| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Luca Liviu Rus | + 1756 word(s) | 1756 | 2020-09-07 03:36:35 |
Video Upload Options
Biomedicine represents one of the main study areas for dendrimers, which have proven to be valuable both in diagnostics and therapy, due to their capacity for improving solubility, absorption, bioavailability and targeted distribution. Molecular cytotoxicity constitutes a limiting characteristic, especially for cationic and higher-generation dendrimers. Antineoplastic research of dendrimers has been widely developed, and several types of poly(amidoamine) and poly(propylene imine) dendrimer complexes with doxorubicin, paclitaxel, imatinib, sunitinib, cisplatin, melphalan and methotrexate have shown an improvement in comparison with the drug molecule alone. The anti-inflammatory therapy focused on dendrimer complexes of ibuprofen, indomethacin, piroxicam, ketoprofen and diflunisal. In the context of the development of antibiotic-resistant bacterial strains, dendrimer complexes of fluoroquinolones, macrolides, beta-lactamines and aminoglycosides have shown promising effects. Regarding antiviral therapy, studies have been performed to develop dendrimer conjugates with tenofovir, maraviroc, zidovudine, oseltamivir and acyclovir, among others. Furthermore, cardiovascular therapy has strongly addressed dendrimers. Employed in imaging diagnostics, dendrimers reduce the dosage required to obtain images, thus improving the efficiency of radioisotopes. Dendrimers are macromolecular structures with multiple advantages that can suffer modifications depending on the chemical nature of the drug that has to be transported. The results obtained so far encourage the pursuit of new studies.
1. Introduction
Dendrimers are synthetic polymers characterized by branched repeating units that emerge from a focal point and possess a large number of exposed anionic, neutral or cationic terminal functionalities on the surface, which leads to hydrophilic or hydrophobic compounds [1]. They are nanometric molecules that are radially symmetric, globular, mono-dispersed and homogenous [2].
The properties of dendrimers are different in comparison to conventional polymers. Due to their size, dendrimers are used in nanomedicine research. They are found to be useful as delivery or carrier systems for drugs and genes, but studies have shown that some dendrimers have medicinal uses of their own, mostly due to their antifungal, antibacterial and cytotoxic properties [3][4].
The benefits of many drugs cannot be exploited because of their poor solubility, toxicity or stability problems. The use of dendrimers as carriers of these compounds can solve these problems, thus improving their clinical applications [5].
The valorization of dendrimers represents an important progress in the current therapeutic field, and the biodegradable properties of these polymers can significantly increase their applicability [6].
Compared to traditional surfactants, when they are used as carriers, dendrimers possess numerous advantages, like a high loading capacity of the drug through numerous functional surface groups and internal cavities, the high bioavailability of the attached drug through covalent or non-covalent bonds, and the high penetrability of biological barriers and cell membranes [7][8][9][10].
2. Biomedical Dendrimer Profile-Cytotoxicity
In order to introduce a new substance in therapeutics and the diagnostics of human illnesses, its properties have to be well documented. Beside the physicochemical characteristics and the pharmacological profile, the toxicological risk/benefit ratio must also be evaluated. Dendrimers, as biocompatible nanoparticle macromolecules, are used for their unique properties as carriers of other molecular structures, in order to improve the activity and efficiency of an active drug molecule and also to reduce its toxicity.
It has been shown that the cytotoxicity of the dendrimer depends on the generation to which it belongs and also on the nature of its surface, given by terminal functional groups. Cytotoxicity was highlighted in cationic, amine dendrimers. Studies also showed a correlation between cytotoxicity and dendrimer generation[11][12]. For example, the cytotoxicity of poly(amidoamine) (PAMAM) and poly(propylene imine) (PPI) dendrimers is directly proportional to concentration and generation, due to the presence of primary amines terminal zones. Grafted polyethylene carbosilane dendrimers are less toxic and so are anionic terminal group dendrimers [13][14][15]. Thus, the surface modification of cationic dendrimers in order to neutralize or completely modify them to anions is directly linked to reduced cytotoxicity [16].
3. Biomedical Applications of Dendrimers
Several dendrimers possess intrinsic pharmacodynamic properties [3][4]. In order to be used for their biomedical activity, dendrimers must meet certain conditions, as follows: a) they must show low toxicity, b) low immunogenicity, and c) high permeability, so that they can cross biological barriers, have a proper presence in the systemic circulation and be capable of specific targeting [17]. The limiting characteristic in relation to the medical use of many dendrimers is their cytotoxicity [18].
