Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | LEONG SING WONG | + 4739 word(s) | 4739 | 2022-02-28 10:20:23 | | | |
| 2 | Vivi Li | -147 word(s) | 4592 | 2022-02-28 10:59:51 | | | | |
| 3 | Vivi Li | Meta information modification | 4592 | 2022-03-07 02:19:48 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Wong, L.S. Durability Performance of Geopolymer Concrete. Encyclopedia. Available online: https://encyclopedia.pub/entry/19981 (accessed on 07 February 2026).
Wong LS. Durability Performance of Geopolymer Concrete. Encyclopedia. Available at: https://encyclopedia.pub/entry/19981. Accessed February 07, 2026.
Wong, Leong Sing. "Durability Performance of Geopolymer Concrete" Encyclopedia, https://encyclopedia.pub/entry/19981 (accessed February 07, 2026).
Wong, L.S. (2022, February 28). Durability Performance of Geopolymer Concrete. In Encyclopedia. https://encyclopedia.pub/entry/19981
Wong, Leong Sing. "Durability Performance of Geopolymer Concrete." Encyclopedia. Web. 28 February, 2022.
Copy Citation
Geopolymer concrete is produced from the geopolymerization process, in which molecules known as oligomers integrate to form geopolymer networks with covalent bonding. Its production expends less thermal energy and results in a smaller carbon footprint compared to Ordinary Portland Cement (OPC) concrete. It requires only an alkaline activator to catalyze its aluminosilicate sources such as metakaolin and fly ash, to yield geopolymer binder for the geopolymerization to take place. Because of its eco-friendly technology and practical application, current research interest is mainly concentrated on the endurance of geopolymer concrete to resist heat and chemical aggressions.
geopolymer concrete
alkaline activator
aluminosilicate sources
durability
compressive strength
chloride ion penetrability
acid attack
abrasion
1. Introduction
Approximately 5 to 7% of global carbon dioxide (CO2) emissions can be attributed to Ordinary Portland Cement (OPC), which has traditionally been used as the primary binder in concrete [1]. A typical mixture of concrete has a cement content ranging from 15 to 20%. In fact, cement manufacturing consumes a lot of energy, and a high temperature of 1450 °C is required for heating its raw materials in a cement kiln. OPC production demands high levels of energy consumption, mainly due to the high temperatures needed for clinker production but also due to the milling of the raw materials and the clinker [2]. This causes a massive emission of CO2 to the environment. Until now, cement has been widely used as a binding agent in the construction industry because of its capability to bind aggregate in the presence of water to form cement-based materials of various sizes and shapes [3]. Such a consequence accelerates global warming and promotes climate change. Phenomenal global warming and a consequent rise in seawater level are causing earth land to shrink [4]. This may affect the quality of life of the world’s population [5].
Geopolymerization is viewed as an attractive process for producing eco-friendly concrete. Geopolymers are important alternative materials for use in support of recycling and sustainability [6]. The geopolymerization of aluminosilicates constitutes a radical change in construction materials chemistry and synthesis pathways compared with the calcium silicate hydrate chemistry which underpins Portland cement [7]. The binder of geopolymer concrete is geopolymer cement, which is formulated from alkaline activated aluminosilicates of natural clays or by-products from the industry. Alkaline activation requires essentially two components: a precursor and an alkaline activator [8]. In regard to that, aluminosilicates such as rice husk ash, silica fume, fly ash, and metakaolin are conventionally applied as precursors of geopolymer cement. Unlike OPC, less energy utilization of raw materials is required for geopolymer cement production. The source of energy consumption, which is required to produce geopolymer concrete, could be due to sodium hydroxide production, the activating solution preparation, and external heat for curing if it is presented [9]. In addition, sodium silicate requires less thermal energy in its manufacturing. As such, it produces less carbon emission than OPC. The usage of thermal energy for the production of sodium silicate requires a temperature of 1100 °C, which is 350 °C lower than that needed for the making of OPC. Geopolymer concrete is considered as a possible alternative to OPC concrete [10]. Unlike normal concrete, the matrix formation and strength development of geopolymer concrete do not need calcium silicate hydrate (C-S-H) gel to be form [11]. The cementation phenomenon of geopolymer concrete is caused by the polycondensation of aluminosilicate. Marked structural, mechanical, and physical differences have been identified in activated binders using precursors from different sources, as a consequence of the chemical and physical differences between slag and fly ash precursors and the influences of different activator concentrations and chemistries, and the microstructures of alkali-activated slag and of fly ash geopolymers have been extensively studied in systems based on sole precursors [12]. Fly ash has been used for producing geopolymeric cement with mechanical strength up to around 60 MPa [13]. Provis et al. [14] have identified ten types of precursor that can be applied to develop geopolymerized materials. They are silico-manganese slag, mineral processing tailings, catalyst residues, coal bottom ash, rice husk ash, palm oil fuel ash, waste glass, waste ceramic, incineration products of sludges, and natural minerals.
Based on geopolymerization, the durability of geopolymer concrete is attributable to the development of sodium aluminosilicate hydrate (N-A-S-H) gel. Lodeiro et al. [15] provided a detailed description on the geopolymerization mechanism that involved three sequential stages, namely, destruction-coagulation, coagulation-condensation, and condensation-crystallization. The process of geopolymerization starts with the degradation of the aluminosilicate structure by hydroxide ions from an alkaline activator. Such process is governed by the amount of dissolution of silicate and aluminate species. Interactions between the small dissolved species, and involving any silicate initially supplied by the activating solution, lead to the formation of aluminosilicate oligomers [16]. The dissolution of the species continues until the dissolved aluminate is concentrated enough to weaken the dissolved silicate, which eventually causes precipitation of N-A-S-H gel. Under such conditions, the development of N-A-S-H gel progresses with time to form solid N-A-S-H crystals, which eventually contribute to geopolymerized bonding. The duration required for the N-A-S-H crystallization is dependent on the design of the geopolymer admixture and the temperature of curing.
It has been shown that geopolymerization can transform a wide range of waste aluminosilicate materials into building and mining materials with excellent chemical and physical properties, such as fire and acid resistance [17]. Self-compacting geopolymer concrete with zero cement and zero superplasticizers cured under ambient conditions was tested to have 40 MPa compressive strength after 28 days of curing, which is comparable to that of M40 grade conventional concrete [18]. The strength of geopolymer concrete was reported to increase with an increase of curing time and a rise in temperature, as reported in several studies [19][20][21]. However, Zhang et al. [22] clarified that the strength of geopolymer concrete deteriorated after exposure to an optimal curing temperature. The dehydration of the geopolymer was identified by Zhang et al. [22] as a reason for the strength loss of the geopolymer concrete after exposure to a temperature of higher than 600 °C. Chen et al. [23] reported that durability of geopolymer concrete is better than OPC concrete, and calcium content has a great effect on the durability mechanism. Any concrete structure should be durable and able to fend off the processes of debilitation to which it is anticipated to be exposed [24].
Although Chen et al. [23] have reviewed the durability of geopolymer concrete exposed to an aggressive environment, comprehensive literature with regard to the source of aluminosilicate, the type of reinforcing raw material, and the mix design that can optimize long-term robustness of the construction material are lacking. Saranya et al. [25] found that a mixture of ground-granulated blast furnace slag and dolomite had a profound effect on improving the load carrying capacity of geopolymer concrete beam-column joints under monotonic loading. The geopolymer concrete beam-column joints were tested to have better ductility, greater energy absorptivity, and higher toughness with the inclusion of steel fibers. Since steel-fiber-reinforced alkali-activated geopolymer concrete can achieve higher mechanical performance and produce less carbon emission as compared to the conventional concrete, it is considered to be a potential construction material solution for the buried tunnel subjected to gas explosion threatening [26]. In another study, Alrshoudi et al. [27] added glass and carbon-fiber-reinforced polymers to high-strength geopolymer concrete to enhance its compressive strength and ductility. Fiber-reinforced concrete also demonstrates other fair qualities of durability in terms of drying shrinkage, chloride ion penetration, water permeability, abrasion resistance, and impact resistance [28]. In the study of Chithambar Ganesh et al. [29], plastic wastes in the form of polyethylene terephthalate (PET) bottles were ground into powder and partially incorporated in geopolymer concrete. The compressive strength and split tensile strength of the geopolymer concrete were reported to increase by 5.8 and 24%, respectively, with a 10% usage of the plastic powder as a partial replacement of sand. Geopolymers blended recycled concrete (OPC, fly ash, rice husk ash, river sand, Cupola furnace slag, and crushed granite) was discovered by Alabi and Mahachi [30] to have better penetration resistance to chloride ion than other concrete mixes in the study and are therefore more durable. With low chloride ion penetrability, it could be affirmed that the geopolymer-blended recycled concrete offered good protection to steel reinforcement against corrosion. It is notable that there are a number of published research papers [31][32][33][34][35][36][37][38][39][40][41][42][43][44][45][46][47] on the mechanical properties of geopolymer concrete without revealing the findings on its durability characteristics.
