Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | John DE VOS | + 2312 word(s) | 2312 | 2021-12-15 07:25:19 | | | |
| 2 | Amina Yu | -24 word(s) | 2288 | 2022-03-01 04:23:00 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
De Vos, J. Mesenchymal Cells in the Lung. Encyclopedia. Available online: https://encyclopedia.pub/entry/20001 (accessed on 10 January 2026).
De Vos J. Mesenchymal Cells in the Lung. Encyclopedia. Available at: https://encyclopedia.pub/entry/20001. Accessed January 10, 2026.
De Vos, John. "Mesenchymal Cells in the Lung" Encyclopedia, https://encyclopedia.pub/entry/20001 (accessed January 10, 2026).
De Vos, J. (2022, February 28). Mesenchymal Cells in the Lung. In Encyclopedia. https://encyclopedia.pub/entry/20001
De Vos, John. "Mesenchymal Cells in the Lung." Encyclopedia. Web. 28 February, 2022.
Copy Citation
Mesenchymal cells are an essential cell type because of their role in tissue support, their multilineage differentiation capacities and their potential clinical applications. They play a crucial role during lung development by interacting with airway epithelium, and also during lung regeneration and remodeling after injury. However, much less is known about their function in lung disease.
mesenchyme
lung
1. Origin of Pulmonary Mesenchymal Cells
At the bilaminar disc stage in the second week of human development, gastrulation starts with the formation of the primitive streak [1]. Cells forming the epiblast undergo epithelial to mesenchymal transition (EMT) and migrate through the primitive streak to form the endoderm and mesoderm cell layers (Figure 1A) [2]. Specifically, the first wave of cells integrates the hypoblast layer and forms the endoderm [1], from which the lung will derive. A second group of cells migrates between the epiblast and endoderm layers and constitutes the mesoderm layer that will give rise to a large variety of tissues, such as skeletal muscle, bone, cartilage, and many mesenchymal cell types (for example, fibroblasts, chondroblasts, osteoblasts, blood cells). The mesoderm is a major contributor to trunk and limb stromal cells, but neural crest cells also contribute to mesenchymal cell lineages [3][4], although their specific role in lung mesenchyme is still poorly defined.
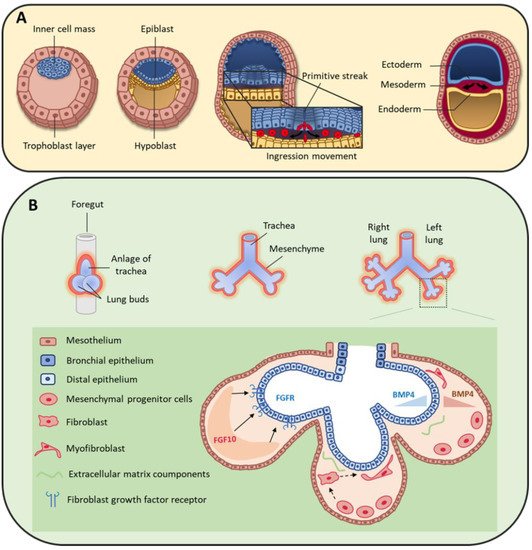
Figure 1. (A) During early human embryo development, the inner cell mass differentiates and becomes organized in the epiblast and hypoblast layers. Primitive streak formation leads to an ingression movement of epiblastic cells that elongate and detach from each other via a critical epithelial–mesenchymal transition [2]. This results in the formation of the three germ layers: ectoderm, mesoderm and endoderm. (B) On day 22, the foregut forms a ventral outgrowth leading to the formation of larynx and trachea in its proximal part, and lung buds in the distal part. Bifurcation and splitting of the lung buds give rise to the future right and left lungs. These structures grow ventrally to caudally through the surrounding mesenchyme. Mesenchymal progenitor cells secrete many factors, including fibroblast growth factor 10 (FGF10) that interacts with fibroblast growth factor receptor (FGFR) expressed by distal epithelial cells. Moreover, some cytokines, such as bone morphogenic protein 4 (BMP4), are secreted by both epithelial and mesenchymal cells.
2. Mesenchymal Cells during Lung Organogenesis
At the beginning of the fourth week of development, the anterior foregut endoderm develops at the cranial extremity after the cephalocaudal folding of the embryo. The foregut produces a ventral evagination that leads to lung bud development. These buds are surrounded by the splanchnopleuric mesoderm that is part of the lateral mesoderm and will contribute to lung vascularization, cartilage, muscles and conjunctive tissue (Figure 1B). In addition, the embryonic lateral splanchnic mesoderm generates mesothelial cells that form a thin layer of squamous-like cells lining the visceral pleura (mesothelium) [5][6][7]. Then, at day 26, the lung buds divide into right and left primitive bronchial buds, which are the precursors of the two lungs (Figure 1B).
