Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Takahiko Sato | + 1868 word(s) | 1868 | 2022-02-18 06:53:37 | | | |
| 2 | Rita Xu | Meta information modification | 1868 | 2022-03-01 03:00:54 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Sato, T. Tceal5 and Tceal7 Function. Encyclopedia. Available online: https://encyclopedia.pub/entry/19988 (accessed on 08 February 2026).
Sato T. Tceal5 and Tceal7 Function. Encyclopedia. Available at: https://encyclopedia.pub/entry/19988. Accessed February 08, 2026.
Sato, Takahiko. "Tceal5 and Tceal7 Function" Encyclopedia, https://encyclopedia.pub/entry/19988 (accessed February 08, 2026).
Sato, T. (2022, February 28). Tceal5 and Tceal7 Function. In Encyclopedia. https://encyclopedia.pub/entry/19988
Sato, Takahiko. "Tceal5 and Tceal7 Function." Encyclopedia. Web. 28 February, 2022.
Copy Citation
Loss- or gain- of function studies with Tceal5 and Tceal7 indicated that they have the potential to regulate myogenic differentiation via exosomes in fetal bovine serum.
myogenic differentiation
exosome
serum
Tceal5
Tceal7
1. Introduction
Growth arrest and terminal differentiation of skeletal muscle cells to postmitotic myotubes occur when the level of serum is decreased in the cell-culture medium. Thus, in vitro studies with myogenic cells indicate that myogenic differentiation is controlled by serum depletion [1]. Myogenic differentiation in cell culture is usually inhibited by placing myoblasts into a mitogen-rich medium, in contrast to a mitogen-poor medium that promotes differentiation. These effects are due to several growth factors such as insulin-like growth factors, fibroblast growth factors, transforming growth factor-beta, platelet-derived growth factors, and so on [2][3][4][5][6].
It has recently been reported that serum contains not only various hormones and growth factors with specific actions on the proliferation and differentiation of myogenic cells, but also exogenous exosomes that are extracellular membrane micro-vesicles derived from many different types of cells and released into body fluids including blood [7][8]. Exosomes originate from endosome and carry a complex cargo of proteins, lipid, and nucleic acids, including DNA, mRNAs, and non-coding RNAs, functioning in intercellular communication [9].
2. Myogenic Cell Growth without Exosomes in Serum
The proliferation and differentiation of skeletal muscle cells in culture are usually controlled by serum components. To define the appropriate serum condition for the growth of C2C12 myoblast cells, we grew these cells in culture medium supplemented with different concentrations of serum. Cells cultured in 10% of FBS (fetal bovine serum) in DMEM reached confluence within a few days (Figure 1a, upper left panel), not observed with less than 10% of FBS in DMEM, as mentioned in many protocols (Figure 1a). It has been reported that many growth factors in serum can stimulate myogenic proliferation and differentiation, and recently exosomes, as well as growth factors, have been identified in serum. To evaluate the effect of exosomes in serum on myogenic cell growth, we prepared exosome-depleted serum, which is available as a commercial product, for proliferating C2C12 cells (Figure 1b, middle panels). There was no apparent morphological difference with or without exosomes in these cultures (Figure 1b, left and middle panels). C2C12 cells did not grow in non-serum conditions although the same number of cells were initially plated (Figure 1b, right panels). Under conditions where exosomes were or were not present in the serum, the number of growing cells was similar, and the level of transcripts for markers of myogenic stem cells, myogenic commitment to differentiation, and the cell cycle showed no difference (Figure 1c,d).