2. Compressive Strength of Geopolymer Concrete at Elevated Temperatures
Residual compressive strength of a material is referred to as the maximum compressive stress that the damaged material can sustain under a loading application. The resistance of geopolymer concrete to crack propagation influences its residual compressive strength. The fracture toughness and residual compression resistance of geopolymer concrete tend to decrease when it is exposed to a very high temperature. High temperature, i.e., heat exposure, is one of the most important parameters that affects the surface characteristics, surface outlook, shape, and color of concrete [48]. Therefore, residual compressive strength is an important durability characteristic of geopolymer concrete. The residual compression resistance of geopolymer concrete at elevated temperatures were investigated in numerous studies.
Zhang et al. [22] evaluated the residual compression resistance of four types of geopolymer concrete, which were exposed to high temperatures ranging from 20 to 1000 °C for 2 h. The heating rate applied to the geopolymer concrete was 5 °C min−1. The four types of concrete are ambient-cured normal-strength geopolymer concrete (GPN-A), heat-cured normal strength geopolymer concrete (GPN-H), ambient-cured high-strength geopolymer concrete (GPH-A), and heat-cured high-strength geopolymer concrete (GPH-H). The ambient and heat curing temperatures are 25 and 80 °C, respectively. The respective sodium-silicate-to-sodium-hydroxide ratios for the normal and high-strength geopolymer concrete are 2.5 and 3.0. Figure 1 shows the compressive strength and crushing index of coarse aggregate of the geopolymer concrete specimens at elevated temperatures. Between 20 and 400 °C, the compressive strength values of all the geopolymer concrete specimens were found to be above their original compressive strength values. This could be attributed to the low crushing index of coarse aggregate within the temperature range depicted in Figure 1. The findings reflected the ability of coarse aggregate in the geopolymer concrete specimens to withstand thermal stress up to 400 °C. As expected, the GPH-H had the highest compressive strength of 89.9 MPa at 200 °C. At the same temperature, its ratio of elevated temperature compressive strength to original compressive strength was found to be 123.2%, which is the lowest among all the geopolymer concrete specimens tested (Figure 2). The study revealed that the maximum temperature that all the geopolymer concrete specimens can endure before strength loss is 600 °C. Hence, Zhang et al. [22] clarified that the threshold crushing index of coarse aggregate of the geopolymer concrete specimens is 7.7% at 600 °C. Beyond 600 °C, the geopolymer concrete specimens experienced cracking and spalling due to thermal degradation of the coarse aggregate, which caused their compressive strength to decrease even further. It was found that the percentage residual strength of GPH-A was higher than that of GPH-H at all the temperatures, indicating that ambient-cured geopolymer concrete performed better compressive strength enhancement and lower strength deterioration than the heat-cured ones [22].
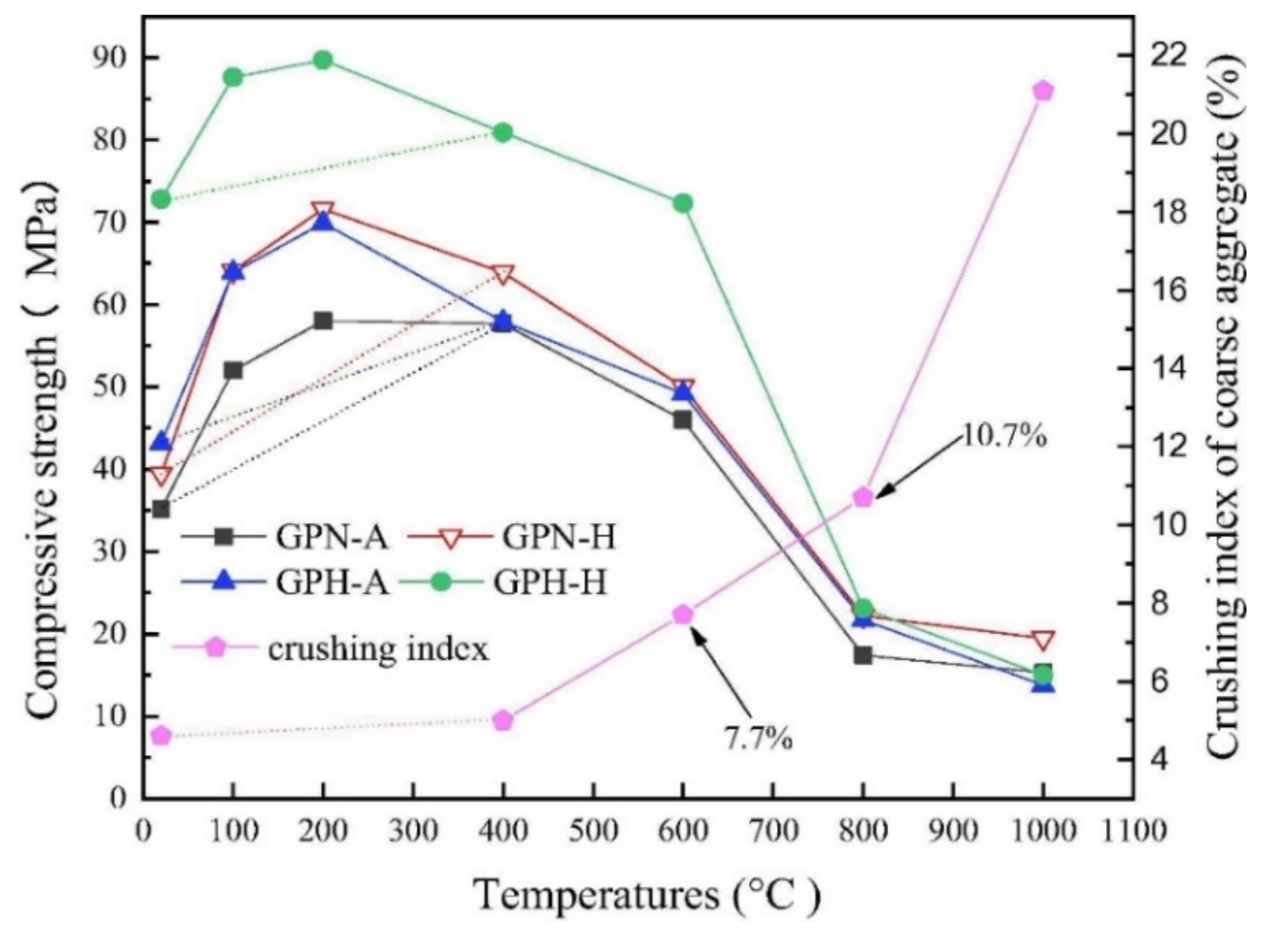
Figure 1. Compressive strength of geopolymer concrete specimens and crushing index of coarse aggregate at elevated temperatures (note: GPN-A, ambient-cured normal strength geopolymer concrete; GPN-H, heat-cured normal strength geopolymer concrete; GPH-A, ambient-cured high-strength geopolymer concrete; and GPH-H, heat-cured high-strength geopolymer concrete). Reproduced with permission from [22], [Construction and Building Materials]; published by [Elsevier], [2020].
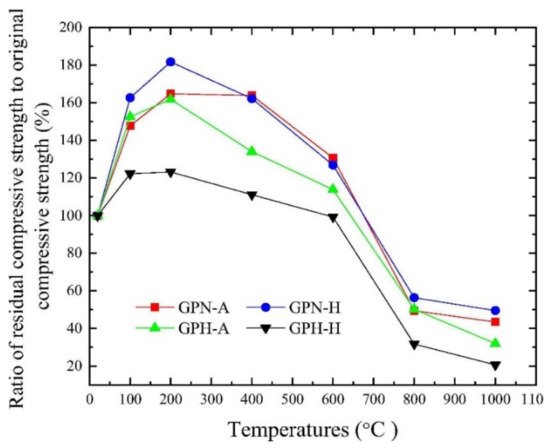
Figure 2. The ratios of residual to original compressive strength of geopolymer concrete specimens at elevated temperatures based on Figure 1, Reproduced with permission from [22], [Construction and Building Materials]; published by [Elsevier], [2020].
In another development, Luhar et al. [49] investigated the compressive strength of fly-ash-based control and rubberized geopolymer concrete specimens (CONTROL GPC and RUBBERIZED GPC) at room temperature, and at 200, 400, 600, and 800 °C. Starting from room temperature, each geopolymer concrete specimen was heated at a rate of 4.4 °C min−1 until an elevated temperature was achieved, and after that the elevated temperature was maintained for a duration of 2 h. The trend of the compressive strength development of the geopolymer concrete specimens is depicted in Figure 3. It is observed in Figure 3 that for all the temperatures, the compressive strength reductions of the RUBBERIZED GPC were slightly lower than the CONTROL GPC. The percentage reductions of compressive strength for the RUBBERIZED GPC were noticed to be 31.23 and 52.43% at the respective temperatures of 200 and 800 °C. On the other hand, the percentage values of the strength reduction for the CONTROL GPC were realized to be 27.38 and 45.22% at 200 and 800 °C, respectively. This was attributed to the soft and light-weight nature of the rubber tire fibers, which could induce cracks of the geopolymer concrete specimens at the initial compression stage. The tendency of the rubber tire fibers to entrap air and form pores in the RUBBERIZED GPC resulted in their higher strength reductions at elevated temperatures compared to the CONTROL GPC.