During the pseudo-glandular stage, a second division at week 5 of development leads to the formation of the future pulmonary lobes by creating three secondary bronchial buds on the right and two on the left side. Each lung bud and the surrounding splanchnopleuric mesoderm grow, elongate and branch until the formation of the terminal bronchioles (17th branching generation) to create the respiratory tree (Figure 1B) [8]. At this stage, the tracheobronchial tree is coated by prismatic epithelial cells, the precursors of ciliated and secretory cells. Bronchioles appears during the canalicular stage (week 16 to 25), forming the basis of the gas exchange units. This is accompanied by geometric modifications of epithelial cells that flatten and by the appearance of capillaries throughout the mesenchyme that surround the bronchioles. Finally, at the saccular stage (week 24 to 40), alveolar ducts start to form. Their formation will continue after birth, and will terminate only in adulthood [9]. Of note, the development of the gas exchange units in utero and during early childhood is critical for achieving full adult lung function [10]. Lung development is the consequence of an interweaved relationship between embryonic lung epithelial and mesenchymal cells, through direct interactions and also indirectly via the secretion of extracellular matrix (ECM) components and growth factors [11][12][13]. Moreover, the mesothelium plays an important role during lung development [7][14][15], partly by secreting fibroblast growth factor (FGF) 9 [6][16][17].
Fetal airway smooth muscle (ASM) development begins early during gestation (from week 5–6 in human airways) [18]. Fetal ASM surrounds the airways and guides lung development and branching. ASM cells spontaneously contract early in fetal life, with proximal to distal peristaltic-like contractions that displace the amniotic liquid along the lumen [19][20]. At the pseudo-glandular and canalicular stages in pigs [20], the mechanical distention and stretching of the developing lungs, produced by ASM contractions, influence lung growth via mechanotransduction, through the pressure exerted across the airway wall and the surrounding parenchyma. Furthermore, the transmural pressure regulates the rate of airway epithelial bud branching [21].
2.1. Peptide Growth Factors
2.1.1. Fibroblast Growth Factors
The large FGF family plays an important role in the regulation of cell differentiation, proliferation and development, including lung branching [22]. Several studies have identified FGFs implicated in the bidirectional signaling between epithelium and mesenchyme during lung development [16][22][23]. For example, FGF10 is expressed in the distal submesothelial mesenchyme and activates FGF receptor 2b (FGFR2b) in the adjacent epithelial cells (Figure 2) to induce lung budding, epithelial cell expansion and migration, and ECM organization [24][25][26]. Indeed, in Fgf10−/− mice, the lung does not develop below the trachea [27][28]. Moreover, Bellusci et al. have identified, in mice, a subtype of Axin2+/FGF10+-resident mesenchymal alveolar niche cells that are close to alveolar type 2 (AT2) stem cells and that control the proliferation and differentiation of AT2 cells [29].
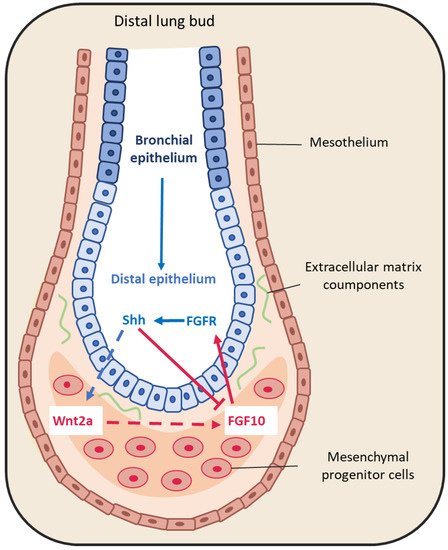
Figure 2. Representative schema of epithelium-mesenchyme interactions during lung budding. Many factors are secreted and exchanged between the lung epithelium (blue arrows) and mesenchyme (red arrows). For instance, fibroblast growth factor 10 (FGF10) is locally expressed by distal submesothelial mesenchymal cells and interacts with its receptor expressed in the distal epithelium. Sonic hedgehog (SHH) is expressed by epithelial cells, and downregulates FGF10 expression through its receptor Patched 1, but also activates FGF10 through the Wnt pathway.