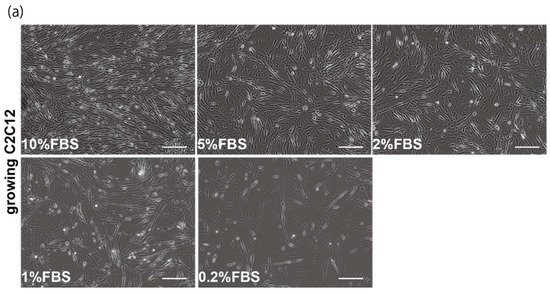
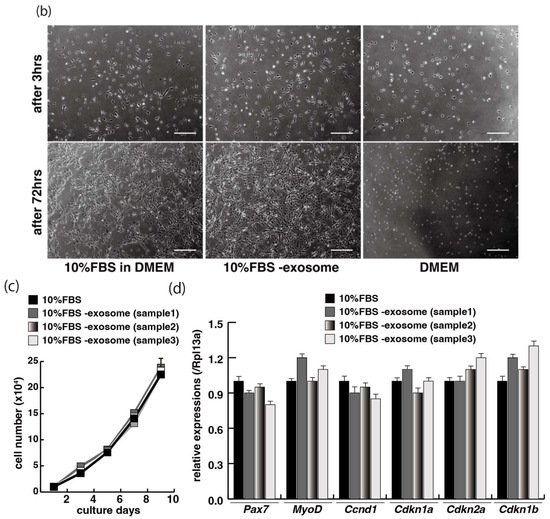
Figure 1. Exosome-depleted serum does not affect myogenic cell growth. (a) Cultured C2C12 cells with different dilutions of fetal bovine serum (FBS); (b) morphology of growing C2C12 cells with or without exosome in FBS (10%FBS in DMEM or 10%FBS−exosome) and without serum (DMEM); (c) C2C12 cells grow in the condition with or without exosomes (sample1–3); (d) relative mRNA expression of myogenic stem cell (Pax7), myogenic commitment to differentiation (MyoD), and cell cycle (Ccnd1, Cdkn1a, cdkn2a, Cdkn1b) markers in C2C12 cells cultured with or without exosomes in FBS. All Scale bar = 100 μm. Data represent means ± SEM (n = 3). The statistical analysis was performed by Mann–Whitney U test.
3. Myogenic Differentiation with Exosomes from Serum
The incubation of C2C12 cells in low-serum conditions can promote myogenic differentiation and we next investigated whether this is impacted by exogenous exosomes from serum impact. Cells cultured in 10% of FBS in DMEM reached semi-confluence around 80% within a few days (Figure 2a, upper panels), and elongated myotubes appeared on the second day after switching to the low serum condition of 0.2% of FBS in DMEM (Figure 2a, lower middle panel). When exosomes isolated from FBS were added to the low-serum medium, negative morphological effects on myogenic differentiation were observed (Figure 2a, lower right panel). In this differentiated phase, myosin heavy chain (MyHC) staining, as a myogenic differentiation marker, was present in a lower percentage of total cells in the presence of additional exosomes (Figure 2b,c). Lower level of transcripts of the myogenic differentiation factor (myogenin: Myog), fusion (myomaker: Mymk), and maturation markers (myosin heavy polypeptide1: Myh1, Creatine kinase: Ckm) were also observed under these conditions (Figure 2d). These data suggest that exosomes in fetal bovine serum function to myogenic differentiation, not to cell growth.
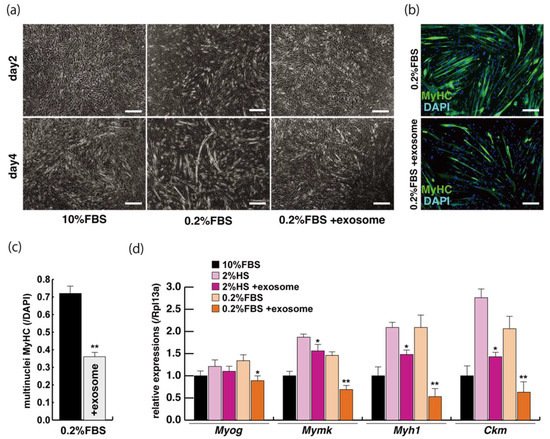
Figure 2. Addition of exosomes derived from serum affects myogenic differentiation. (a) Approximately 80% of semi-confluent C2C12 cells with 10% or 0.2% of fetal bovine serum (FBS) with or without additional exosomes; (b) differentiated C2C12 cells (day4) were immunostained with anti-MF20 (MyHC, green). All nuclei were stained with 4′6-diamidino-2-phenylindole (DAPI, blue); (c) the ratio of DAPI-positive multinuclei staining present in single MyHC-positive myofibers with or without additional exosomes; (d) relative expression of myogenic differentiation (Myog), fusion (Mymk), and differentiated markers (Myh1, Ckm) in C2C12 cells cultured in FBS with or without additional exosomes. All Scale bar = 100 μm. Data represent means ± SEM (n = 3). The statistical analysis was performed with Mann–Whitney U test. * p < 0.05, ** p < 0.01.