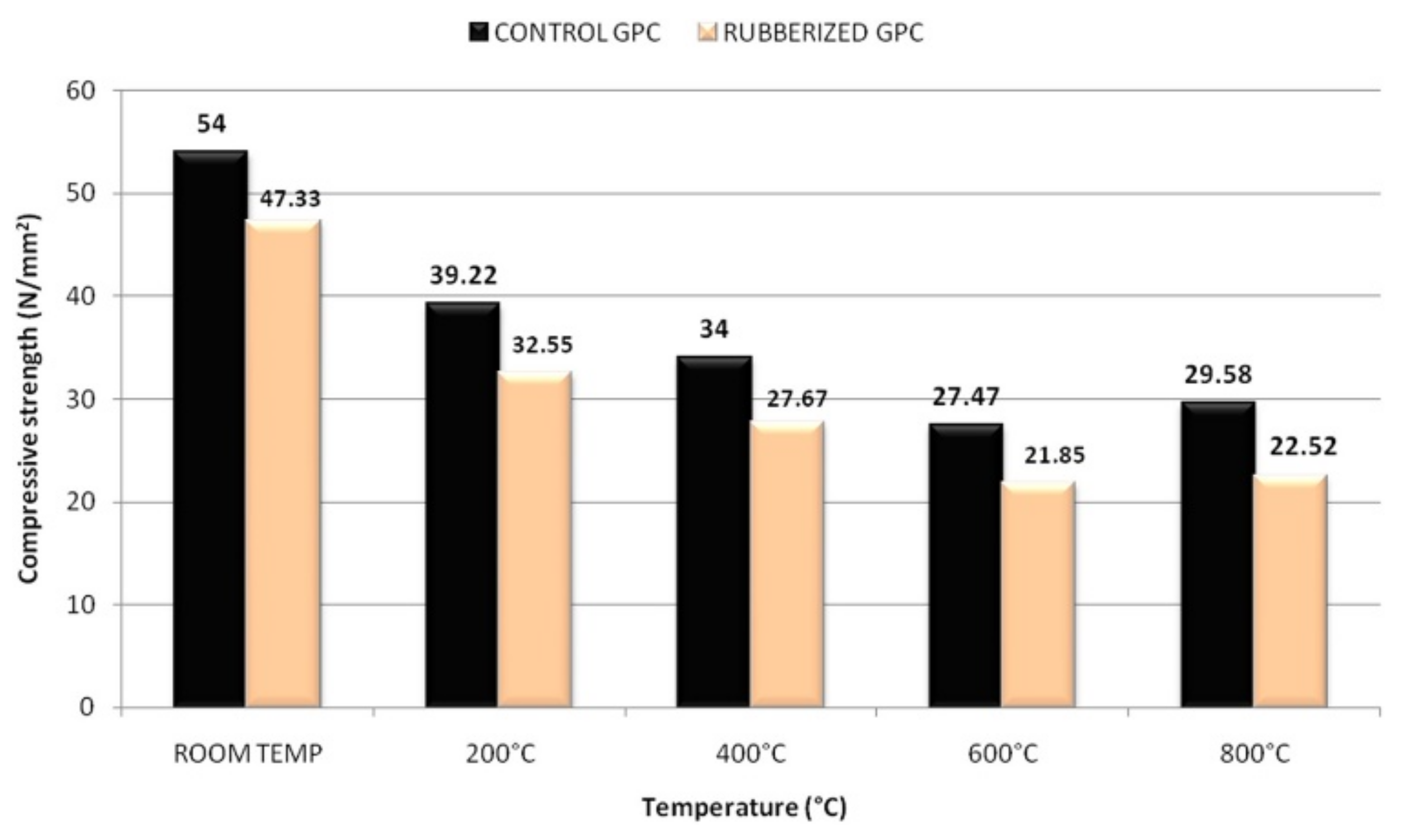
Figure 3. Compressive strength of control and rubberized geopolymer concrete specimens after exposure to elevated temperature (note: GPC, geopolymer concrete). Reproduced with permission from [49], [Journal of Building Engineering]; published by [Elsevier], [2018].
Kantarci et al. [50] studied the compressive strength of volcanic-tuff-based geopolymer concrete using nano-silica, micro-silica, and styrene-butadiene latex at elevated temperatures. Figure 4 indicates the compression resistance of the improved geopolymer concrete samples compared to the control ones at the temperatures of 23, 100, 300, 500, and 700 °C. Each geopolymer concrete sample was heated at an elevated temperature for 1 h. The compressive strength of all geopolymer concrete specimens increased as the temperature rose from 23 to 300 °C. The increase in the compressive strength may be due to promotion of polycondensation between tetrahedral aluminosilicate gels [50]. The process involved the condensation reactions that caused geomonomers to combine and form the geopolymers in the concrete, and this was accompanied by the expulsion of water during the process of heating. At a temperature of 300 °C, the geopolymer concrete sample with 2% micro-silica additive had the highest compressive strength of 28.45 MPa compared to all the other samples of geopolymer concrete under study. At the same temperature, the geopolymer concrete samples with 2% nano-silica additive, without additive, and with 5% styrene-butadiene latex were tested to have compressive strength of 26.30, 25.60, and 22.09 MPa, respectively. This proved that the type of additive in the geopolymer concrete sample played an important role in enhancing the polycondensation reactivity that resulted in its high compressive strength at the elevated temperature. When the geopolymer concrete samples were exposed to a temperature higher than 300 °C, their compressive strength declined due to the formation of micro-cracks in them as a result of their degradation caused by extreme heat.
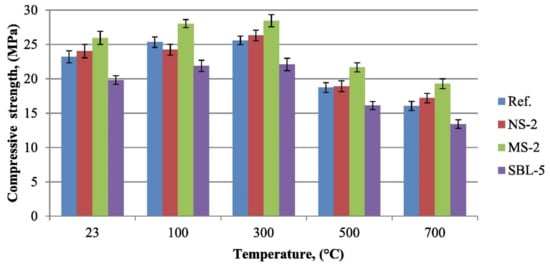
Figure 4. Compressive strength of geopolymer concrete samples after elevated temperatures (note: Ref, control geopolymer concrete; NS-2, geopolymer concrete with 2% nano-silica additive; MS-2, geopolymer concrete with 2% micro-silica additive; and SBL-5, geopolymer concrete with 5% styrene-butadiene latex). Reproduced with permission from [50], [Construction and Building Materials]; published by [Elsevier], [2021].
Based on three literature studies, the impact of geopolymerization methods on the compressive strength of geopolymer specimens at elevated temperatures can be further examined from Figure 5. The descriptions for the geopolymerization methods and chemical compositions of the geopolymer specimens are summarized in Table 1. After investigating the application of Class F fly ash as a precursor for geopolymerization, Rahmadina and Ekaputri [51] found that compressive strength of geopolymer specimen peaked at 71.00 MPa after exposure to 200 °C. It then decreased by 8.45, 43.66, 57.75, 55.63, and 90.14% when the temperatures were elevated at 400, 600, 800, 1000, and 1200 °C respectively. Using the same precursor for geopolymerization but with a higher molar concentration of sodium hydroxide and higher water content, Jiang et al. [52] discovered that compressive strength of geopolymer specimen reached its peak at 64.70 MPa after exposure to 300 °C. The strength was later reduced by 21.02 and 37.40% at the respective temperature of 500 and 800 °C. This indicates that a careful design of the geopolymer specimen admixture is required to maximize its compressive strength and optimize its elevated temperature. In the study of Payakaniti et al. [53], the method of alkaline activation by sodium hydroxide and sodium silicate solutions with high calcium (Class C) lignite fly ash as a precursor was applied for the making of geopolymer specimens. The highest compressive strength of the geopolymer specimen under the study is 50.24 MPa after exposure to 100 °C. The strength declined consistently by 11.01, 34.95, 63.28, 82.84, and 97.37% at 300, 500, 800, 1000, and 1200 °C respectively. This reflects that different types of precursors induced different levels of geopolymerization reactivity. There was a threshold temperature for the geopolymerization reaction to peak and failure was noticed in the geopolymer specimens after exposure beyond the threshold temperature due to thermal pressure. As a result of the thermal instability due to high temperatures, cracks were induced in the geopolymer specimens. This led to the decline in their compressive strength. A similar trend of compressive strength development of geopolymer specimens at elevated temperatures was also reported by Liu et al. [54]. In the study of Liu et al. [54], it was clarified that metakaolin-based geopolymer specimens were covered with ductile crack markedly after high-temperature exposure at 300 °C, and the crack further developed into fork crack after 500 and 700 °C exposure.