Dendrimers have been investigated in relation to medical tasks, the targeted release of active molecules, or gene therapy, due to the malleability of their structure which permits the tailoring of their physicochemical properties [19][20][21]. This possibility confers the uniqueness of dendrimers compared to other nanoparticles, their structure on generations (dendrons—branched concentric layers) (offering the possibility of synthesizing dendrimers as monodisperse systems), and the terminal groups offering possibilities for further interaction [22][23][24].
4. Dendrimers in Drug Therapy
4.1. Dendrimers in Antineoplastic Therapy
Conventional antineoplastic therapy is associated with many important side effects. Commonly indicated radiotherapy can lead to the development of secondary gene mutations, which could cause complications and future new malignancies. Chemotherapy, immunotherapy and gene therapy are generally characterized by significant nonspecificity, which limits the bioavailability of the drug at the tumor site [25][26].
Dendrimers transport active drug molecules using various strategies: a) physical interactions based on the inclusion of the active drug molecule in the central structure of the dendrimer through non-covalent associations, hydrogen bonds, hydrophobic or electrostatic interactions [27]; b) chemical interactions involving the covalent conjugation of drugs with the functional end groups of dendrimers [28], on the other hand, are much more stable.
4.2. Dendrimers in Anti-Inflammatory Therapy
The interest in the studying of dendrimers as carriers of active non-steroidal anti-inflammatory drugs (NSAIDs) is increasing. NSAIDs are one of the most widely used classes of drugs, but their use is often limited because of the considerable level of toxicity and associated side effects. Most NSAIDs are hydrophobic molecules, poorly soluble, and have low bioavailability [29]. To improve the solubility of this class of drugs, numerous studies have been performed using water-soluble dendrimers, such as poly(amidoamine) (PAMAM) or poly(propylene imine) (PPI) dendrimers [30][31][32][33]. Due to the presence of amino-terminal groups in these dendrimers, the solubilization of hydrophobic NSAID molecules is possible by using encapsulation technologies, while improving the bioavailability of NSAIDs as well [34][35]. The main mechanism of interaction between the active NSAID molecule and the dendrimer takes place between the dendrimer’s amino groups and the NSAIDs carboxyl groups [36][37].
4.3. Dendrimers in Antibacterial Therapy
The encapsulation of antibiotics in dendrimeric systems can improve their therapeutic efficacy and reduce their side effects to a minimum. The main objectives in the design of dendrimers as delivery systems are the control of particle size, the properties of the surface, the functionality and branch length/density, and the release of drugs in order to obtain the wanted effect at the marked site of action [38]. The active molecules can be condensed inside the dendrimers, physically adsorbed, or chemically attached to the surface of the dendrimer. These structures lead to an improvement in the pharmacokinetic and pharmacodynamic properties of drugs, and can be used in combination with traditional drugs [39].
4.4. Dendrimers in Antiviral Therapy
In antiviral therapy, numerous studies have been performed for the development of dendrimeric conjugates with active substances, which offer multiple advantages, such as increased specificity and bioavailability, prolonged half-life, and the reduced toxicity of the drug [40]. In the last decade, in anti-HIV therapy, nanotechnology using polyanionic carbosilane dendrimers (PCD) has been a promising approach in improving the characteristics of antiretroviral drugs, using dendrimeric nanoparticles with dimensions between 1 and 40 nm [41] and different generations G1-S4, G2-S16 and G3-S16 [42]. These compounds are characterized by the sulfonate groups in the peripheral structures, as follows: G1-S4 PCDs have four peripheral sulfonate groups, and G2-S16 and G3-S16 have 16 groups [43][44]. The number of repeated layers of atoms of silicon determines the generation of dendrimers.
4.5. Dendrimers in Cardiovascular Therapy
In cardiovascular pathologies, due to the low bioavailability of drugs, dendrimeric conjugates have been studied.
A small interfering RNA (siRNA) delivery system was studied. It was comprised of two cell-penetrating peptides, oligo-arginine and a transactivator of transcription, linked to a G4 PAMAM dendrimer through a PEG crosslinker [45]. The loading of siRNA in this delivery system had effective downregulation effects on the expression of AT1R in cardiomyocytes in vitro. In vivo, the delivery of siRNA prevented the increase in the AT1R levels, and it improved the recovery of the cardiac function after IR injury, compared to the groups treated with saline solution or dendrimers alone [45][46].
