Legionella pneumophila is defined as a bacterium that can cause severe pneumonia. It is found in the natural environment and in water, and is often found in water tanks. It can be an integral part of biofilms in nature, and the protozoa in which it can live provide it with food and protect it from harmful influences; therefore, it has the ability to move into a sustainable but uncultured state (VBNC). L. pneumophila has been shown to cause infections in dental practices. The most common transmission route is aerosol generated in dental office water systems, which can negatively affect patients and healthcare professionals. The most common way of becoming infected with L. pneumophila in a dental office is through water from dental instruments, and the dental unit. In addition to these bacteria, patients and the dental team may be exposed to other harmful bacteria and viruses. Therefore, it is vital that the dental team regularly maintains and decontaminates the dental unit, and sterilizes all accessories that come with it. In addition, regular water control in dental offices is necessary.
1. Introduction
Members of the
Legionellaceae family are small, Gram-negative, aerobic bacilli that do not form spores, are capsule-free, and possess the enzymes catalase and oxidase (
Table 1)
[1]. Amino acids are their primary carbon and energy sources for bacterial growth in the intracellular environment
[2]. Amino acids are catabolized by the Krebs cycle, and gluconeogenetic enzymes synthesize sugars from the Embden–Meyerhof–Parnas pathway.
Legionella is non-saccharolytic and possesses the enzyme protease. The guanine and cytosine content in its DNA ranges from 38% to 52%
[3].
Of the known 15 serogroups of
Legionella pneumophila (
L. pneumophila), serogroup 1 is present in 84% of cases worldwide (
Table 2)
[4][5][6]. Serogroups 2 to 15 account for 16 to 20% of
Legionella pneumonia cases
[7]. Patients with
L. pneumophila of serogroups 2 to 15 showed typical symptoms of
Legionella pneumonia, although
Legionella urinary antigen detection tests were negative
[8][9][10]. In addition, specific differences in the virulence of different serogroups were observed; for instance, Buse et al. showed a significant difference in the mobility of
L. pneumophila between serogroups
[11]. A characteristic of the
L. pneumophila genome is the presence of many different eukaryotic-like proteins and protein domains that are probably acquired by horizontal gene transfer
[12][13][14].
Table 1. Classification of Legionella pneumophila.
| Characteristics |
Legionella pneumophila |
References |
| Family |
Legionellaceae |
[15] |
| Form |
bacillus |
[1] |
| Coloring per gram |
Gram (-) |
[1] |
| Metabolism |
Aerobic |
[1] |
| pH |
5–8.5 |
[16] |
| Habitat |
Aquatic habitats (biofilm, within multicellular organisms) |
[17] |
| Reproduction temperature |
25–37 °C |
[18] |
| Survival temperature |
0–63 °C |
[16] |
| Nutrients |
Amino acids (L-cysteine), iron |
[17] |
| Sensitivity |
Drying, chlorine, UV radiation |
[19] |
Table 2. Legionella species, serogroup details, and their ability to cause human infection and mortality rate
[7][17][20][21].
| Legionella sp. |
Serogroups Associated with Human Disease |
Diseases |
Mortality Rate |
| L. anisa |
|
Pleural infection |
|
| L. bozemanii |
1 and 2 |
Pneumonia |
|
| L. cardiaca |
|
Native endocarditis |
|
| L. cincinnatiensis |
|
Pneumonia |
|
| L. clemsonensis |
|
Pneumonia |
|
| L. dumoffii |
|
Legionnaires’ disease |
|
| Legionella feeleii |
1 and 2 |
Pontiac fever |
|
| L. hackeliae |
1 and 2 |
Pneumonia |
|
| L. jordanis |
|
Endocarditis |
|
| L. lansingensis |
|
Pneumonia |
|
| Legionella longbeachae |
1 and 2 |
Pneumonia |
|
| L. maceachernii |
|
Pneumonia |
|
| L. micdadei |
|
Opportunistic pneumonia |
|
| L. parisiensis |
|
Pneumonia |
|
| L. pneumophila |
1–15 |
Pontiac fever, Legionnaires’ disease, and pneumonia |
7–25% |
Legionella is found in the natural or artificial aquatic environment
[22]. It occurs in planktonic form or as part of biofilms
[23] and has the ability to move into a sustainable but uncultured state (VBNC)
[19]. Elevated temperature, inorganic and organic water content, and the presence of protozoa play a crucial role in their growth and spread
[24]. The most significant number of
Legionella is found in water samples with temperatures from 30 °C to 40 °C
[25]. Infections in humans occur exclusively by inhalation of contaminated aerosols, which can occur in air conditioning systems, cooling towers, spas, fountains, ice machines, plant sprayers, dental appliances, and showerheads
[26]. The material from which the pipeline system is built significantly influences the appearance of high concentrations of bacteria (
Figure 1)
[5]. The use of copper as a plumbing material has been shown to help reduce the risk of Legionnaires’ disease, while plastic materials support many
L. pneumophila bacteria
[27].