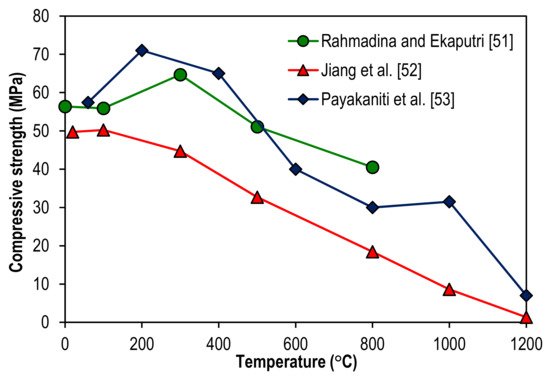
Table 1. Methods of geopolymerization and chemical compositions of geopolymer specimen admixture related to Figure 5.
| Published Work | Method of Geopolymerization | Chemical Composition of Geopolymer Specimen Admixture |
|---|---|---|
| Rahmadina and Ekaputri [51] | Alkaline activation by sodium hydroxide and sodium silicate solutions with Class F fly ash as a precursor. | 8 molar concentration of sodium hydroxide, 18.5% sodium oxide, 36.4% silica, and 45.1% water. |
| Jiang et al. [52] | Alkaline activation by sodium hydroxide and sodium silicate solutions with Class F fly ash as a precursor. | 10 molar concentration of sodium hydroxide, 8.3% sodium oxide, 28.7% silica, and 63% water. |
| Payakaniti et al. [53] | Alkaline activation by sodium hydroxide and sodium silicate solutions with high calcium (Class C) lignite fly ash as a precursor. | 10 molar concentration of sodium hydroxide, 12.53% sodium silicate by weight of sodium oxide, 30.24% silica, and 57.23% water. |
In summary, it can be deduced from section 2 that the compressive strength of geopolymer concrete at an elevated temperature could be optimized using additives such as fly ash, nano-silica and micro-silica. Inclusion of rubber tire fibers could slightly decrease the compressive strength of geopolymer concrete at an elevated temperature due to its role at causing concrete cracks upon initial loading. The negative residual strength impact of the rubber tire fibers on the geopolymer concrete was due to the fibers’ capability to capture air and form pores during compression test. As such, it is interesting to explore other additive such as ultra-fine calcium carbonate on the compressive strength development of geopolymer concrete for a range of high temperatures. Geopolymer composites, if designed with the correct compatibility between matrix and filler characteristics, can act as an inexpensive castable composite refractory [55].
3. Morphological and Chemical Properties of Durable Geopolymer Concrete
Dense structure is responsible for the increased strength characteristics of the concrete due to geopolymerization [56]. In this section, the morphological properties of durable geopolymer concrete samples were visualized from a detailed review of their scanning electron micrographs. Their chemical properties after acid exposure were evaluated by reviewing their X-ray diffraction (XRD) test results.
Zhang et al. [22] assessed the microstructures of fly-ash-based geopolymer concrete samples at elevated temperatures. Figure 6 shows the scanning electron micrographs of the geopolymer concrete samples exposed to 200, 800, and 1000 °C. At 200 °C, the microstructures of the geopolymer concrete sample are characterized by a dense and intact morphology with little pore spaces (Figure 6a,b). Similarly, dense and flat microstructures were observed in fly-ash-based geopolymer concrete researched by Zhao et al. [57] and Le et al. [58]. Zhao et al. [57] further discovered that that the dense microstructure of the geopolymer concrete remained intact after an exposure to 400 °C. After an exposure to 800 °C, however, some thermal micro-cracks could be seen in the microstructures of the geopolymer concrete samples (Figure 6c,d). A few unreacted fly ash particles could be observed in the micrographs as well. Additionally, loose microstructure was noticed on the surface of geopolymer concrete sample after it was subjected to a temperature of 800 °C based on the study of Zhao et al. [57]. The thermal micro-cracks were caused by extreme heat exposure, which reflected the serious deterioration of the geopolymer concrete specimen. At 1000 °C, no unreacted fly ash particle could be observed in the micrographs of the geopolymer concrete sample (Figure 6e,f). The big pores on the micrographs of the geopolymer concrete sample signified the destruction of its geopolymer matrix due to thermal stress. Figure 7a,b indicates the thermal macro-cracks on part of the geopolymer concrete specimen cross-section at elevated temperatures. Three types of thermal macro-crack were identified from the figure. They are radial, tangential, and inclusion cracks. According to Zhang et al. [22], the appearance of inclusion cracks indicated that the coarse aggregate of the geopolymer concrete specimen lost its ability to stand the thermal stress at 800 and 1000 °C. The thermal macro-cracks are more visible on the cross section of geopolymer concrete specimen exposed to 1000 °C compared to the one exposed to 800 °C, implying a more severe thermal degradation at the higher temperature. The porosity increased significantly after exposure to 400 °C and reached a maximum at 1000 °C, which could be directly attributed to increasing visible cracks [59].
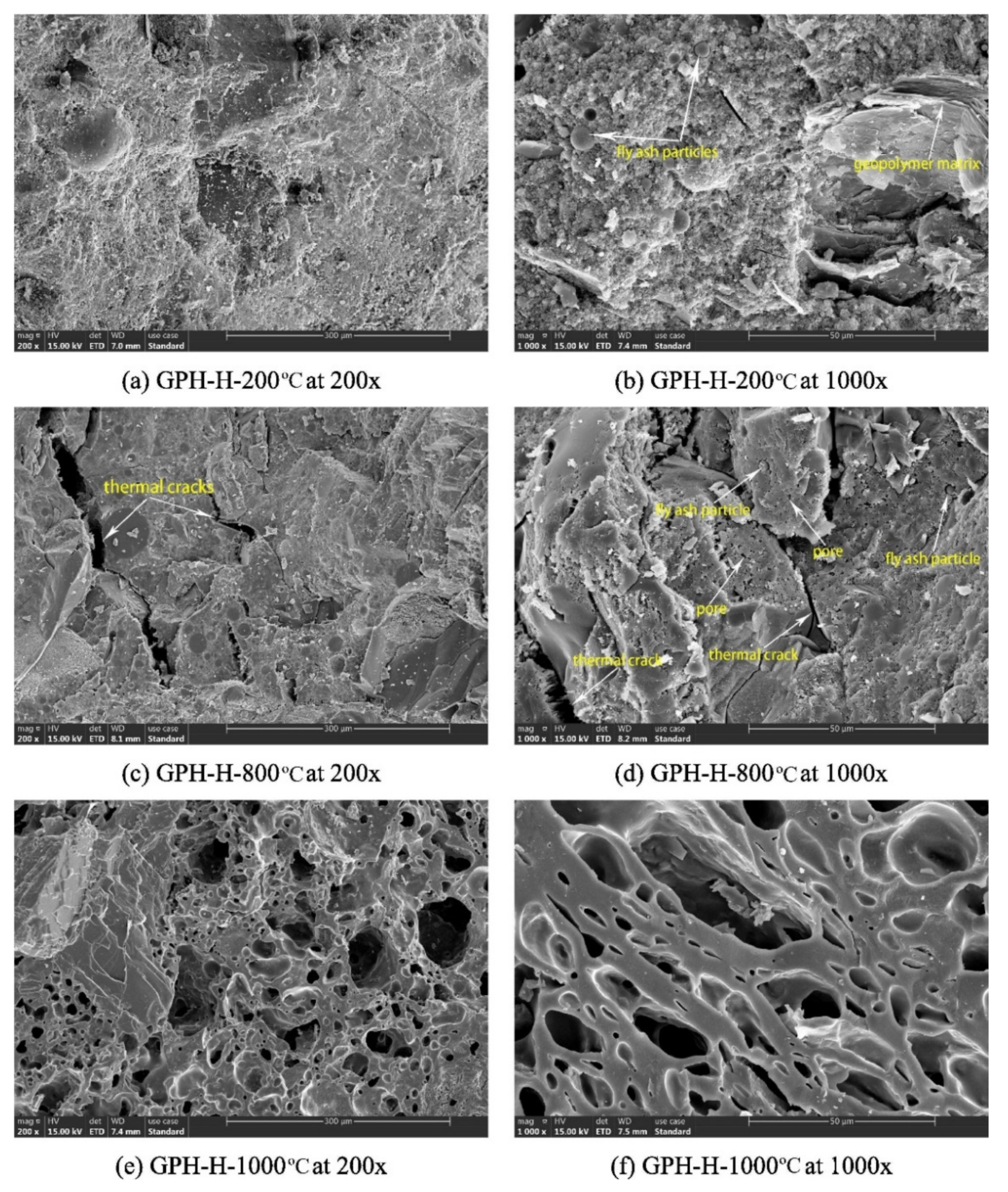
Figure 6. Scanning electron micrographs of GPH-H group geopolymer concrete samples at different elevated temperatures (Note: GPH-H, mix proportions: 1201 kg m−3 coarse crushed basalt aggregate, 539 kg m−3 natural siliceous sand, 460 kg m−3 fly ash, 150 kg m−3 sodium silicate, 50 kg m−3 sodium hydroxide, 3-sodium-silicate-to-sodium-hydroxide ratio, sodium hydroxide solution molarity of 14, 80 °C curing temperature, and 5.21% water content; 200×, 200× magnification; 1000×, 1000× magnification). Reproduced with permission from [22], [Construction and Building Materials]; published by [Elsevier], [2020].