FGF9 is another FGF family member that is expressed in the mesothelium from the pseudo-glandular stage (week 5 to 17) onwards, regulating the local activation of Wnt signaling to promote mesenchymal cell proliferation [6][16] (Figure 2). FGF9 also plays a critical role in lung development, as indicated by the finding that Fgf9−/− mice die at the neonatal stage due to lung hypoplasia caused by very reduced mesenchymal cell expansion [30].
2.1.2. Bone Morphogenic Protein 4 (BMP4)
BMP4 belongs to the transforming growth factor superfamily. Bmp4−/− mice die early during development, mainly due to an absence of mesoderm differentiation [31]. During lung development, BMP4 expression is detected in the distal epithelium buds and in the adjacent mesenchyme already at the pseudo-glandular stage (week 5 to 17) [32][33]. Conversely, BMP type I receptor (BMPR1) is expressed in both epithelium and mesenchyme (Figure 1B) [34]. In association with sonic hedgehog (SHH), BMP4 antagonizes FGF10 that is expressed in the surrounding mesenchyme [35]. Conditional knock-out of Bmpr 1a, the gene encoding the BMP4 receptor in the epithelium, leads to abnormal lung development with reduced cell proliferation, increased apoptosis and abnormal lung morphogenesis. This indicates that BMP4 plays important roles in lung development [36]. Moreover [37], in cultured mouse embryonic lung, reduction of gremlin expression, a BMP4 antagonist, using antisense oligonucleotides promotes epithelial cell proliferation and abnormal lung formation/function. Finally, BMP4 overexpression in the distal bud tips leads to lung hypoplasia, reduction of AT2 cells, and enlargement of the terminal buds [34]. However, the exact role of this signaling pathway during lung development remains debated, because mathematical models to mimic the FGF10-SHH interaction accurately model bronchial branching independently of BMP4 expression [38][39].
2.1.3. Sonic Hedgehog
SHH also is part of a key developmental signaling pathway. It is implicated in central nervous system patterning, and limb, digit and lung development [40]. In Shh−/− mice, a single lobe, lung hypoplasia with absence of left and right asymmetry, enhanced cell death and decreased lung mesenchymal cell proliferation are observed [41]. SHH is expressed with BMP4 in the distal bud epithelium during lung development. It binds to and activates its receptor Patched 1 (PTCH1) that is localized in the adjacent mesenchyme. Patched 1 activation downregulates FGF10 expression [16][24][42]. Indeed, in Shh−/− mice, FGF10 expression in the mesenchyme immediately adjacent to the epithelium is increased [43]. In addition, BMP4 is overexpressed and wingless-related integration site family member 2 (WNT2) is downregulated in the mesenchyme. In 2013, Peng et al. [44] identified a cardiopulmonary mesoderm progenitor population that is defined by the expression of WNT2, glioma-associated oncogene 1 (Gli1) and Islet 1, and gives rise to the lung mesenchyme and cardiac lineage in the mouse. This population is regulated by Hedgehog signaling because Gli proteins are the main transcriptional effectors of this pathway, and start to be expressed at the lung organogenesis step [44]. This suggests that SHH is broadly involved in mesenchymal signaling in the developing lung.
2.1.4. Epidermal Growth Factor (EGF)
EGF and its tyrosine kinase receptor EGF-R are expressed in the epithelial and mesenchymal compartments during lung development. EGF stimulates lung branching in fetal mice [12]. In agreement, in Egfr−/− mice, neonatal lethality is high and epithelial cell development is impaired in several organs, including the lung [45]. Importantly, lung branching is reduced and alveolarization and septation are deficient in Egfr−/− mice [46]. Similarly, mouse lung branching can be inhibited in ex-vivo cultures using antisense oligonucleotides against EGF [12][47]. Furthermore, the interplay between retinoid acid (RA) and EGFR during fetal lung development stimulates lung branching [48].
2.1.5. Retinoic Acid (RA)
RA is essential for normal embryo development, including lung development [49]. In the mouse, retinoic acid receptor (RAR) double knock-out (RARα-/-/RARβ2-/-) leads to agenesis of the left lung and hypoplasia of the right lung [50]. RA is produced by the mesenchyme surrounding the lung primordia, and also by the epithelial compartment of the proximal bronchi [51]. Importantly, a RA-transforming growth factor (TGF)-β-FGF10 interaction has been described during lung bud induction, where RA downregulates TGF-β to allow FGF10 expression [52].