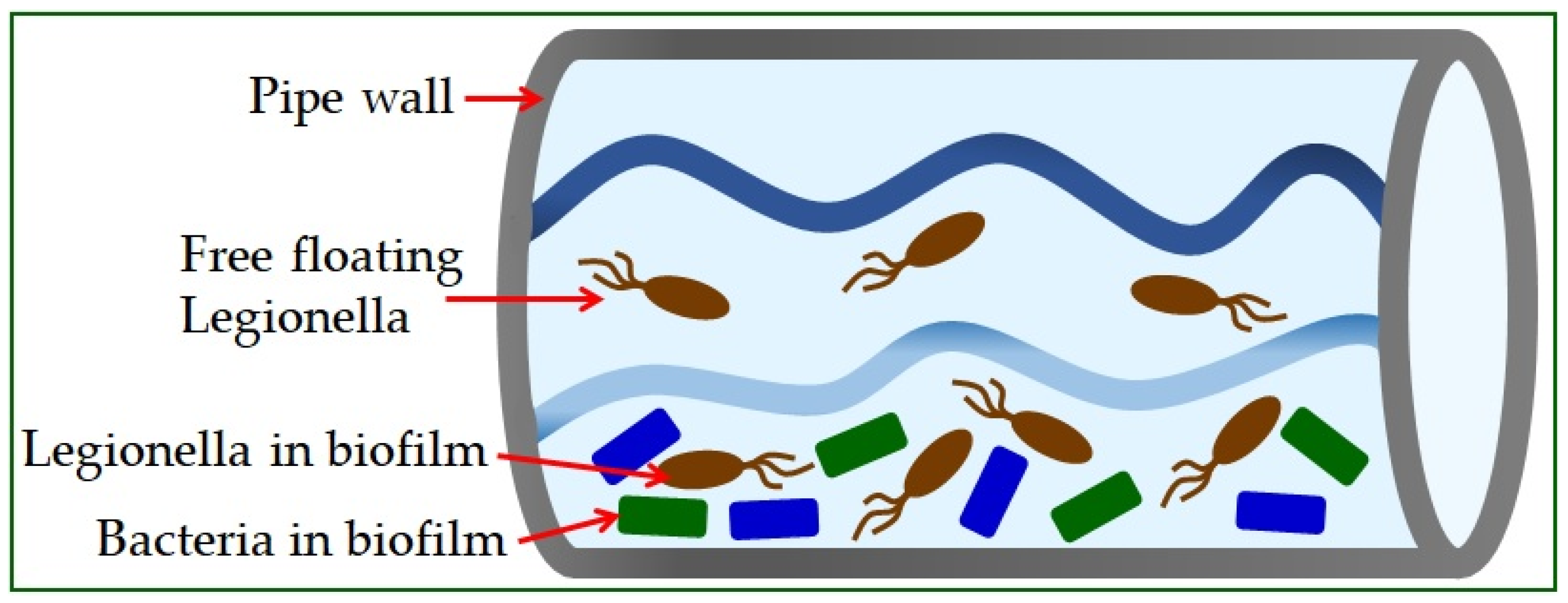
Figure 1. Legionella in water supply systems.
Legionella can be found in water distribution cooling towers, where it can replicate within protozoa. It is often
Vermamoeba vermiformis that protects
Legionella pneumophila from the effects of heat and disinfectants, which can result in nosocomial infections
[28]. Coevolution with multiple protozoan species has resulted in the development of mechanisms that allow
L. pneumophila to occupy different hosts, and the possibility of human cell infection
[2][29][30].