4. Tceal5 and Tceal7 in Differentiated C2C12 Cells
To examine the effect of exosome in serum on myogenic differentiation, we performed next-generation sequencing analyses (NGS) with C2C12 cells in growing conditions with 10% of FBS, or in differentiating conditions with 0.2% of FBS or 2% of horse serum with or without added exosomes (Figure 3a,b). Among downregulated mRNAs expressed in differentiating C2C12 cells with the addition of exosomes, the expression levels of Tceal5 and Tceal7 were significantly lower in both NGS and RT-qPCR analyses (Figure 3c–e). These data suggest that Tceal5 and Tceal7 are upregulated in differentiating myogenic cells and that exogenous exosomes in fetal bovine serum downregulated less than half of these transcripts.
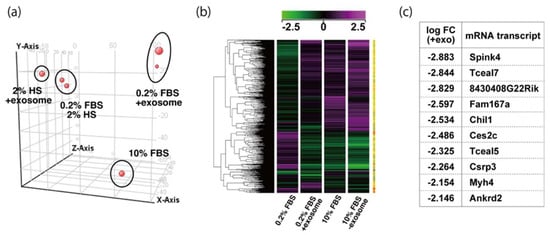
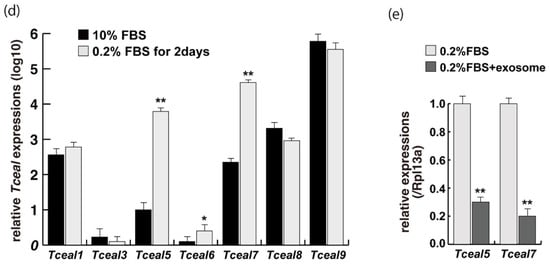
Figure 3. Identification of downregulated genes in differentiating myogenic cells cultured in the presence of added exosomes. (a) Principal component analysis, (b) heatmap of NGS data, illustrating differentially expressed messenger RNAs between 0.2% of FBS differentiating medium (0.2% FBS), 0.2% of FBS supplemented with exosomes (0.2% FBS + exosome), 10% of FBS (10% FBS), and 10% of exosome-depleted FBS (10% FBS −exosome); (c) TOP10 downregulated genes in differentiating C2C12 cells cultured in the presence of additional exosomes; (d) transcriptional changes in the Tceal family of genes in growing (10% FBS) and differentiating (0.2% FBS) C2C12 cells; (e) relative RT-qPCR of Tceal5 and Tceal7 transcripts in differentiating muscle cells with or without additional exosomes. Data represent means ± SEM (n = 3). The statistical analysis was performed by Mann–Whitney U test. * p < 0.05, ** p < 0.01.
5. Attenuated Tceal5 and Tceal7 Expression Effects to Myogenic Differentiation
We next examined whether the knockdown of Tceal5/7 mRNAs with small interfering RNAs (siRNAs) affected differentiating C2C12 cells in a similar manner to the addition of exosomes. The locations of target positions of siRNAs in the coding sequence (CDS) and 3′UTR of Tceal5 and Tceal7 genes are indicated in Figure 4a. Treatment with each siRNA showed the repression of the targeted transcripts specifically (Figure 4b). C2C12 cells grown with these siRNAs were morphologically normal (Figure 4c), and their level of transcripts related to myogenesis, or the cell cycle was almost identical to that of control C2C12 cells (Figure 4d).