Due to its low water solubility over a pH range of 4–13, nifedipine possesses a low bioavailability in the human body. PAMAM dendrimers from G0 to G3, with amine or ester surface functional groups, increased the water solubility of nifedipine at a pH of 7. The ester surface functional groups had a greater efficiency than the amine ones. Thus, PAMAM dendrimers could act as solubilizers for nifedipine, in order to increase its therapeutic effects [46].
4.6. Dendrimers in Imaging Diagnostics
Nanotechnology-based imaging is a promising field of interest for overcoming some limitations to the use of imaging agents, and especially for enhancing permeation and retention (EPR), because of the possibility of improving the specificity and the sensitivity of imaging [47].
The advantage of using nanomaterials imaging agents is that they can penetrate and accumulate specifically in tumor tissue through the EPR effect, due to dysfunctional vascularization and lymphatic drainage in the tumor microenvironment [48][49]. The EPR effect, also called “the passive tumor targeting effect”, can augment the concentration of the imaging agent in the tumor, thus increasing the sensitivity and the resolution of the image [50][51].
Systems obtained via the self-assembly of supramolecular nanostructures formed by amphiphilic dendrimers represent innovative and efficient drug delivery systems [52]. The use of these amphiphilic dendrimers offers the advantage of well-defined structures and the stability of dendrimers in generating nanostructures of appropriate dimensions, and the possibility of high drug loading [53][54].
5. Toxicity Reports Regarding Dendrimers
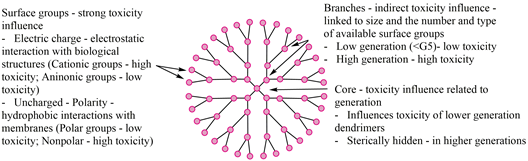
Toxicity, as well as all the other properties of a compound, are directly linked to its structure. Specific elements composing a dendrimer (core, branch, surface groups) contribute to the increase or limitation of its toxicity (Figure 1).
Figure 1. Schematic representation of the toxicity of dendrimers based on their structure [55][56][57].
6. Conclusions
Dendrimers possess many applications due to their functional and structural versatility. They can be used in different fields, like photodynamic therapy, biomedicine, the delivery of genes and siRNA, pharmacy, biopharmacy, the conjugation of oligonucleotides, immunology and imaging. As this study has shown, dendrimers are macromolecular structures with multiple advantages that can suffer modifications in order to ensure drug transport and targeted drug delivery. The toxicity of different dendrimers constitutes a limitation of their applications in biomedicine, and has triggered the development of different toxicity reduction strategies.
The article has been published on 10.3390/molecules25173982
References
- Lyu, Z.; Ding, L.; Huang, A.Y.T.; Kao, C.L.; Peng, L. Poly(amidoamine)dendrimers: Covalent andsupramolecular synthesis. Mater. Today Chem. 2019, 13, 34–48.
- Sohail, I.; Bhatti, I.A.; Ashar, A.; Sarim, F.M.; Mohsin, M.; Naveed, R.; Yasir, M.; Iqbal, M.; Nazir, A.Polyamidoamine (PAMAM) dendrimers synthesis, characterization and adsorptive removal of nickel ionsfrom aqueous solution. J. Mater. Res. Technol. 2020, 9, 498–506.
- Pandita, D.; Poonia, N.; Kumar, S.; Lather, V.; Madaan, K. Dendrimers in drug delivery and targeting:Drug-dendrimer interactions and toxicity issues. J. Pharm. Bioallied Sci. 2014, 6, 139–150.
- Cheng, Y.; Zhao, L.; Li, Y.; Xu, T. Design of biocompatible dendrimers for cancer diagnosis and therapy:Current status and future perspectives. Chem. Soc. Rev. 2011, 40, 2673–2703.
- Sherje, A.P.; Jadhav, M.; Dravyakar, B.R.; Kadam, D. Dendrimers: A versatile nanocarrier for drug deliveryand targeting. Int. J. Pharm. 2018, 548, 707–720.
- Cojocaru, F.D.; Botezat, D.; Gardikiotis, I.; Uritu, C.M.; Dodi, G.; Trandafir, L.; Rezus, C.; Rezus, E.;Tamba, B.I.; Mihai, C.T. Nanomaterials designed for antiviral drug delivery transport across biologicalbarriers. Pharmaceutics 2020, 12, 171.