Figure 7. Thermal macro-cracks on part of the geopolymer concrete specimen cross-section at (a) 800 °C and (b) 1000 °C (Note: GPH-H, Mix proportions: 1201 kg m−3 coarse crushed basalt aggregate, 539 kg m−3 natural siliceous sand, 460 kg m−3 fly ash, 150 kg m−3 sodium silicate, 50 kg m−3 sodium hydroxide, 3-sodium-silicate-to-sodium-hydroxide ratio, sodium hydroxide solution molarity of 14, 80 °C curing temperature, and 5.21% water content; 200×, 200× magnification; 1000×, 1000× magnification). Reproduced with permission from [22], [Construction and Building Materials]; published by [Elsevier], [2020].
Sontia Metekong et al. [60] performed X-ray diffraction analysis on volcanic ash and calcined-laterite-based geopolymer concrete samples. In the study, volcanic ash and calcined laterite were applied as the aluminosilicate sources of the geopolymer concrete. The results are shown in Figure 8. GL20 and GL40 are the two types of geopolymer concrete sample that were evaluated for their X-ray diffraction patterns. Their mixtures’ proportions are specified in the description of Figure 8. The mix designs of GL20 and GL40 were developed to have 20 and 40% calcined laterite by weight percentage of the aluminosilicate sources in the geopolymer concrete specimens respectively. In both GL20 and GL40 samples, the minerals of the volcanic ash inclusive of quartz, hematite, and anorthoclase could be traced from their high intensity peaks of X-ray diffraction. This could be justified by the fact that these mineral phases have been unaltered or partially dissolved in alkaline medium [60]. The unreacted minerals could function as filling agents that reinforced the geopolymer concrete, and this contributed to the concrete robustness. The geopolymer concrete samples were exposed to sulfuric acid at a pH of 1 for 19 weeks. The optical images of GL40 samples before and after the acid attack are shown in Figure 9. The microscopic image of Figure 9a shows that the particles of the geopolymer concrete were closely packed to each other to form intact and dense surface. Sontia Metekong et al. [60] further clarified that this densification could be due to the fact that the introduction of calcined laterite as additive in volcanic-ash-based geopolymer concrete extensively increased the reactive or amorphous phase, which allowed high polycondensation/polymerization between Si- and Al- oligomers, resulting in high Na-A-S-H geopolymer binder, contributing to good cohesion between aggregates and other particles. However, after the GL40 sample was exposed to the acid, pores were observed on the surface of the sample as indicated in the microscopic image of Figure 9b. The presence of these pores can be explained by the rupturing of polysialate (Si–O–Al) bonds by chemical attack of the matrix [60]. This caused leaching to take place, which gradually destroyed the geoploymer matrix and loosened the bonding among the geopolymer particles and aggregate, thereby reducing the strength of the geopolymer concrete.
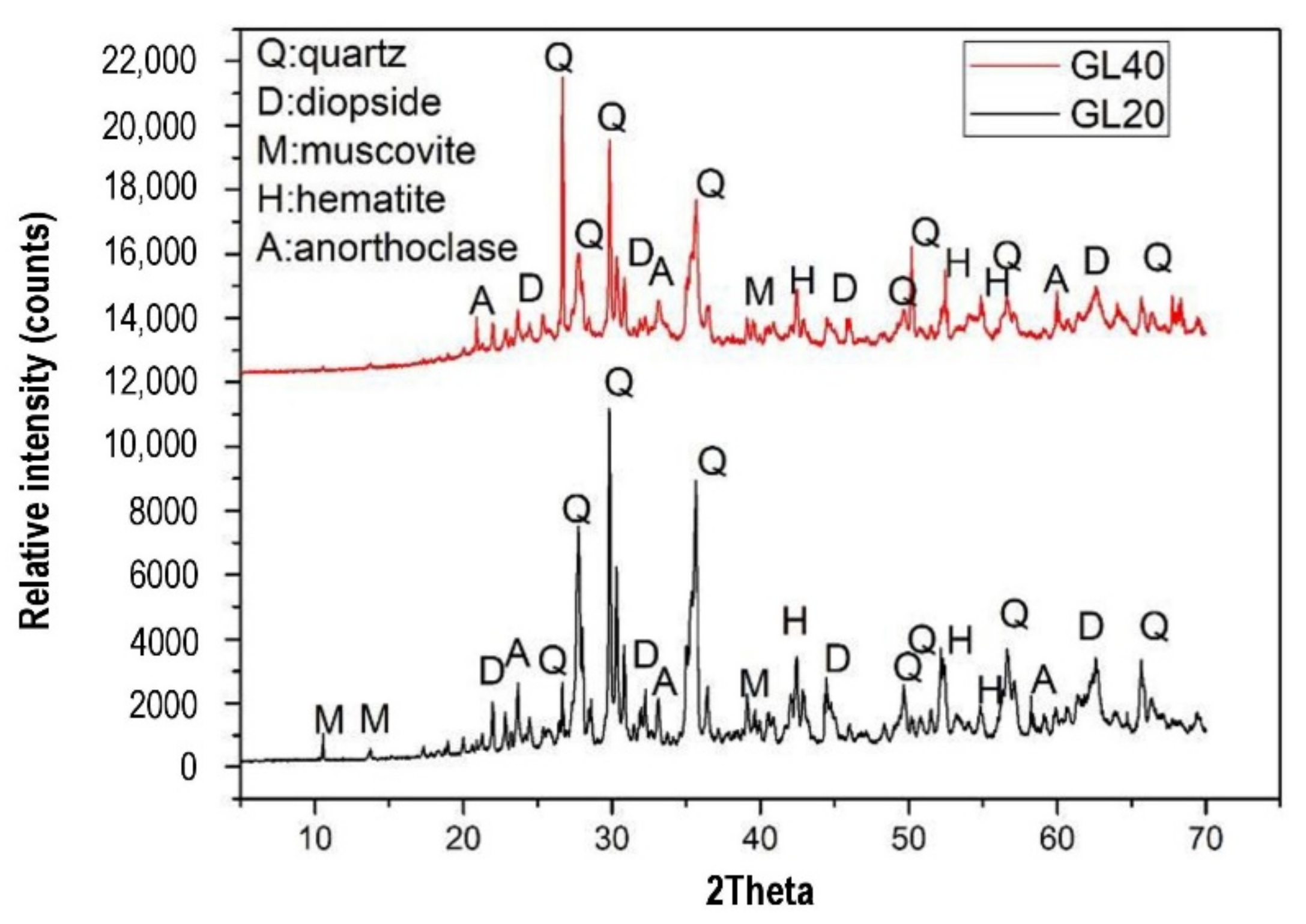
Figure 8. X-ray diffraction patterns of volcanic-ash-laterite-based geopolymer concrete samples cured at room temperature (28 °C). (Note: GL40, Mix proportions: 122.09 kg m−3 volcanic ash, 83.54 kg m−3 laterite, 357.15 kg m−3 river sand, 714.30 kg m−3 granite aggregate, and 0.80-sodium-silicate-to-sodium-hydroxide ratio; GL20, Mix proportions: 162.79 kg m−3 volcanic ash, 41.77 kg m−3 laterite, 357.15 kg m−3 river sand, 714.30 kg m−3 granite aggregate, and 0.80-sodium-silicate-to-sodium-hydroxide ratio). Reproduced with permission from [60], [Journal of Building Engineering]; published by [Elsevier], [2021].