Legionella pneumophila is responsible for most cases of legionellosis and is one of the major causes of community-acquired and nosocomial-acquired atypical cases of pneumonia, with a mortality rate between 7% and 25%
[20][31]. In comparison, the mortality rate of non-
Legionella pneumophila species is 5%
[31]. Legionnaires’ disease is a pulmonary form of legionellosis with an incubation period of two to fourteen days, and involves severe pneumonia and systemic infection
[32][33]. A benign flu-like condition is called Pontiac fever
[34]. It is a non-pneumonic disease with unclear pathogenesis requiring no antimicrobial treatment
[32]. The mortality rate of adequately treated patients with Legionnaires’ disease varies from 7% to 24%, with immunocompromised and elderly patients being the most susceptible
[34]. It is estimated that 25,000 to 100,000 people are diagnosed with legionellosis each year in the United States
[35]. In North America and Western Europe, 1–13% of all types of pneumonia were associated with this pathogen
[36].
2. Legionella pneumophila in Dental Practice
Dental staff may be at high risk of
Legionella infection, and therefore, an occupational risk assessment is required. In addition to many dentists, other healthcare professionals in the dental clinic, such as dental assistants and hygienists, are also exposed to the occupational risk of
Legionella infection. It is estimated that the occupational risk of
Legionella infection may affect 1 to 2 million healthcare professionals worldwide
[37].
Based on research in dental offices conducted in 1986 in Austria, the presence of
Legionella pneumophila serogroup 1 was determined in 10% of water supply systems. The first death of a dentist due to Legionnaires’ disease was in 1995, and
Legionella was discovered in the plumbing system of his office
[37]. In 2012, an 83-year-old patient from Italy died of Legionnaires’ disease, and the source of the infection was contaminated water in the dental office she visited
[38]. In addition, in the same year, an elderly, immunocompromised man in Sweden died due to
Legionella in the cup filler outlet used for rinsing at the dental ward
[39].
Legionella pneumophila is fatal in many cases
[20]. However, the cause of the fatal outcome related to the dental practice was determined only in the patients shown. According to a study by Kevorkyan et al., antibodies to
Legionella were significantly higher in medical and dental professionals than in non-professionally exposed subjects
[40]. The possibility of contamination in a dental unit water system with microorganisms has been discussed since the beginning of dental chair use. Due to the constant exposure of patients and staff in the dental team to the aerosol produced during operations, the microbial quality of the water is critical. Water supply systems might contain opportunistic and pathogenic bacteria, mostly Gram-negative species that pose a particular risk in immunocompromised individuals
[41][42]. The water systems of the dental unit can be initially contaminated through water coming into the system or by pulling and sucking saliva and other fluids from the patient’s mouth. Dental chairs can receive water through a public water supply network or special tanks built into the chair to pour liquid. Other factors that might influence this are the work unit’s model and the time of its construction, whether there is a built-in system against the return flow of patient fluids, infection prevention measures, used disinfection methods, etc.
[42][43].
The guidelines for the allowable number of bacteria in the dental unit’s water are different and mostly coincide with the number of microorganisms allowed in the drinking water. In Europe, this number is up to 100 colony-forming units per milliliter of water (CFU/mL). The American Dental Association (ADA) set the allowable number of microorganisms in the water for dental supply at ≤200 CFU/mL, while the Centers for Disease Control and Prevention (CDC) recommended that the number be ≤500 CFU/mL
[42][43][44][45][46][47]. The risk of infection arises because most instruments that are necessary for work in dentistry, such as micromotors, turbines, sonic and ultrasonic scalers, water/air syringes, etc., produce an aerosol in the inhalation zone. The presence of
Legionella spp. in saliva and dental plaque biofilm has also been shown
[48][49].