- Shadrack, D.; Swai, H.; Munissi, J.; Mubofu, E.; Nyandoro, S. Polyamidoamine dendrimers for enhancedsolubility of small molecules and other desirable properties for site specific delivery: Insights fromexperimental and computational studies. Molecules 2018, 23, 1419.
- Shadrack, D.; Mubofu, E.; Nyandoro, S. Synthesis of polyamidoamine dendrimer for encapsulatingtetramethylscutellarein for potential bioactivity enhancement. Int. J. Mol. Sci. 2015, 16, 26363–26377.
- Prajapati, R.N.; Tekade, R.K.; Gupta, U.; Gajbhiye, V.; Jain, N.K. Dendimer-mediated solubilization,formulation development and in vitro-in vivo assessment of piroxicam. Mol. Pharm. 2009, 6, 940–950.
- Otto, D.P.; de Villiers, M.M. Poly(amidoamine) dendrimers as a pharmaceutical excipient. Are We There yet?J. Pharm. Sci. 2018, 107, 75–83.
- Abd-El-Aziz, A.S.; Agatemor, C. Emerging opportunities in the biomedical applications of dendrimers.J. Inorg. Organomet. Polym. Mater. 2018, 28, 369–382.
- Janaszewska, A.; Lazniewska, J.; Trzepi ´ nski, P.; Marcinkowska, M.; Klajnert-Maculewicz, B. Cytotoxicity ofdendrimers. Biomolecules 2019, 9, 330.
- Tomalia, D.A.; Huang, B.; Swanson, D.R.; Brothers, H.M.; Klimash, J.W. Structure control withinpoly(amidoamine) dendrimers: Size, shape and regio-chemical mimicry of globular proteins. Tetrahedron2003, 59, 3799–3813.
- Jevprasesphant, R.; Penny, J.; Jalal, R.; Attwood, D.; McKeown, N.B.; D’Emanuele, A. The influence of surfacemodification on the cytotoxicity of PAMAM dendrimers. Int. J. Pharm. 2003, 252, 263–266.
- Malik, N.; Wiwattanapatapee, R.; Klopsch, R.; Lorenz, K.; Frey, H.; Weener, J.W.; Meijer, E.W.; Paulus,W.;Duncan, R. Dendrimers: Relationship between structure and biocompatibility in vitro, and preliminarystudies on the biodistribution of 125I-labelled polyamidoamine dendrimers in vivo. J. Control. Release 2000,65, 133–148.
- Ciolkowski, M.; Petersen, J.F.; Ficker, M.; Janaszewska, A.; Christensen, J.B.; Klajnert, B.; Bryszewska, M.Surface modification of PAMAM dendrimer improves its biocompatibility. Nanomed. Nanotechnol. Biol. Med.2012, 8, 815–817.
- Boas, U.; Christensen, J.B.; HeegaardJ, P.M.H. Dendrimers in medicine and biotechnology: New moleculartools. RCS Publ. 2006, 2, 28–61.
- Yang, W.; Cheng, Y.; Xu, T.; Wang, X.; Wen, L.P. Targeting cancer cells with biotin-dendrimer conjugates.Eur. J. Med. Chem. 2009, 44, 862–868.
- Shao, N.; Su, Y.; Hu, J.; Zhang, J.; Zhang, H.; Cheng, Y. Comparison of generation 3 polyamidoaminedendrimer and generation 4 polypropylenimine dendrimer on drug loading, complex structure, releasebehavior, and cytotoxicity. Int. J. Nanomed. 2011, 6, 3361–3372.
- Pourianazar, N.T.; Mutlu, P.; Gunduz, U. Bioapplications of poly(amidoamine) (PAMAM) dendrimers innanomedicine. J. Nanopart. Res. 2014, 16, 1–38.
- Gardikis, K.; Micha-Screttas, M.; Demetzos, C.; Steele, B.R. Dendrimers and the development of new complexnanomaterials for biomedical applications. Curr. Med. Chem. Curr. Med. Chem. 2012, 19, 4913–4928.
- Yu, G.S.; Bae, Y.M.; Choi, H.; Kong, B.; Choi, I.S.; Choi, J.S. Synthesis of PAMAM dendrimer derivativeswith enhanced buffering capacity and remarkable gene transfection efficiency. Bioconjug. Chem. 2011, 22,1046–1055.