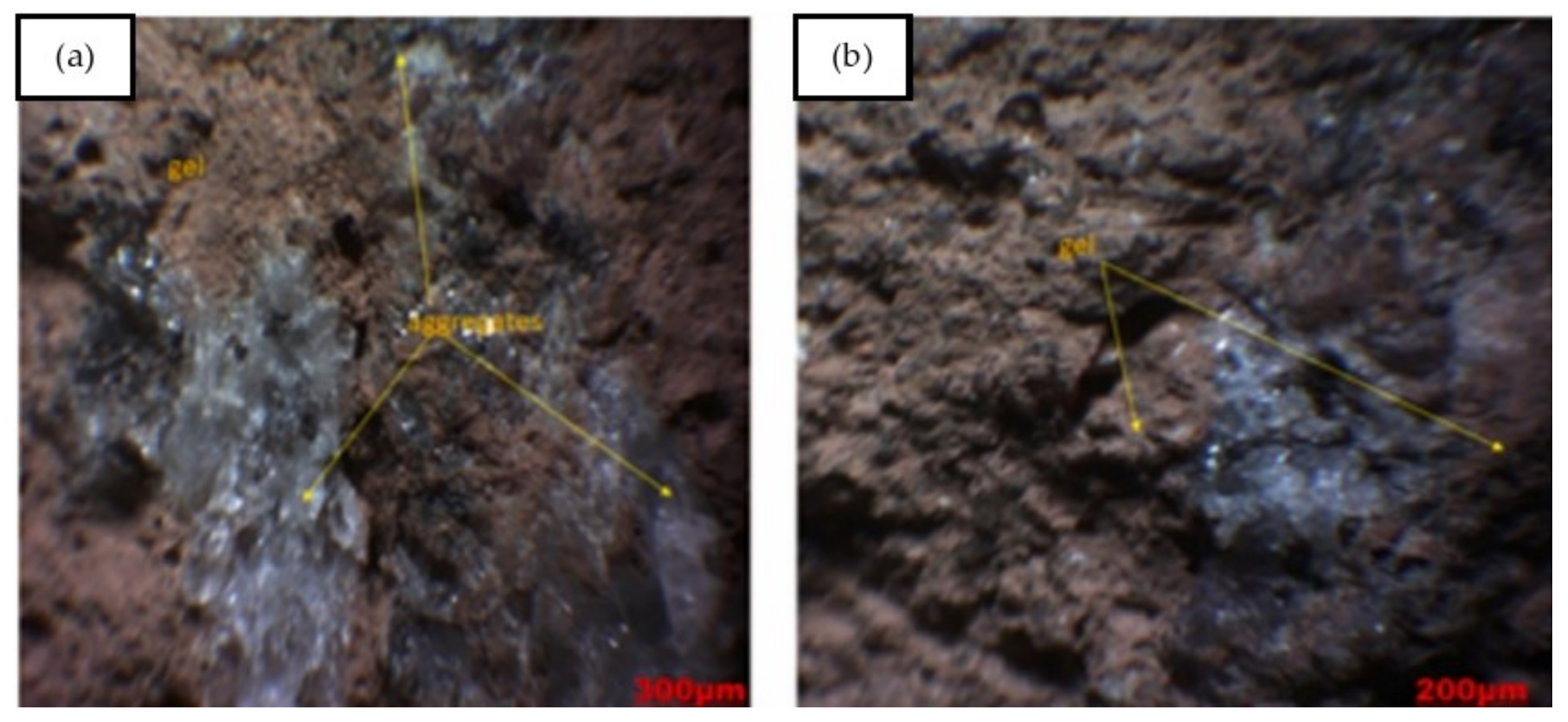
Figure 9. Optical observation of GL40 samples: (a) before and (b) after acid attack (note: GL40, mix proportions: 122.09 kg m−3 volcanic ash, 83.54 kg m−3 laterite, 357.15 kg m−3 river sand, 714.30 kg m−3 granite aggregate, and 0.80-sodium-silicate-to-sodium-hydroxide ratio). Reproduced with permission from [60], [Journal of Building Engineering]; published by [Elsevier], [2021].
The long-term effect of sulfuric acid attack on the morphology and X-ray diffraction pattern of fly-ash-based geopolymer concrete was reported by Mehta and Siddique [61]. Figure 10 reveals the scanning electron micrographs (SEM) of geopolymer concrete samples unexposed and exposed to sulfuric acid solution for 365 days. It can be observed from the micrograph of Figure 10a that the surface of the geopolymer concrete sample without the acid exposure was characterized by tightly packed particles with some voids and unreacted fly ash. The unreacted fly ash particles served as fillers of the geopolymer concrete that ensured its low porosity. Compared with the geopolymer concrete sample that was not exposed to acid, the geopolymer concrete sample exposed to acid had a lower particle packing efficiency (Figure 10b). However, in the geopolymer concrete sample exposed to acid, the main geopolymer binder remained intact. The geopolymer binder was the strength-contributing product, with strong alumina and silica bonds that were less affected by acid exposure [61].
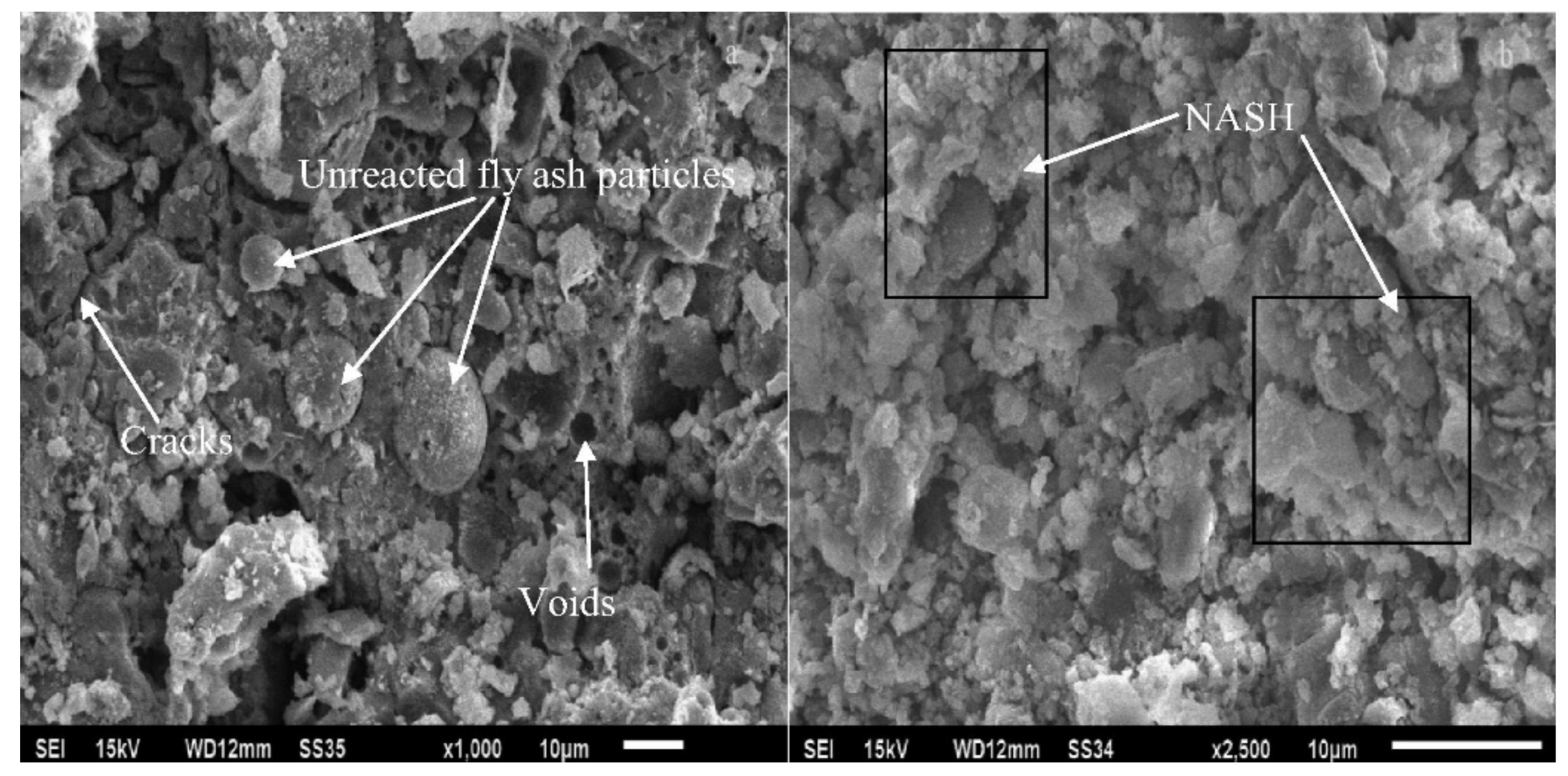
Figure 10. Scanning electron micrographs (SEM) of geopolymer concrete samples: (a) unexposed and (b) exposed to sulfuric acid solution for 365 days (note: mix proportions: 310 kg fly ash, 1204 kg coarse aggregate (crushed stone), 649 kg fine aggregate (river sand), 171 kg alkali solution (2.5-sodium-silicate-to-sodium-hydroxide ratio), and 6.2 kg naphthalene-based superplasticizer). Reproduced with permission from [61], [Construction and Building Materials]; published by [Elsevier], [2017].