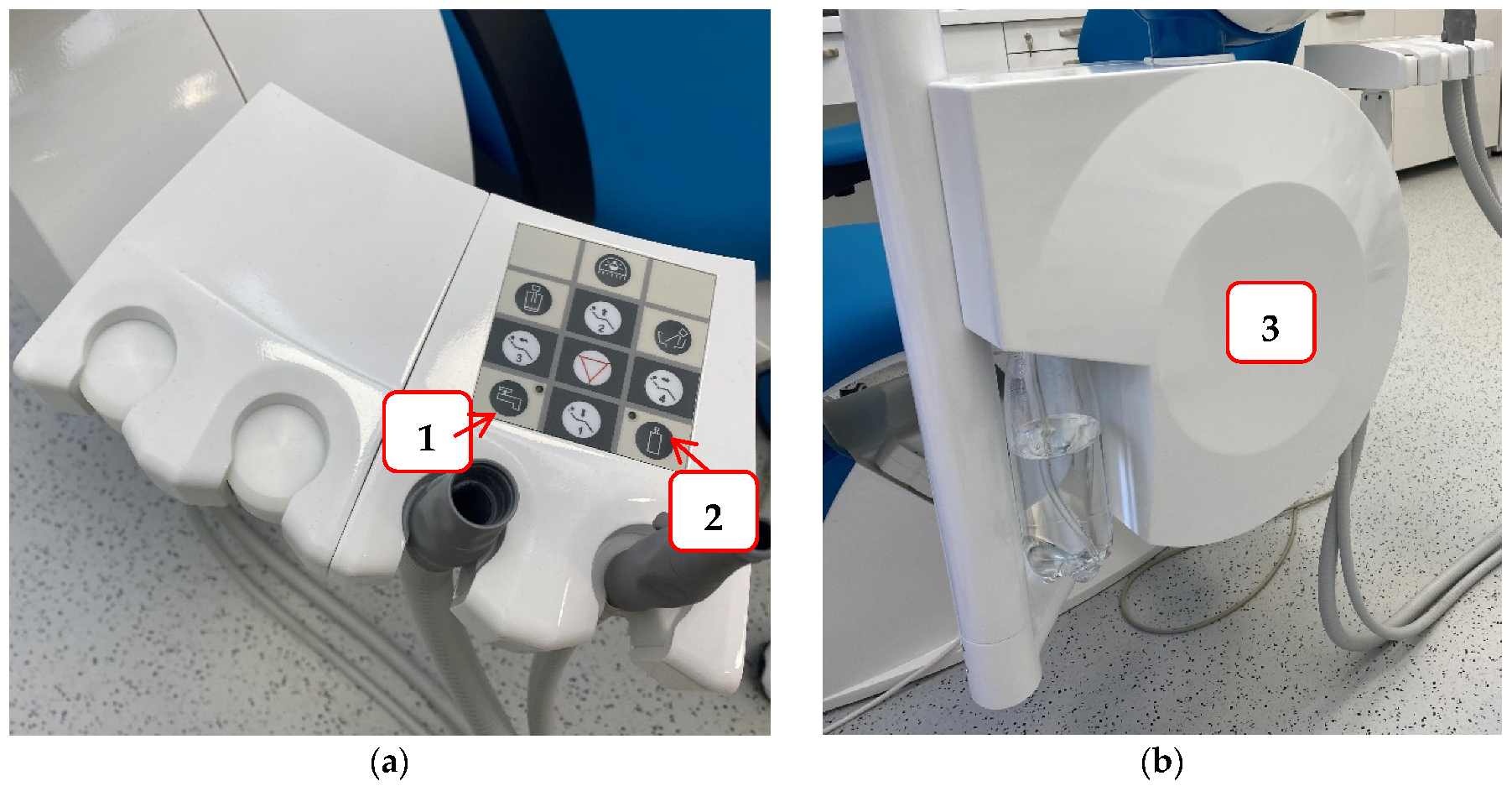
To reduce the possibility of infection and protect staff and patients in the dental office, it is necessary to try to prevent contamination of the hydraulic system of the dental unit. Some of the measures that can be achieved are: a dental unit that can be connected to sterile or distilled water (
Figure 2); flushing water through the instruments at the beginning and end of the working day, and also between each patient to prevent cross-contamination and water stagnation; continuous disinfection; procurement of thick filters, etc.
[50]. For this reason, dentists are required to conduct a legal risk assessment of their hydraulic systems, identify and assess the sources of risk, and prepare guidelines for the prevention and control of the risk of
Legionella infection. Furthermore, they must monitor the quality of their hydraulic systems once a year to ensure that the hydraulic systems are free of
Legionella [50].
Figure 2. An example of a dental unit with a choice of water supply: Panel (a) the buttons that allow the selection of water supply either from the public network (1) or a bottle of distilled water (2); Panel (b) water tank for distilled water.