2.1.6. TGF-β
TGF-β, a pleiotropic growth factor and a key EMT inducer, is another modulator of epithelium–mesenchyme interactions in the developing lung [53]. Its three isoforms play a crucial role during lung organogenesis [54]. Their expression is well characterized in the mouse where TGF-β1 is expressed in the mesenchyme, TGF-β2 in the distal epithelium, and TGF-β3 in the proximal mesenchyme and pulmonary mesothelium [55][56]. In Tgf-β2−/− mice, the distal airways are collapsed and the proximal airways are dilated [54], whereas in Tgf-β3−/− mice lung development is delayed, causing their death [57]. Conversely, Tgfr-β2 ablation in mesodermal tissue results in abnormal lung branching and lung development, while its ablation in epithelial cells that produce surfactant protein C results in a decrease of alveolar type I (AT1) epithelial cells during post-natal alveolarization [58]. TGF-β also plays a role in FGF10 regulation by RA [52] as illustrated by the finding that ectopic TGF-β1 expression inhibits FGF10-induced lung morphogenesis in cultured embryonic lung endodermal explants [59].
2.1.7. The Hippo Pathway
The Hippo pathway and its downstream targets Yes-associated protein (YAP) and transcriptional coactivator with PDZ-binding motif (TAZ) play major roles in tissue homeostasis, organ development, and organ size [60][61] by regulating various processes, such as cell proliferation/survival, the response to mechanical stress and cell geometry. This pathway involves a kinase cascade and adaptors that ultimately regulate YAP/TAZ activities. The name comes from the Drosophila kinase Hippo, the mammalian orthologs of which are the kinases MST1/2. Upon activation of the Hippo signaling pathway, MST1/2 phosphorylates LATS1/2, thereby activate these kinases that in turn phosphorylate YAP and TAZ. Phosphorylated YAP and TAZ are retained in the cytoplasm or degraded by the ubiquitin system. Conversely, when the Hippo pathway is inactive, YAP and TAZ shuttle to the nucleus where they bind to TEA domain (TEAD) transcription factors and regulates the transcription of many downstream genes [61]. Taz knockout mice show abnormal alveolarization, leading to airway enlargement that mimics human emphysema [62][63]. Conditional Yap knockout in lung epithelium causes disruption of bronchial morphogenesis [63]. During alveologenesis, YAP/TAZ inactivation by cell crowding orients NKX2.1 activity towards AT2 cells, a mechanism thought to be a negative feedback to limit AT1 cell expansion [64]. In bronchia, YAP is activated in distal airways, and its induction prevents multi-ciliated cell differentiation [65]. These observations suggest a general role of YAP/TAZ in favoring the stem cell compartment at the expenses of terminal differentiation. In line, YAP overexpression in adult tracheal cells results in basal cell hyperplasia and stratification [66]. Interestingly, injury in airway epithelial cells leads to downregulation of hippo signaling that increases the concentration of nuclear YAP [10], and induces FGF10 secretion by the adjacent mesenchymal cells [67]. Moreover, in the absence of Yap, epithelial progenitors cannot respond to local TGF-β signaling [65]. Overall, the Hippo pathway plays a critical role in lung development and response to injury directly in epithelial cells or indirectly through epithelium/mesenchyme signaling.
Other factors also are involved in lung development, such as components of the vascular endothelial growth factor (VEGF) and WNT signaling pathways. Their expression is highly regulated in space and time, allowing optimal lung development, at least partly through epithelium-mesenchyme interactions. Single-cell transcriptomic analyses will help to identify all the factors involved in lung development.
3. Extracellular Matrix Compounds
The ECM exerts functions of support, structure and stabilization that are essential for organ development and homeostasis. By directly binding to growth factors, it can also modulate the activity of secreted factors [68]. At the end of the human pseudo-glandular stage, proteoglycans, such as decorin and lumican, are located between the epithelium and the mesenchyme compartments, along with collagen type I, III and VI [69]. In ex-vivo cultures of mouse embryonic lungs, decorin binds to and neutralizes exogenous TGF-β with high affinity, thus inhibiting TGF-β signaling [70]. Furthermore, heparan sulfates present in the EMC can stabilize the interaction between growth factors and receptors. For example, Izvolsky et al. [71] showed that endogenous gradients of heparan sulfates, especially highly sulfated heparan sulfates, help lung budding induced by FGF10.
References
- Solnica-Krezel, L.; Sepich, D.S. Gastrulation: Making and Shaping Germ Layers. Annu. Rev. Cell Dev. Biol. 2012, 28, 687–717.