The chemical proofs of the fly-ash-based geopolymer concrete samples before and after sulfuric acid exposure could be traced from their X-ray diffraction patterns as illustrated in Figure 11. Without the acid exposure, there were significant peaks of X-ray diffraction of quartz (Q), mullite (M), nepheline (N), and calcium aluminosilicate hydrate (C-A-S-H) (Figure 11a). The presence of quartz in the sample showed the evidence of the aggregate in the geopolymer concrete. The existence of mullite (M), nepheline (N), and calcium aluminosilicate hydrate (C-A-S-H) in the sample revealed the proof of geopolymer binder with strong aluminosilicate bonds, which were responsible for the cementation of the geopolymer concrete. However, after the geopolymer concrete sample was exposed to the acid for 365 days, these minerals could still be observed in the sample but with lower X-ray diffraction intensities (Figure 11b). Such evidence pointed out that some degradation happened in the geopolymer concrete due to its geopolymer depolymerization as a result of the acid attack. Despite that, it was shown by Thokchom [62] that geopolymer concrete is better than OPC concrete to resist the degrading action caused by acid.
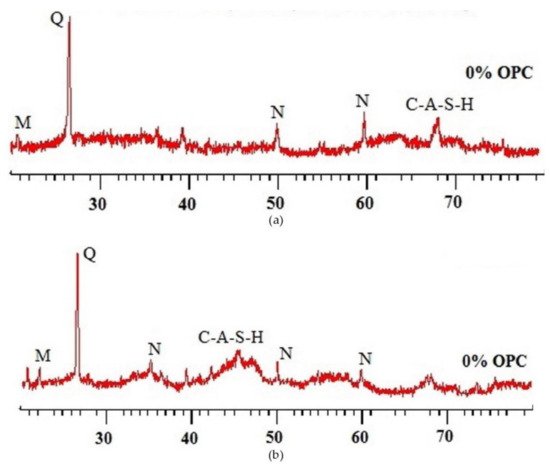
Figure 11. X-ray diffraction (XRD) patterns of geopolymer concrete samples: (a) unexposed and (b) exposed to sulfuric acid solution for 365 days (note: mix proportions: 310 kg fly ash, 1204 kg coarse aggregate (crushed stone), 649 kg fine aggregate (river sand), 171 kg alkali solution (2.5-sodium-silicate-to-sodium-hydroxide ratio), and 6.2 kg naphthalene-based superplasticizer). Reproduced with permission from [61], [Construction and Building Materials]; published by [Elsevier], [2017].
In section 3, it can be summarized that in terms of durability, geopolymer concrete demonstrated more robust morphology and chemical composition compared to OPC concrete. This is explainable by the fact the sodium aluminosilicate bonds in geopolymer concrete are strong and therefore, putting up high resistance against both thermal and acid deteriorations. On the other hand, the calcium hydroxide of the OPC concrete is reactive to acid, and this caused leaching to occur. The leaching process could weaken the cementation bonds of the OPC concrete, resulting in the decrease of its strength.
References
- Shobeiri, V.; Bennet, B.; Xie, T.; Visintin, P. A comprehensive assessment of the global warming potential of geopolymer concrete. J. Clean. Prod. 2021, 297, 126669.
- Fernández-Jiménez, A.; Cristelo, N.; Miranda, T.; Palomo, A. Sustainable alkali activated materials: Precursor and activator derived from industrial wastes. J. Clean. Prod. 2017, 162, 1200–1209.
- Wong, L.S. Microbial cementation of ureolytic bacteria from the genus Bacillus: A review of the bacterial application on cement-based materials for cleaner production. J. Clean. Prod. 2015, 93, 5–17.
- Ting, M.Z.Y.; Wong, K.S.; Rahman, M.E.; Meheron, S.J. Deterioration of marine concrete exposed to wetting-drying action. J. Clean. Prod. 2021, 278, 123383.
- Iqbal, D.M.; Wong, L.S.; Kong, S.Y. Bio-cementation in construction materials: A review. Materials 2021, 14, 2175.
- Aygörmez, Y.; Canpolat, O.; Al-mashhadani, M.M. Assessment of geopolymer composites durability at one year age. J. Build. Eng. 2020, 32, 101453.
- Van Deventer, J.S.J.; Provis, J.L.; Duxson, P. Technical and commercial progress in the adoption of geopolymer cement. Miner. Eng. 2012, 29, 89–104.
- Lodeiro, I.G.; Cristelo, N.; Palomo, A.; Fernández-Jiménez, A. Use of industrial by-products as alkaline cement activators. Constr. Build. Mater. 2020, 253, 119000.
- Assi, L.; Carter, K.; Dever, E.; Anay, R.; Ziehl, P. Sustainable concrete: Building a greener future. J. Clean. Prod. 2018, 198, 1641–1651.
- Cheng, Z.; Zhao, R.; Yuan, Y.; Li, F.; Castel, A.; Xu, T. Ageing coefficient for early age tensile creep of blended slag and low calcium fly ash geopolymer concrete. Constr. Build. Mater. 2020, 262, 119855.
- Zannerni, G.M.; Fattah, K.P.; Al-Tamimi, A.K. Ambient-cured geopolymer concrete with single alkali activator. Sustain. Mater. Technol. 2020, 23, e00131.
- Ismail, I.; Bernal, S.A.; Provis, J.L.; Nicolas, R.S.; Hamdan, S.; van Deventer, J.S.J. Modification of phase evolution in alkali-activated blast furnace slag by the incorporation of fly ash. Cem. Concr. Compos. 2014, 45, 125–135.
- Palomo, A.; Grutzeck, M.W.; Blanco, M.T. Alkali-activated fly ashes: A cement for the future. Cem. Concr. Res. 1999, 29, 1323–1329.
- Provis, J.L.; Palomo, A.; Shi, C. Advances in understanding alkali-activated materials. Cem. Concr. Res. 2015, 78, 110–125.
- Lodeiro, I.G.; Palomo, A.; Fernández-Jiménez, A. An overview of the chemistry of alkali-activated cement-based binders. In Handbook of Alkali-Activated Cements, Mortars and Concretes, 1st ed.; Pacheco-Torgal, F., Labrincha, J.A., Leonelli, C., Palomo, A., Chindaprasirt, P., Eds.; Elsevier Limited: Amsterdam, The Netherlands, 2015; pp. 19–47.
- Provis, J.L.; Yong, S.L.; Duxson, P. Nanostructure/microstructure of metakaolin geopolymers. In Geopolymers: Structures, Processing, Properties and Industrial Applications, 1st ed.; Provis, J.L., Van Deventer, J.S.J., Eds.; Woodhead Publishing Limited: Cambridge, UK, 2009; pp. 72–88.
- Xu, H.; Van Deventer, J.S.J. The geopolymerisation of alumino-silicate minerals. Int. J. Miner. Process. 2000, 59, 247–266.
- Rahman, S.K.; Al-Ameri, R. A newly developed self-compacting geopolymer concrete under ambient condition. Constr. Build. Mater. 2021, 267, 121822.
- Kastiukas, G.; Ruan, S.; Liang, S.; Zhou, X. Development of precast geopolymer concrete via oven and microwave radiation curing with an environmental assessment. J. Clean. Prod. 2020, 255, 120290.
- Poloju, K.K.; Srinivasu, K. Impact of GGBS and strength ratio on mechanical properties of geopolymer concrete under ambient curing and oven curing. Mater. Today 2021, 42, 962–968.
- Albibah, A.; Alghannam, M.; Abbas, H.; Almusallam, T.; Al-Salloum, Y. Characteristics of metakaolin-based geopolymer concrete for different mix design parameters. J. Mater. Res. Technol. 2021, 10, 84–98.
- Zhang, H.; Li, L.; Yuan, C.; Wang, Q.; Sarker, P.K.; Shi, X. Deterioration of ambient-cured and heat-cured fly ash geopolymer concrete by high temperature exposure and prediction of its residual compressive strength. Constr. Build. Mater. 2020, 262, 120924.
- Chen, K.; Wu, D.; Xia, L.; Cai, Q.; Zhang, Z. Geopolymer concrete durability subjected to aggressive environments—A review of influence factors and comparison with ordinary Portland cement. Constr. Build. Mater. 2021, 279, 122496.
- Wong, L.S.; Oweida, A.F.M.; Kong, S.Y.; Iqbal, D.M.; Regunathan, P. The surface coating mechanism of polluted concrete by Candida ethanolica induced calcium carbonate mineralization. Constr. Build. Mater. 2020, 257, 119482.
- Saranya, P.; Nagarajan, P.; Shashikala, A.P. Behaviour of GGBS-dolomite geopolymer concrete beam-column joints under monotonic loading. Structures 2020, 25, 47–55.
- Meng, Q.; Wu, C.; Hao, H.; Li, J.; Wu, P.; Yang, Y.; Wang, Z. Steel fibre reinforced alkali-activated geopolymer concrete slabs subjected to natural gas explosion in buried utility tunnel. Constr. Build. Mater. 2020, 246, 118447.
- Alrshoudi, F.; Abbas, H.; Abadel, R.; Albidah, A.; Altheeb, A.; Al-Salloum, Y. Compression behavior and modeling of FRP-confined high strength geopolymer concrete. Constr. Build. Mater. 2021, 283, 122759.