Particular attention should be paid to prevention measures in these times of the COVID-19 pandemic. COVID patients are more susceptible to secondary infections for several months during recovery. Lock-down and government measures such as staying at home and delaying the procedures also result in prolonged water standing in the dental unit’s supply tanks. Hence, the biofilm accumulation in the system is more likely
[49][51][52].
2.1. Resistance of L. pneumophila Biofilms to Biocides
L. pneumophila poses a constant threat to human health in anthropogenic water sources
[21]. Due to the intracellular lifestyle within protozoa, it is difficult to assess whether the resistance of
L. pneumophila in environmental biofilms is due to the structure of the biofilm, its association with amoebae, or both
[53]. However, the fact is that
L. pneumophila, which is found in biofilms, is highly resistant to the action of biocides
[54].
Numerous disinfection methods were used to limit the growth of
L. pneumophila, but none succeeded in complete eradication; namely, recolonization occurred very soon after treatment
[53][55]. In addition, some studies showed that the biocide action on the
L. pneumophila biofilm could lead to the transition of the bacterium to the VBNC state
[54]. The most common biocides used in
L. pneumophila water disinfection protocols are chlorine and chlorine derivatives. However, they show efficacy only on planktonic cells, but not on biofilm
[56].
Two reasons for this are the resistance of
L. pneumophila to disinfectants; one is due to its ability to survive within the biofilm, and the other is that it possesses an intra-amoebic lifestyle
[29]. Namely, vesicles containing intracellular
L. pneumophila released by the amoeba are resistant to biocidal treatments. It is important to note that these vesicles remain viable for several months
[54]. Chlorine dioxide, unlike chlorine, can penetrate the biofilm and can also inactivate free-living amoebae which
L. pneumophila inhabits. Therefore, it is concluded that chlorine dioxide can be used as a secondary disinfectant to reduce the risk of Legionnaires’ disease in hospital systems
[19].
The use of phages in the treatment of biofilm infections is known in many bacterial pathogens
[57], so the addition of specific phages can be used to control the growth of
L. pneumophila. However, the phage can degrade polysaccharides and destabilize the biofilm
[58].
The antimicrobial activity of silver has long been known, and silver is an increasingly frequent target of research to find new antimicrobial agents
[59]. When it comes to a significant reduction in the volume of
L. pneumophila biofilm, silver nanoparticles have shown outstanding results
[60].
Natural compounds that have demonstrated antimicrobial efficacy on
Legionella strains are antimicrobial peptides, biosurfactants, and essential oils
[19]. Different filtration methods are possible, but as filters have a certain lifespan, this could significantly increase the cost of maintenance in the hospital system
[61].
2.2. Antimicrobial Therapy
Legionella possesses the enzyme ß-lactamase. Therefore, beta-lactam antibiotics are ineffective in treating legionellosis
[62]. For that reason, azithromycin and fluoroquinolones, including levofloxacin and moxifloxacin, are recommended for
Legionella pneumonia in some guidelines
[63][64]. In addition, treatment of legionellosis is long-term, from 7 to 14 days, while the symptoms themselves are not present for too long
[65][66].
5. Conclusions
In dental offices, along with many other potential causes of infections for both staff and patients, there is a possibility of exposure to the bacterium Legionella pneumophila, which can cause severe pneumonia—Legionnaires’ disease. Vulnerable patients are the immunocompromised and elderly, and chronic disease patients such as those with chronic obstructive pulmonary disease, cardiovascular disease, and diabetes. Legionella infection could be fatal for patients on hemodialysis and with kidney transplants. Smoking and alcoholism are risk factors for Legionnaires’ disease. The most common reservoir of Legionella in dental practices are water tanks, and the route of spread is through contaminated aerosol generated during the use of dental instruments. Understanding the molecular mechanisms responsible for intra-amoebic related resistance is necessary, and would result in the development of new strategies for eradicating L. pneumophila. It is essential to know the breeding characteristics of L. pneumophila, as well as its virulence factors, and spread methods. Knowing the hygienic measures and that disinfectants can be used to prevent the spread of L. pneumophila is imperative. It is necessary to carry out water control, appropriate sampling in dental offices, and microbiological processing of samples.