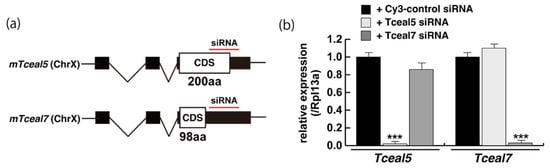
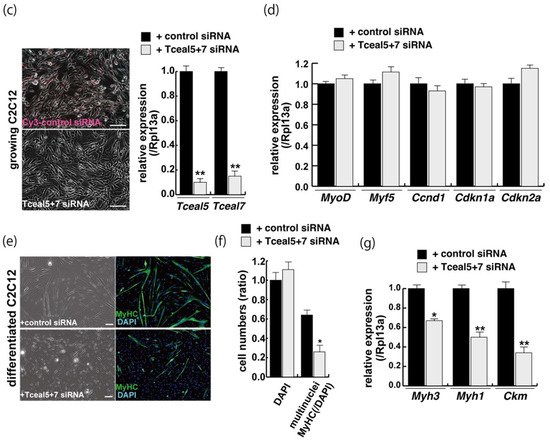
Figure 4. Downregulation of Tceal5/7 reduced myogenic differentiation. (a) Schematic representation of mouse Tceal5 (mTceal5, coding sequence; 200 amino acids) and Tceal7 (mTceal7, coding sequence; 98 amino acids) on chromosome X (ChrX); (b) RT-qPCR analyses after siRNA transfection into C2C12 cells. Each siRNA represses transcripts from the targeted gene specifically; (c) morphological features with both Tceal5 and Tceal7 siRNA treatment in growing C2C12 cells. Cy3-labelled siRNA was used as a control (left). RT-qPCR analyses after both siRNAs transfection into C2C12 cells (right). (d) Relative RT-qPCR analyses of myogenic determination (MyoD, Myf5) and cell cycle genes (Ccnd1, Cdkn1a, Cdkn2a) in growing C2C12 cells with or without both siRNAs; (e) differentiated C2C12 cells with siRNAs were immunostained with anti-MF20 (MyHC, green). All nuclei were stained with 4′6-diamidino-2-phenylindole (DAPI, blue); (f) relative cell numbers of differentiated C2C12 cells with or without Tceal5/7 siRNAs, counted with anti-MyHC antibody and DAPI in (e); (g) relative RT-qPCR of mature myogenic transcripts (Myh1, Myh3, Ckm) in differentiating muscle cells with or without Tceal5/7 siRNAs; All Scale bar = 100 μm. Data represent means ± SEM (n = 3). The statistical analysis was performed by Mann–Whitney U test. * p < 0.05, ** p < 0.01, *** p < 0.001.
Downregulation of single Tceal5 or Tceal7 did not show significant reduction of myogenic differentiation. In contrast, the treatment with both Tceal5 and Tceal7 siRNAs had an impact on differentiated myofibers in C2C12 cultures, shown as lower staining efficiency with MyHC antibodies and a reduction in transcripts of myogenic maturation markers compared to non-treated cells (Figure 4e,f). These results suggest that the attenuation of Tceal5 and Tceal7 gene expression had an effect on myogenic differentiation.
6. Increased Expression of Tceal5 and Tceal7 Rescues Myogenic Differentiation from the Negative Effects of Exosomes
These studies suggest that Tceal5 and Tceal7 genes are downregulated by exosomes. To investigate further their functional role in myogenic differentiation, we examined the effects of Tceal5/7 overexpression, using vectors producing, Tceal5, HA-tagged Tceal7, and 2A peptide-based bicistronic Tceal5 and Tceal7 proteins, driven by a CAG promoter (Figure 5a). Tceal5 and Tceal7 overexpression were confirmed by RT-qPCR (Figure 5b), cleaved Tceal7-HA-tag protein was detected by Western blot analyses (Figure 5c) and Tceal7-HA was localized in the nucleus by immunostaining with anti-HA antibody. To further investigate their functional roles in myogenic differentiation, C2C12 cells were seeded in growth condition with or without additional expression of Tceal5/7 factors and then exposed to myogenic differentiating condition with or without additional exosomes as shown in Figure 2a. Differentiating medium with added exosomes suppressed myogenic differentiation, however this effect was partially rescued by Tceal5/7-overexpression as shown by morphological elongation of myogenic cells and MyHC staining (Figure 5d–f). Transcripts of differentiated myogenic markers were upregulated in cells grown under the condition of Tceal5/7 overexpression (Figure 5g). The upregulation of single Tceal5 or Tceal7 did not rescue the phenotype that exosomes repressed myogenic differentiation. These results support the hypothesis that exosomes in fetal bovine serum downregulate the expression of Tceal5 and Tceal7, which are necessary for myogenic differentiation.