- Xu, H.; Wang, Z.; Shao, Z.; Cai, L.; Jin, H.; Zhang, Z.; Qiu, Z.; Rui, X.; Chen, T. Experimental study on durability of fiber reinforced concrete: Effect of cellulose fiber, polyvinyl alcohol fiber and polyolefin fiber. Constr. Build. Mater. 2021, 306, 124867.
- Chithambar Ganesh, A.; Deepak, N.; Deepak, V.; Ajay, S.; Pandian, A.; Karthik. Utilization of PET bottles and plastic granules in geopolymer concrete. Mater. Today 2021, 42, 444–449.
- Alabi, S.A.; Mahachi, J. Chloride ion penetration performance of recycled concrete with different geopolymers. Mater. Today 2021, 38, 762–766.
- Noori, A.S.; Oweed, K.M.; Raouf, R.M.; Abdulrehman, M.A. The relation between destructive and non-destructive tests of geopolymer concrete. Mater. Today 2021, 42, 2125–2133.
- Cárdenas-Pulido, J.; Reyes, J.C.; Carillo, J.; Ramírez, F. Shear behavior of geopolymer concrete panels under diagonal tensile stresses. Eng. Struct. 2020, 212, 110518.
- Rahman, S.S.; Khattak, M.J. Roller compacted geopolymer concrete using recycled concrete aggregate. Constr. Build. Mater. 2021, 283, 122624.
- Khan, I.; Xu, T.; Castel, A.; Gilbert, R.I.; Babaee, M. Roller compacted geopolymer concrete using recycled concrete aggregate. Constr. Build. Mater. 2019, 229, 116840.
- Mesgari, S.; Akbarnezhad, A.; Xiao, J.Z. Recycled geopolymer aggregates as coarse aggregates for Portland cement concrete and geopolymer concrete: Effects on mechanical properties. Constr. Build. Mater. 2020, 236, 117571.
- Mayhoub, O.A.; Nasr, E.A.R.; Ali, Y.; Kohail, M. Properties of slag based geopolymer reactive powder concrete. Ain Shams Eng. J. 2021, 12, 99–105.
- Shi, J.; Liu, B.; Liu, Y.; Wang, E.; He, Z.; Xu, H.; Ren, X. Preparation and characterization of lightweight aggregate foamed geopolymer concretes aerated using hydrogen peroxide. Constr. Build. Mater. 2020, 256, 119442.
- Sáez-Pérez, M.P.; Brümmer, M.; Durán-Suárez, J.A. Effect of the state of conservation of the hemp used in geopolymer and hydraulic lime concretes. Constr. Build. Mater. 2021, 285, 122853.
- Saloni; Singh, A.; Sandhu, V.; Jatin; Parvin. Effects of alccofine and curing conditions on properties of low calcium fly ash-based geopolymer concrete. Mater. Today 2020, 32, 620–625.
- Mathew, G.; Issac, B.M. Effect of molarity of sodium hydroxide on the aluminosilicate content in laterite aggregate of laterised geopolymer concrete. J. Build. Eng. 2020, 32, 101486.
- Jindal, B.B.; Jangra, P.; Garg, A. Effects of ultra fine slag as mineral admixture on the compressive strength, water absorption and permeability of rice husk ash based geopolymer concrete. Mater. Today 2020, 32, 871–877.
- Alabi, S.A.; Mahachi, J. Estimating the compressive strength of geopolymer recycled concrete. Mater. Today 2021, 43, 1973–1976.
- Erfanimanesh, A.; Sharbatdar, M.K. Mechanical and microstructural characteristics of geopolymer paste, mortar, and concrete containing local zeolite and slag activated by sodium carbonate. J. Build. Eng. 2020, 32, 101781.
- Bellum, R.R.; Muniraj, K.; Indukuri, C.S.R.; Madduru, S.R.C. Investigation on performance enhancement of fly ash—GGBFS based graphene geopolymer concrete. J. Build. Eng. 2020, 32, 101659.
- Pasupathy, K.; Ramakrishnan, S.; Sanjayan, J. Influence of recycled concrete aggregate on the foam stability of aerated geopolymer concrete. Constr. Build. Mater. 2021, 271, 121850.
- Talha Ghafoor, M.; Khan, Q.S.; Qazi, A.U.; Sheikh, M.N.; Hadi, M.N.S. Influence of alkaline activators on the mechanical properties of fly ash based geopolymer concrete cured at ambient temperature. Constr. Build. Mater. 2021, 273, 121752.
- Ouda, A.S. A preliminary investigation on gamma-ray attenuation of alkali-activated concrete waste based-geopolymer modified with pozzocrete-fly ash. Prog. Nucl. Energy. 2021, 134, 103681.
- Ishak, S.; Lee, H.S.; Singh, J.K.; Ariffin, M.A.M.; Lim, N.H.A.S.; Yang, H.M. Performance of fly ash geopolymer concrete incorporating bamboo ash at elevated temperature. Materials 2019, 12, 3404.
- Luhar, S.; Chaudhary, S.; Luhar, I. Thermal resistance of fly ash based rubberized geopolymer concrete. J. Build. Eng. 2018, 19, 420–428.
- Kantarci, F.; Türkmen, I.; Ekinci, E. Improving elevated temperature performance of geopolymer concrete utilizing nano-silica, micro-silica and styrene-butadiene latex. Constr. Build. Mater. 2021, 286, 122980.
- Rahmadina, A.; Ekaputri, J.J. Mechanical properties of geopolymer concrete exposed to combustion. In Green Infrastructure for Future World, Proceedings of the 6th International Conference of Euro Asia Civil Engineering Forum 2017, Hanyang University; Seoul, Korea, 22–25 August 2017, Park, J.-W., Ayli, H., Hardjasaputra, H., Thayaalan, P., Eds.; EDP Sciences: Seoul, Korea, 2017; Volume 138, pp. 1–10.
- Jiang, X.; Zhang, Y.; Xiao, R.; Polaczyk, P.; Zhang, M.; Hu, W.; Bai, Y.; Huang, B. A comparative study on geopolymers synthesized by different classes of fly ash after exposure to elevated temperatures. J. Clean. Prod. 2020, 270, 122500.
- Payakaniti, P.; Chuewangkam, N.; Yensano, R.; Pinitsoontorn, S.; Chindaprasirt, P. Changes in compressive strength, microstructure and magnetic properties of a high-calcium fly ash geopolymer subjected to high temperatures. Constr. Build. Mater. 2020, 265, 120650.
- Liu, X.; Jiang, J.; Zhang, H.; Li, M.; Wu, Y.; Guo, L.; Wang, W.; Duan, P.; Zhang, W.; Zhang, Z. Thermal stability and microstructure of metakaolin-based geopolymer blended with rice husk ash. Appl. Clay Sci. 2020, 196, 105769.
- Bernal, S.A.; Bejarano, J.; Garzón, C.; de Gutiérrez, M.; Delvasto, S.; Rodríguez, E.D. Performance of refractory aluminosilicate particle/fiber-reinforced geopolymer composites. Compos. B Eng. 2012, 43, 1919–1928.
- Kathirvel, P.; Sreekumaran, S. Sustainable development of ultra high performance concrete using geopolymer technology. J. Build. Eng. 2021, 39, 102267.
- Zhao, J.; Wang, K.; Wang, S.; Wang, Z.; Yang, Z.; Shumuye, E.D.; Gong, X. Effect of elevated temperature on mechanical properties of high-volume fly ash-based geopolymer concrete, mortar and paste cured at room temperature. Polymers 2021, 13, 1473.
- Le, H.B.; Bui, Q.B.; Tang, L. Geopolymer recycled aggregate concrete: From experiments to empirical models. Materials 2021, 14, 1180.
- Ye, J.; Zhang, W.; Shi, D. Effect of elevated temperature on the properties of geopolymer synthesized from calcined ore-dressing tailing of bauxite and ground-granulated blast furnace slag concrete. Constr. Build. Mater. 2014, 69, 41–48.
- Sontia Metekong, J.V.; Kaze, C.R.; Deutou, J.G.; Venyite, P.; Nana, A.; Kamseu, E.; Melo, U.C.; Tatietse, T.T. Evaluation of performances of volcanic-ash-laterite based blended geopolymer concretes: Mechanical properties and durability. J. Build. Eng. 2021, 34, 101935.
- Mehta, A.; Siddique, R. Sulfuric acid resistance of fly ash based geopolymer concrete. Constr. Build. Mater. 2017, 146, 136–143.
- Thokchom, S. Fly ash geopolymer pastes in sulphuric acid. Int. J. Eng. Innov. Res. 2014, 3, 943–947.
More
Information
Subjects:
Polymer Science; Engineering, Civil
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.3K
Revisions:
3 times
(View History)
Update Date:
07 Mar 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