- Thiery, J.P.; Acloque, H.; Huang, R.Y.J.; Nieto, M.A. Epithelial-Mesenchymal Transitions in Development and Disease. Cell 2009, 139, 871–890.
- Douarin, N.M.L.; Creuzet, S.; Couly, G.; Dupin, E. Neural crest cell plasticity and its limits. Development 2004, 131, 4637–4650.
- Takashima, Y.; Era, T.; Nakao, K.; Kondo, S.; Kasuga, M.; Smith, A.G.; Nishikawa, S.-I. Neuroepithelial Cells Supply an Initial Transient Wave of MSC Differentiation. Cell 2007, 129, 1377–1388.
- Que, J.; Wilm, B.; Hasegawa, H.; Wang, F.; Bader, D.; Hogan, B.L.M. Mesothelium contributes to vascular smooth muscle and mesenchyme during lung development. Proc. Natl. Acad. Sci. USA 2008, 105, 16626–16630.
- Yin, Y.; Wang, F.; Ornitz, D.M. Mesothelial- and epithelial-derived FGF9 have distinct functions in the regulation of lung development. Development 2011, 138, 3169–3177.
- Batra, H.; Antony, V.B. The pleural mesothelium in development and disease. Front. Physiol. 2014, 5, 284.
- Schittny, J.C. Development of the lung. Cell Tissue Res. 2017, 367, 427–444.
- Bishop, A.E. Pulmonary epithelial stem cells. Cell Prolif. 2004, 37, 89–96.
- Lange, P.; Celli, B.; Agustí, A.; Boje Jensen, G.; Divo, M.; Faner, R.; Guerra, S.; Marott, J.L.; Martinez, F.D.; Martinez-Camblor, P.; et al. Lung-Function Trajectories Leading to Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 2015, 373, 111–122.
- Nogawa, H.; Ito, T. Branching Morphogenesis of Embryonic Mouse Lung Epithelium in Mesenchyme-Free Culture. Development 1995, 121, 1015–1022.
- Warburton, D.; Seth, R.; Shum, L.; Horcher, P.G.; Hall, F.L.; Werb, Z.; Slavkin, H.C. Epigenetic role of epidermal growth factor expression and signalling in embryonic mouse lung morphogenesis. Dev. Biol. 1992, 149, 123–133.
- Warburton, D.; El-Hashash, A.; Carraro, G.; Tiozzo, C.; Sala, F.; Rogers, O.; De Langhe, S.; Kemp, P.J.; Riccardi, D.; Torday, J.; et al. Lung Organogenesis. Curr. Top. Dev. Biol. 2010, 90, 73–158.
- Koopmans, T.; Rinkevich, Y. Mesothelial to mesenchyme transition as a major developmental and pathological player in trunk organs and their cavities. Commun. Biol. 2018, 1, 1–14.
- Ariza, L.; Carmona, R.; Cañete, A.; Cano, E.; Muñoz-Chápuli, R. Coelomic epithelium-derived cells in visceral morphogenesis. Dev. Dyn. 2016, 245, 307–322.
- White, A.C.; Xu, J.; Yin, Y.; Smith, C.; Schmid, G.; Ornitz, D.M. FGF9 and SHH signaling coordinate lung growth and development through regulation of distinct mesenchymal domains. Development 2006, 133, 1507–1517.
- Yin, Y.; Ornitz, D.M. FGF9 and FGF10 activate distinct signaling pathways to direct lung epithelial specification and branching. Sci. Signal. 2020, 13, 621.
- Nakamura, K.T.; McCray, P.B. Fetal airway smooth-muscle contractility and lung development. A player in the band or just someone in the audience? Am. J. Respir. Cell Mol. Biol. 2000, 23, 3–6.
- Fayon, M.; Andrieux, A.; Bara, I.; Rebola, M.; Labbé, A.; Marthan, R.; Berger, P. An Age-Wise Comparison of Human Airway Smooth Muscle Proliferative Capacity. PLoS ONE 2015, 10, e0122446.
- Bokka, K.K.; Jesudason, E.C.; Lozoya, O.A.; Guilak, F.; Warburton, D.; Lubkin, S.R. Morphogenetic Implications of Peristalsis-Driven Fluid Flow in the Embryonic Lung. PLoS ONE 2015, 10, e0132015.
- Nelson, C.M.; Gleghorn, J.P.; Pang, M.-F.; Jaslove, J.M.; Goodwin, K.; Varner, V.D.; Miller, E.; Radisky, D.C.; Stone, H.A. Microfluidic chest cavities reveal that transmural pressure controls the rate of lung development. Development 2017, 144, 4328–4335.
