nanocomposite as adsorbent. J. Hazard. Mater. 2009, 164, 1517–1522.
- Chen, C.; Tsyusko, O.V.; McNear, D.H., Jr.; Judy, J.; Lewis, R.W.; Unrine, J.M. Effects of biosolids from a wastewater treatment plant receiving manufactured nanomaterials on Medicago truncatula and associated soil microbial communities at low nanomaterial concentrations. Sci. Total Environ. 2017, 609, 799–806.
- Chauhan, R.; Yadav, H.O.S.; Sehrawat, N. Nanobioremediation: A new and a versatile tool for sustainable environmental clean up-Overview. J. Mater. Environ. Sci. 2020, 11, 564–573.
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691.
- Misra, M.; Ghosh Sachan, S. Nanobioremediation of heavy metals: Perspectives and challenges. J. Basic Microbiol. 2021, 1–16.
- Abdi, O.; Kazemi, M. A review study of biosorption of heavy metals and comparison between different biosorbents. J. Mater. Environ. Sci. 2015, 6, 1386–1399.
- Ayangbenro, A.S.; Babalola, O.O. A new strategy for heavy metal polluted environments: A review of microbial biosorbents. Int. J. Environ. Res. Public Health 2017, 14, 94.
- Usman, M.; Farooq, M.; Wakeel, A.; Nawaz, A.; Cheema, S.A.; Rehman, H.; Ashraf, I.; Sanaullah, M. Nanotechnology in agriculture: Current status, challenges and future opportunities. Sci. Total Environ. 2020, 721, 137778.
- Abebe, B.; Murthy, H.C.A.; Amare, E. Summary on adsorption and photocatalysis for pollutant remediation: Mini review. J. Encapsulation Adsorpt. Sci. 2018, 8, 225–255.
- Desiante, W.L.; Minas, N.S.; Fenner, K. Micropollutant biotransformation and bioaccumulation in natural stream biofilms. Water Res. 2021, 193, 116846.
- Filote, C.; Roșca, M.; Hlihor, R.M.; Cozma, P.; Simion, I.M.; Apostol, M.; Gavrilescu, M. Sustainable application of biosorption and bioaccumulation of persistent pollutants in wastewater treatment: Current practice. Processes 2021, 9, 1696.
- Alissa, E.M.; Ferns, G.A. Heavy metal poisoning and cardiovascular disease. J. Toxicol. 2011, 2011, 870125.
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60.
- Rasmussen, L.D.; Sørensen, S.J.; Turner, R.R.; Barkay, T. Application of a mer-lux biosensor for estimating bioavailable mercury in soil. Soil Biol. Biochem. 2000, 32, 639–646.
- Mukhopadhyay, S.; Maiti, S.K. Phytoremediation of metal mine waste. Appl. Ecol. Environ. Res. 2010, 8, 207–222.
- Chaplygin, V.A.; Minkina, T.M.; Mandzhieva, S.S.; Nazarenko, O.G.; Zimulina, I.V.; Bauer, T.V.; Litvinov, Y.A.; Rajput, V. Heavy metals in agricultural crops of Rostov region through the example of soft wheat (Triticum aestivum). IOP Conf. Ser. Earth Environ. Sci. 2021, 624, 012204.
- Huang, H.; Ullah, F.; Zhou, D.-X.; Yi, M.; Zhao, Y. Mechanisms of ROS Regulation of Plant Development and Stress Responses. Front. Plant Sci. 2019, 10, 193–198.
- Enez, A.; Hudek, L.; Bräu, L. Reduction in trace element mediated oxidative stress towards cropped plants via beneficial microbes in irrigated cropping systems: A review. Appl. Sci. 2018, 8, 1953.
- Arif, N.; Yadav, V.; Singh, S.; Singh, S.; Ahmad, P.; Mishra, R.K.; Sharma, S.; Tripathi, D.K.; Dubey, N.K.; Chauhan, D.K. Influence of high and low levels of plant-beneficial heavy metal ions on plant growth and development. Front. Environ. Sci. 2016, 4, 69.
- Suman, J.; Uhlik, O.; Viktorova, J.; Macek, T. Phytoextraction of heavy metals: A promising tool for clean-up of polluted environment? Front. Plant Sci. 2018, 9, 1476.
- Rajput, V.D.; Minkina, T.; Kimber, R.L.; Singh, V.K.; Shende, S.; Behal, A.; Sushkova, S.; Mandzhieva, S.; Lloyd, J.R.; Semrau, J.D. Insights into the Biosynthesis of Nanoparticles by the Genus Shewanella. Appl. Environ. Microbiol. 2021, 87, e01390-21.
- Capeness, M.J.; Echavarri-Bravo, V.; Horsfall, L.E. Production of biogenic nanoparticles for the reduction of 4-Nitrophenol and oxidative laccase-like reactions. Front. Microbiol. 2019, 10, 997.