- Mukherjee, S.P.; Davoren, M.; Byrne, H.J. In vitro mammalian cytotoxicological study of PAMAMdendrimers—Towards quantitative structure activity relationships. Toxicol. Vitr. 2010, 24, 169–177.
- Mukherjee, S.P.; Lyng, F.M.; Garcia, A.; Davoren, M.; Byrne, H.J. Mechanistic studies of in vitro cytotoxicity ofpoly(amidoamine) dendrimers in mammalian cells. Toxicol. Appl. Pharmacol. 2010, 248, 259–268.
- He, Y.; Zha, J.; Wang, Y.; Liu, W.; Yang, X.; Yu, P. Tissue damage-associated “danger signals” influence T-cellresponses that promote the progression of preneoplasia to cancer. Cancer Res. 2013, 73, 629–639.
- Zitvogel, L.; Galluzzi, L.; Smyth, M.J.; Kroemer, G. Mechanism of action of conventional and targetedanticancer therapies: Reinstating immunosurveillance. Immunity 2013, 39, 74–88.
- Singh, J.; Jain, K.; Mehra, N.K.; Jain, N.K. Dendrimers in anticancer drug delivery: Mechanism of interactionof drug and dendrimers. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1626–1634.
- Caminade, A.M.; Turrin, C.O. Dendrimers for drug delivery. J. Mater. Chem. B 2014, 2, 4055–4066.
- Lipinski, C.A. Poor aqueous solubility—An industry wide problem in drug discovery. Am. Pharm. Res. 2002,19, 1894–1900.
- Leuner, C.; Dressman, J. Improving drug solubility for oral delivery using solid dispersions.Eur. J. Pharm. Biopharm. 2000, 50, 47–60.
- Yiyun, C.; Jeipin, Y. Solubilization of non-steroidal anti-inflammatory drugs in the presence of tween seriessurfactants. Phys. Chem. Liq. 2006, 44, 249–256.
- Yiyun, C.; Tongwen, X. Dendrimers as potential drug carriers. Part I. Solubilization of non-steroidalanti-inflammatory drugs in the presence of polyamidoamine dendrimers. Eur. J. Med. Chem. 2005, 40,1188–1192.
- Ullah, I.; Baloch, M.K.; Durrani, G.F. Solubility of nonsteroidal anti-inflammatory drugs (NSAIDs) in aqueoussolutions of non-ionic surfactants. J. Solut. Chem. 2011, 40, 1341–1348.
- Choudhary, S.; Gupta, L.; Rani, S.; Dave, K.; Gupta, U. Impact of dendrimers on solubility of hydrophobicdrug molecules. Front. Pharmacol. 2017, 8, 261.
- Ihre, H.R.; de Jesús, O.L.P.; Szoka, F.C.; Fréchet, J.M.J. Polyester dendritic systems for drug deliveryapplications: Design, synthesis, and characterization. Bioconjug. Chem. 2002, 13, 443–452.
- Markowicz-Piasecka, M.; Mikiciuk-Olasik, E. Nanobiomaterials in Drug Delivery, 1st ed.; Elsevier: Norwich,NY, USA, 2016; Volume 9, pp. 39–74.
- Beezer, A.E.; King, A.S.H.; Martin, I.K.; Mitchel, J.C.; Twyman, L.J.; Wain, C.F. Dendrimers as potential drugcarriers; encapsulation of acidic hydrophobes within water soluble PAMAM derivatives. Tetrahedron 2003,59, 3873–3880.
- Karthikeyan, R.; Koushik, O.S.; Kumar, P.V. Dendrimeric architecture for effective antimicrobial therapy in:Dendrimers for drug delivery. In Dendrimers for Controlled Release Drug Delivery; Apple Academic Press Inc.:Palm Bay, FL, USA, 2019; pp. 375–405
- Authimoolam, S.; Dziubla, T. Biopolymeric mucin and synthetic polymer analogs: Their structure, functionand role in biomedical applications. Polymers 2016, 8, 71.
- Mhlwatika, Z.; Aderibigbe, B. Application of dendrimers for the treatment of infectious diseases. Molecules2018, 23, 2205.
- Vacas-Córdoba, E.; Maly, M.; De la Mata, F.J.; Gómez, R.; Pion, M.; Muñoz-Fernández, M.Á. Antiviralmechanism of polyanionic carbosilane dendrimers against HIV-I. Int. J. Nanomed. 2016, 11, 1281–1294.