- Ornitz, D.M.; Itoh, N. Fibroblast Growth Factors. Genome Biol. 2001, 2, 3005.1–3005.12.
- Shannon, J.M.; Hyatt, B.A. Epithelial-Mesenchymal Interactions in the Developing Lung. Annu. Rev. Physiol. 2004, 66, 625–645.
- Bellusci, S.; Grindley, J.; Emoto, H.; Itoh, N.; Hogan, B.L. Fibroblast Growth Factor 10 (FGF10) and Branching Morphogenesis in the Embryonic Mouse Lung. Development 1997, 124, 4867–4878.
- Park, W.Y.; Miranda, B.; Lebeche, D.; Hashimoto, G.; Cardoso, W.V. FGF-10 Is a Chemotactic Factor for Distal Epithelial Buds during Lung Development. Dev. Biol. 1998, 201, 125–134.
- Lü, J.; Izvolsky, K.I.; Qian, J.; Cardoso, W.V. Identification of FGF10 Targets in the Embryonic Lung Epithelium during Bud Morphogenesis. J. Biol. Chem. 2005, 280, 4834–4841.
- Sekine, K.; Ohuchi, H.; Fujiwara, M.; Yamasaki, M.; Yoshizawa, T.; Sato, T.; Yagishita, N.; Matsui, D.; Koga, Y.; Itoh, N.; et al. Fgf10 is essential for limb and lung formation. Nat. Genet. 1999, 21, 138–141.
- Min, H.; Danilenko, D.M.; Scully, S.A.; Bolon, B.; Ring, B.D.; Tarpley, J.E.; DeRose, M.; Simonet, W.S. Fgf-10 is required for both limb and lung development and exhibits striking functional similarity to Drosophila branchless. Genes Dev. 1998, 12, 3156–3161.
- Taghizadeh, S.; Heiner, M.; Vazquez-Armendariz, A.I.; Wilhelm, J.; Herold, S.; Chen, C.; Zhang, J.S.; Bellusci, S. Characterization in mice of the resident mesenchymal niche maintaining AT2 stem cell proliferation in homeostasis and disease. Stem Cells 2021, 39, 1382–1394.
- Colvin, J.S.; Green, R.P.; Schmahl, J.; Capel, B.; Ornitz, D.M. Male-to-Female Sex Reversal in Mice Lacking Fibroblast Growth Factor 9. Cell 2001, 104, 875–889.
- Winnier, G.; Blessing, M.; Labosky, P.A.; Hogan, B.L. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 1995, 9, 2105–2116.
- Weaver, M.; Dunn, N.R.; Hogan, B.L. Bmp4 and Fgf10 Play Opposing Roles during Lung Bud Morphogenesis. Development 2000, 127, 2695–2704.
- Weaver, M.; Batts, L.; Hogan, B.L.M. Tissue interactions pattern the mesenchyme of the embryonic mouse lung. Dev. Biol. 2003, 258, 169–184.
- Bellusci, S.; Henderson, R.; Winnier, G.; Oikawa, T.; Hogan, B.L. Evidence from Normal Expression and Targeted Misexpression That Bone Morphogenetic Protein (Bmp-4) Plays a Role in Mouse Embryonic Lung Morphogenesis. Development 1996, 122, 1693–1702.
- Herriges, M.; Morrisey, E.E. Lung development: Orchestrating the generation and regeneration of a complex organ. Development 2014, 141, 502–513.
- Eblaghie, M.C.; Reedy, M.; Oliver, T.; Mishina, Y.; Hogan, B.L.M. Evidence that autocrine signaling through Bmpr1a regulates the proliferation, survival and morphogenetic behavior of distal lung epithelial cells. Dev. Biol. 2006, 291, 67–82.
- Shi, W.; Zhao, J.; Anderson, K.D.; Warburton, D. Gremlin negatively modulates BMP-4 induction of embryonic mouse lung branching morphogenesis. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2001, 280, L1030–L1039.
- Hirashima, T.; Iwasa, Y.; Morishita, Y. Mechanisms for split localization of Fgf10 expression in early lung development. Dev. Dyn. 2009, 238, 2813–2822.
- Menshykau, D.; Kraemer, C.; Iber, D. Branch Mode Selection during Early Lung Development. PLoS Comput. Biol. 2012, 8, e1002377.
- Carballo, G.B.; Honorato, J.R.; de Lopes, G.P.F.; de Sampaio e Spohr, T.C.L. A highlight on Sonic hedgehog pathway. Cell Commun. Signal. 2018, 16, 11.