- Sharma, D.; Kanchi, S.; Bisetty, K. Biogenic synthesis of nanoparticles: A review. Arab. J. Chem. 2019, 12, 3576–3600.
- Alabresm, A.; Chen, Y.P.; Decho, A.W.; Lead, J. A novel method for the synergistic remediation of oil-water mixtures using nanoparticles and oil-degrading bacteria. Sci. Total Environ. 2018, 630, 1292–1297.
- Xie, Y.; He, Y.; Irwin, P.L.; Jin, T.; Shi, X. Antibacterial activity and mechanism of action of zinc oxide nanoparticles against Campylobacter jejuni. Appl. Environ. Microbiol. 2011, 77, 2325–2331.
- Laslo, V.; Pinzaru, S.C.; Zaguła, G.; Kluz, M.; Vicas, S.I.; Cavalu, S. Synergic effect of selenium nanoparticles and lactic acid bacteria in reduction cadmium toxicity. J. Mol. Struct. 2022, 1247, 131325.
- Dong, G.; Wang, Y.; Gong, L.; Wang, M.; Wang, H.; He, N.; Zheng, Y.; Li, Q. Formation of soluble Cr (III) end-products and nanoparticles during Cr (VI) reduction by Bacillus cereus strain XMCr-6. Biochem. Eng. J. 2013, 70, 166–172.
- Ramos-Ruiz, A.; Sesma-Martin, J.; Sierra-Alvarez, R.; Field, J.A. Continuous reduction of tellurite to recoverable tellurium nanoparticles using an upflow anaerobic sludge bed (UASB) reactor. Water Res. 2017, 108, 189–196.
- Ramos-Ruiz, A.; Field, J.A.; Wilkening, J.V.; Sierra-Alvarez, R. Recovery of elemental tellurium nanoparticles by the reduction of tellurium oxyanions in a methanogenic microbial consortium. Environ. Sci. Technol. 2016, 50, 1492–1500.
- Borghese, R.; Baccolini, C.; Francia, F.; Sabatino, P.; Turner, R.J.; Zannoni, D. Reduction of chalcogen oxyanions and generation of nanoprecipitates by the photosynthetic bacterium Rhodobacter capsulatus. J. Hazard. Mater. 2014, 269, 24–30.
- Manickam, N.; Reddy, M.K.; Saini, H.S.; Shanker, R. Isolation of hexachlorocyclohexane-degrading Sphingomonas sp. by dehalogenase assay and characterization of genes involved in γ-HCH degradation. J. Appl. Microbiol. 2008, 104, 952–960.
- Singh, R.; Manickam, N.; Mudiam, M.K.R.; Murthy, R.C.; Misra, V. An integrated (nano-bio) technique for degradation of γ-HCH contaminated soil. J. Hazard. Mater. 2013, 258, 35–41.
- Hung, C.-M.; Huang, C.-P.; Chen, C.-W.; Dong, C.-D. Degradation of organic contaminants in marine sediments by peroxymonosulfate over LaFeO3 nanoparticles supported on water caltrop shell-derived biochar and the associated microbial community responses. J. Hazard. Mater. 2021, 420, 126553.
- Gholami, F.; Shavandi, M.; Dastgheib, S.M.M.; Amoozegar, M.A. Naphthalene remediation from groundwater by calcium peroxide (CaO2) nanoparticles in permeable reactive barrier (PRB). Chemosphere 2018, 212, 105–113.
- Rizwan, M.; Ali, S.; ur Rehman, M.Z.; Adrees, M.; Arshad, M.; Qayyum, M.F.; Ali, L.; Hussain, A.; Chatha, S.A.S.; Imran, M. Alleviation of cadmium accumulation in maize (Zea mays L.) by foliar spray of zinc oxide nanoparticles and biochar to contaminated soil. Environ. Pollut. 2019, 248, 358–367.
- Rajput, V.D.; Minkina, T.; Feizi, M.; Kumari, A.; Khan, M.; Mandzhieva, S.; Sushkova, S.; El-Ramady, H.; Verma, K.K.; Singh, A. Effects of silicon and silicon-based nanoparticles on rhizosphere microbiome, plant stress and growth. Biology 2021, 10, 791.
- Srivastava, S.; Shukla, A.; Rajput, V.D.; Kumar, K.; Minkina, T.; Mandzhieva, S.; Shmaraeva, A.; Suprasanna, P. Arsenic Remediation through Sustainable Phytoremediation Approaches. Minerals 2021, 11, 936.
- Gajić, G.; Djurdjević, L.; Kostić, O.; Jarić, S.; Mitrović, M.; Pavlović, P. Ecological Potential of Plants for Phytoremediation and Ecorestoration of Fly Ash Deposits and Mine Wastes. Front. Environ. Sci. 2018, 6, 124.
More
Information
Subjects:
Environmental Sciences
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.8K
Revisions:
2 times
(View History)
Update Date:
01 Mar 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