- Relaño-Rodríguez, I.; Juárez-Sánchez, R.; Pavicic, C.; Muñoz, E.; Muñoz-Fernández, M.Á. Polyanioniccarbosilane dendrimers as a new adjuvant in combination with latency reversal agents for HIV treatment.J. Nanobiotechnol. 2019, 17, 1–8.
- Rasines, B.; Sánchez-Nieves, J.; Maiolo, M.; Maly, M.; Chonco, L.; Jiménez, J.L.;Muñoz-Fernández, M.Á.; De La Mata, F.J.; Gómez, R. Synthesis, structure and molecular modelling ofanionic carbosilane dendrimers. Dalton Trans. 2012, 41, 12733–12748.
- Arnáiz, E.; Vacas-Córdoba, E.; Galán, M.; Pion, M.; Gómez, R.; Muñoz-Fernández, M.Á.; de la Mata, F.J.Synthesis of anionic carbosilane dendrimers via “click chemistry” and their antiviral properties against HIV.J. Polym. Sci. Part A Polym. Chem. 2014, 52, 1099–1112.
- Liu, J.; Gu, C.; Cabigas, E.B.; Pendergrass, K.D.; Brown, M.E.; Luo, Y.; Davis, M.E. Functionalizeddendrimer-based delivery of angiotensin type 1 receptor siRNA for preserving cardiac function followinginfarction. Biomaterials 2013, 34, 3729–3736.
- Yu, M.; Jie, X.; Xu, L.; Chen, C.; Shen, W.; Cao, Y.; Lian, G.; Qi, R. Recent advances in dendrimer research forcardiovascular diseases. Biomacromolecules 2015, 16, 2588–2598.
- Garriguea, P.; Tang, J.; Ding, L.; Bouhlel, A.; Tintaru, A.; Laurini, E.; Huang, Y.; Lyu, Z.; Zhang, M.;Fernandez, S.; et al. Self-assembling supramolecular dendrimer nanosystem for PET imaging of tumors.Proc. Natl. Acad. Sci. USA 2018, 115, 11454–11459.
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges andopportunities. Nat. Rev. Cancer 2017, 17, 20.
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR e ect inmacromolecular therapeutics: A review. J. Control. Release 2000, 65, 271–284.
- Chow, E.K.H.; Ho, D. Cancer nanomedicine: From drug delivery to imaging. Sci. Transl. Med. 2013, 5, 216rv4.
- Li, C. A targeted approach to cancer imaging and therapy. Nat. Mater. 2014, 13, 110–115.
- Percec, V.; Wilson, D.A.; Leowanawat, P.; Wilson, C.J.; Hughes, A.D.; Kaucher, M.S.; Hammer, D.A.;Levine, D.H.; Kim, A.J.; Bates, F.S.; et al. Self-assembly of Janus dendrimers into uniform dendrimersomesand other complex architectures. Science 2010, 328, 1009–1014.
- Wei, T.; Chen, C.; Liu, J.; Liu, C.; Posocco, P.; Liu, X.; Cheng, Q.; Huo, S.; Liang, Z.; Fermeglia, M.; et al.Anticancer drug nanomicelles formed by self-assembling amphiphilic dendrimer to combat cancer drugresistance. Proc. Natl. Acad. Sci. USA 2015, 112, 2978–2983.
- Sherman, S.E.; Xiao, Q.; Percec, V. Mimicking complex biological membranes and their programmable glycanligands with dendrimersomes and glycodendrimersomes. Chem. Rev. 2017, 117, 6538–6631.
- Castro, R.I.; Forero-Doria, O.; Guzmán, L. Perspectives of dendrimer-based nanoparticles in cancer therapy.An. Acad. Bras. Cienc. 2018, 90, 2331–2346.
- Kitchens, K.M.; Foraker, A.B.; Kolhatkar, R.B.; Swaan, P.W.; Ghandehari, H. Endocytosis and interaction ofpoly (amidoamine) dendrimers with Caco-2 cells. Pharm. Res. 2007, 24, 2138–2145.
- Sebestik, J.; Niederhafner, P.; Jezek, J. Peptide and glycopeptide dendrimers and analogous dendrimericstructures and their biomedical applications. Amino Acids 2011, 40, 301–370.