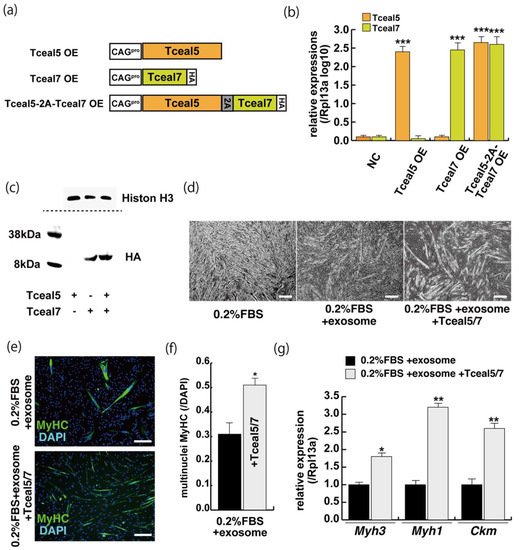
Figure 5. The upregulation of Tceal5/7 rescues differentiating myogenic cells repressed by exosome. (a) Representation of DNA constructs for overexpression of mouse Tceal5 (Tceal5 OE), HA-tagged Tceal7 (Tceal7 OE), and both genes (Tceal5-2A-Tceal7 OE); (b) RT-qPCR analyses with Tceal5 and/or Tceal7 overexpressing in C2C12 cells; (c) Western blot analyses with Tceal5 and/or Tceal7 overexpressing 293 cells. Histone H3 was used as a loading control; (d) morphological changes with both Tceal5 and Tceal7 overexpression in differentiated C2C12 cultures in the presence of additional exosomes; (e) differentiated C2C12 cells treated with additional exosomes and Tceal5/7 overexpressions, were immunostained with anti-MF20 (MyHC, green). All nuclei were stained with 4′6-diamidino-2-phenylindole (DAPI, blue); (f) The ratio of DAPI-positive multinuclei staining present in single MyHC-positive myofibers with additional exosomes and Tceal5/7 overexpression; (g) relative RT-qPCR of mature myogenic transcripts (Myh1, Myh3, Ckm) in differentiating muscle cells with exosomes and Tceal5/7; All Scale bar = 100 μm. Data represent means ± SEM (n = 3). The statistical analysis was performed by Mann–Whitney U test. * p < 0.05, ** p < 0.01, *** p < 0.001.
References
- Schneider, M.D.; Olson, E.N. Control of myogenic differentiation by cellular oncogenes. Mol. Neurobiol. 1988, 2, 1–39.
- Clegg, C.H.; Linkhart, T.A.; Olwin, B.B.; Hauschka, S.D. Growth factor control of skeletal muscle differentiation: Commitment to terminal differentiation occurs in G1 phase and is repressed by fibroblast growth factor. J. Cell Biol. 1987, 105, 949–956.
- Magri, K.A.; Ewton, D.Z.; Florini, J.R. The role of the IGFs in myogenesis differentiation. Adv. Exp. Med. Biol. 1991, 293, 57–76.
- Jin, P.; Sejersen, T.; Ringertz, N.R. Recombinant platelet-derived growth factor-BB stimulates growth and inhibits differentiation of rat L6 myoblasts. J. Biol. Chem. 1991, 266, 1245–1249.
- Zentella, A.; Massague, J. Transforming growth factor beta induces myoblast differentiation in the presence of mitogens. Proc. Natl. Acad. Sci. USA 1992, 89, 5176–5180.
- Yoshiko, Y.; Hirao, K.; Sakabe, K.; Seiki, K.; Takezawa, J.; Maeda, N. Autonomous control of expression of genes for insulin-like growth factors during the proliferation and differentiation of C2C12 mouse myoblasts in serum-free culture. Life Sci. 1996, 59, 1961–1968.
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, biologic function and clinical potential. Cell Biosci. 2019, 9, 19.
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977.
- Schmid, M.; Jensen, T.H. The exosome: A multipurpose RNA-decay machine. Trends Biochem. Sci. 2008, 33, 501–510.
More
Information
Subjects:
Developmental Biology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
598
Revisions:
2 times
(View History)
Update Date:
01 Mar 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