- Litingtung, Y.; Lei, L.; Westphal, H.; Chiang, C. Sonic hedgehog is essential to foregut development. Nat. Genet. 1998, 20, 58–61.
- Cardoso, W.V.; Lü, J. Regulation of early lung morphogenesis: Questions, facts and controversies. Development 2006, 133, 1611–1624.
- Pepicelli, C.V.; Lewis, P.M.; McMahon, A.P. Sonic hedgehog regulates branching morphogenesis in the mammalian lung. Curr. Biol. 1998, 8, 1083–1086.
- Peng, T.; Tian, Y.; Boogerd, C.J.; Lu, M.M.; Kadzik, R.S.; Stewart, K.M.; Evans, S.M.; Morrisey, E.E. Coordination of heart and lung co-development by a multipotent cardiopulmonary progenitor. Nature 2013, 500, 589–592.
- Miettinen, P.J.; Berger, J.E.; Meneses, J.; Phung, Y.; Pedersen, R.A.; Werb, Z.; Derynck, R. Epithelial immaturity and multiorgan failure in mice lacking epidermal growth factor receptor. Nature 1995, 376, 337–341.
- Miettinen, P.J.; Warburton, D.; Bu, D.; Zhao, J.-S.; Berger, J.E.; Minoo, P.; Koivisto, T.; Allen, L.; Dobbs, L.; Werb, Z.; et al. Impaired Lung Branching Morphogenesis in the Absence of Functional EGF Receptor. Dev. Biol. 1997, 186, 224–236.
- Seth, R.; Shum, L.; Wu, F.; Wuenschell, C.; Hall, F.L.; Slavkin, H.C.; Warburton, D. Role of Epidermal Growth Factor Expression in Early Mouse Embryo Lung Branching Morphogenesis in Culture: Antisense Oligodeoxynucleotide Inhibitory Strategy. Dev. Biol. 1993, 158, 555–559.
- Schuger, L.; Varani, J.; Mitra, R.; Gilbride, K. Retinoic acid stimulates mouse lung development by a mechanism involving epithelial-mesenchymal interaction and regulation of epidermal growth factor receptors. Dev. Biol. 1993, 159, 462–473.
- Mark, M.; Ghyselinck, N.B.; Chambon, P. Function of retinoid nuclear receptors: Lessons from Genetic and Pharmacological Dissections of the Retinoic Acid Signaling Pathway During Mouse Embryogenesis. Annu. Rev. Pharmacol. Toxicol. 2006, 46, 451–480.
- Mendelsohn, C.; Lohnes, D.; Décimo, D.; Lufkin, T.; LeMeur, M.; Chambon, P.; Mark, M. Function of the retinoic acid receptors (RARs) during development (II). Multiple abnormalities at various stages of organogenesis in RAR double mutants. Development 1994, 120, 2749–2771.
- Fernandes-Silva, H.; Araújo-Silva, H.; Correia-Pinto, J.; Moura, R.S. Retinoic Acid: A Key Regulator of Lung Development. Biomolecules 2020, 10, 152.
- Chen, F.; Desai, T.J.; Qian, J.; Niederreither, K.; Lü, J.; Cardoso, W.V. Inhibition of Tgfβ signaling by endogenous retinoic acid is essential for primary lung bud induction. Development 2007, 134, 2969–2979.
- Kahata, K.; Dadras, M.S.; Moustakas, A. TGF-β Family Signaling in Epithelial Differentiation and Epithelial–Mesenchymal Transition. Cold Spring Harb. Perspect. Biol. 2018, 10, a022194.
- Saito, A.; Horie, M.; Nagase, T. TGF-β Signaling in Lung Health and Disease. Int. J. Mol. Sci. 2018, 19, 2460.
- Bragg, A.D.; Moses, H.L.; Serra, R. Signaling to the epithelium is not sufficient to mediate all of the effects of transforming growth factor β and bone morphogenetic protein 4 on murine embryonic lung development. Mech. Dev. 2001, 109, 13–26.
- Warburton, D.; Bellusci, S.; De Langhe, S.; Del Moral, P.-M.; Fleury, V.; Mailleux, A.; Tefft, D.; Unbekandt, M.; Wang, K.; Shi, W. Molecular Mechanisms of Early Lung Specification and Branching Morphogenesis. Pediatr. Res. 2005, 57, 26–37.
- Kaartinen, V.; Voncken, J.W.; Shuler, C.; Warburton, D.; Bu, D.; Heisterkamp, N.; Groffen, J. Abnormal lung development and cleft palate in mice lacking TGF-beta 3 indicates defects of epithelial-mesenchymal interaction. Nat. Genet. 1995, 11, 415–421.
- Chen, H.; Zhuang, F.; Liu, Y.-H.; Xu, B.; del Moral, P.; Deng, W.; Chai, Y.; Kolb, M.; Gauldie, J.; Warburton, D.; et al. TGF-β receptor II in epithelia versus mesenchyme plays distinct roles in the developing lung. Eur. Respir. J. 2008, 32, 285–295.
- Xing, Y.; Li, C.; Hu, L.; Tiozzo, C.; Li, M.; Chai, Y.; Bellusci, S.; Anderson, S.; Minoo, P. Mechanisms of TGFβ Inhibition of Lung Endodermal Morphogenesis: The role of TβRII, Smads, Nkx2.1 and Pten. Dev. Biol. 2008, 320, 340–350.
- Edgar, B.A. From Cell Structure to Transcription: Hippo Forges a New Path. Cell 2006, 124, 267–273.
- Meng, Z.; Moroishi, T.; Guan, K.-L. Mechanisms of Hippo pathway regulation. Genes Dev. 2016, 30, 1–17.
- Makita, R.; Uchijima, Y.; Nishiyama, K.; Amano, T.; Chen, Q.; Takeuchi, T.; Mitani, A.; Nagase, T.; Yatomi, Y.; Aburatani, H.; et al. Multiple renal cysts, urinary concentration defects, and pulmonary emphysematous changes in mice lacking TAZ. Am. J. Physiol. Ren. Physiol. 2008, 294, F542–F553.
- Isago, H.; Mitani, A.; Mikami, Y.; Horie, M.; Urushiyama, H.; Hamamoto, R.; Terasaki, Y.; Nagase, T. Epithelial Expression of YAP and TAZ Is Sequentially Required in Lung Development. Am. J. Respir. Cell Mol. Biol. 2020, 62, 256–266.
- Little, D.R.; Lynch, A.M.; Yan, Y.; Akiyama, H.; Kimura, S.; Chen, J. Differential chromatin binding of the lung lineage transcription factor NKX2-1 resolves opposing murine alveolar cell fates in vivo. Nat. Commun. 2021, 12, 2509.
- Mahoney, J.E.; Mori, M.; Szymaniak, A.D.; Varelas, X.; Cardoso, W.V. The Hippo Pathway Effector Yap Controls Patterning and Differentiation of Airway Epithelial Progenitors. Dev. Cell 2014, 30, 137–150.
- Zhao, R.; Fallon, T.R.; Saladi, S.V.; Pardo-Saganta, A.; Villoria, J.; Mou, H.; Vinarsky, V.; Gonzalez-Celeiro, M.; Nunna, N.; Hariri, L.P.; et al. Yap Tunes Airway Epithelial Size and Architecture by Regulating the Identity, Maintenance, and Self-renewal of Stem Cells. Dev. Cell 2014, 30, 151–165.
- Volckaert, T.; Yuan, T.; Chao, C.-M.; Bell, H.; Sitaula, A.; Szimmtenings, L.; El Agha, E.; Chanda, D.; Majka, S.; Bellusci, S.; et al. Fgf10-Hippo epithelial mesenchymal crosstalk maintains and recruits lung basal stem cells. Dev. Cell 2017, 43, 48–59.e5.
- McGowan, S.E. Extracellular matrix and the regulation of lung development and repair1. FASEB J. 1992, 6, 2895–2904.
- Godoy-Guzmán, C.; San Martin, S.; Pereda, J. Proteoglycan and collagen expression during human air conducting system development. Eur. J. Histochem. 2012, 56, e29.
- Zhao, J.; Sime, P.J.; Bringas, P.; Gauldie, J.; Warburton, D. Adenovirus-mediated decorin gene transfer prevents TGF-β-induced inhibition of lung morphogenesis. Am. J. Physiol.-Lung Cell. Mol. Physiol. 1999, 277, L412–L422.
- Izvolsky, K.I.; Shoykhet, D.; Yang, Y.; Yu, Q.; Nugent, M.A.; Cardoso, W.V. Heparan sulfate–FGF10 interactions during lung morphogenesis. Dev. Biol. 2003, 258, 185–200.
More
Information
Subjects:
Developmental Biology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.3K
Revisions:
2 times
(View History)
Update Date:
01 Mar 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




