
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Eduardo Lebeña | + 1285 word(s) | 1285 | 2022-02-17 09:00:07 | | | |
| 2 | Beatrix Zheng | Meta information modification | 1285 | 2022-02-28 10:21:56 | | | | |
| 3 | Beatrix Zheng | Meta information modification | 1285 | 2022-02-28 10:22:34 | | | | |
| 4 | Beatrix Zheng | Meta information modification | 1285 | 2022-03-02 07:12:47 | | |
Video Upload Options
In recent years, much interest has been generated by the idea that nitrosative stress plays a role in the aetiology of human diseases, such as atherosclerosis, inflammation, cancer, and neurological diseases. The chemical changes mediated by reactive nitrogen species (RNS) are detrimental to cell function, because they can cause nitration, which can alter the structures of cellular proteins, DNA, and lipids, and hence, impair their normal function. One of the most potent biological nitrosative agents is peroxynitrite (ONOO−), which is produced when nitric oxide (•NO) and superoxide (•O2−) are combined at extremely rapid rates. Considering the plethora of oxidations by peroxynitrite, this makes peroxynitrite the most prevalent nitrating species responsible for protein, DNA, and lipids nitration in vivo. There is biochemical evidence to suggest that the interactions of the radicals NO and superoxide result in the formation of a redox system, which includes the reactions of nitrosation and nitration, and is a component of the complex cellular signalling network. However, the chemistry involved in the nitration process with peroxynitrite derivatives is poorly understood, particularly for biological molecules, such as DNA, proteins, and lipids.
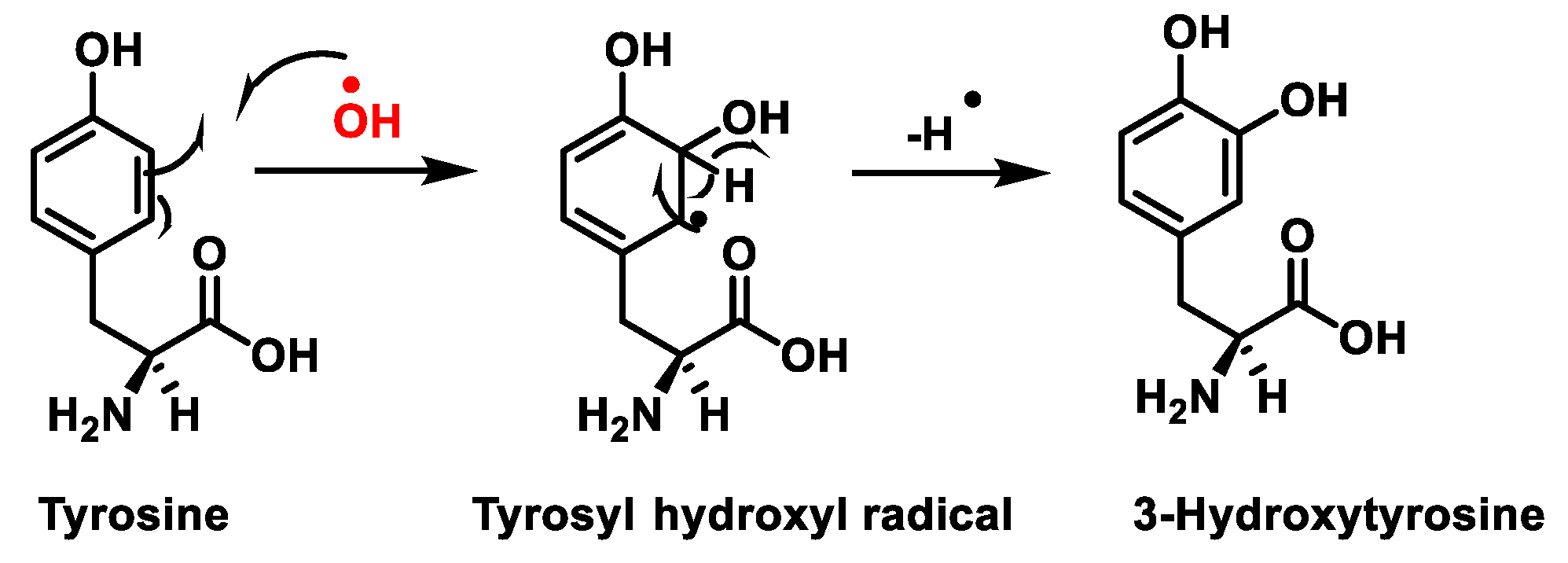
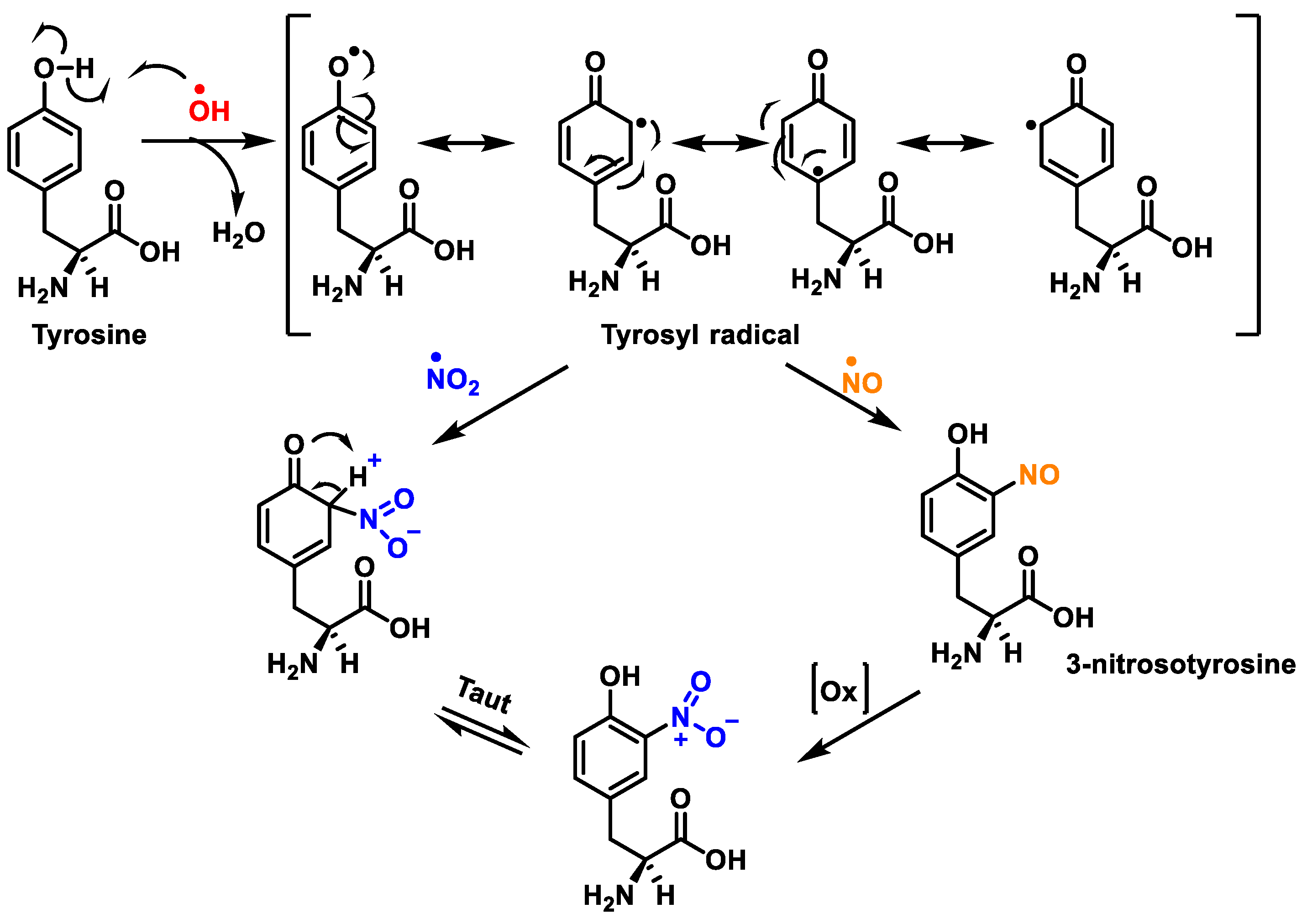
1. Protein Nitration
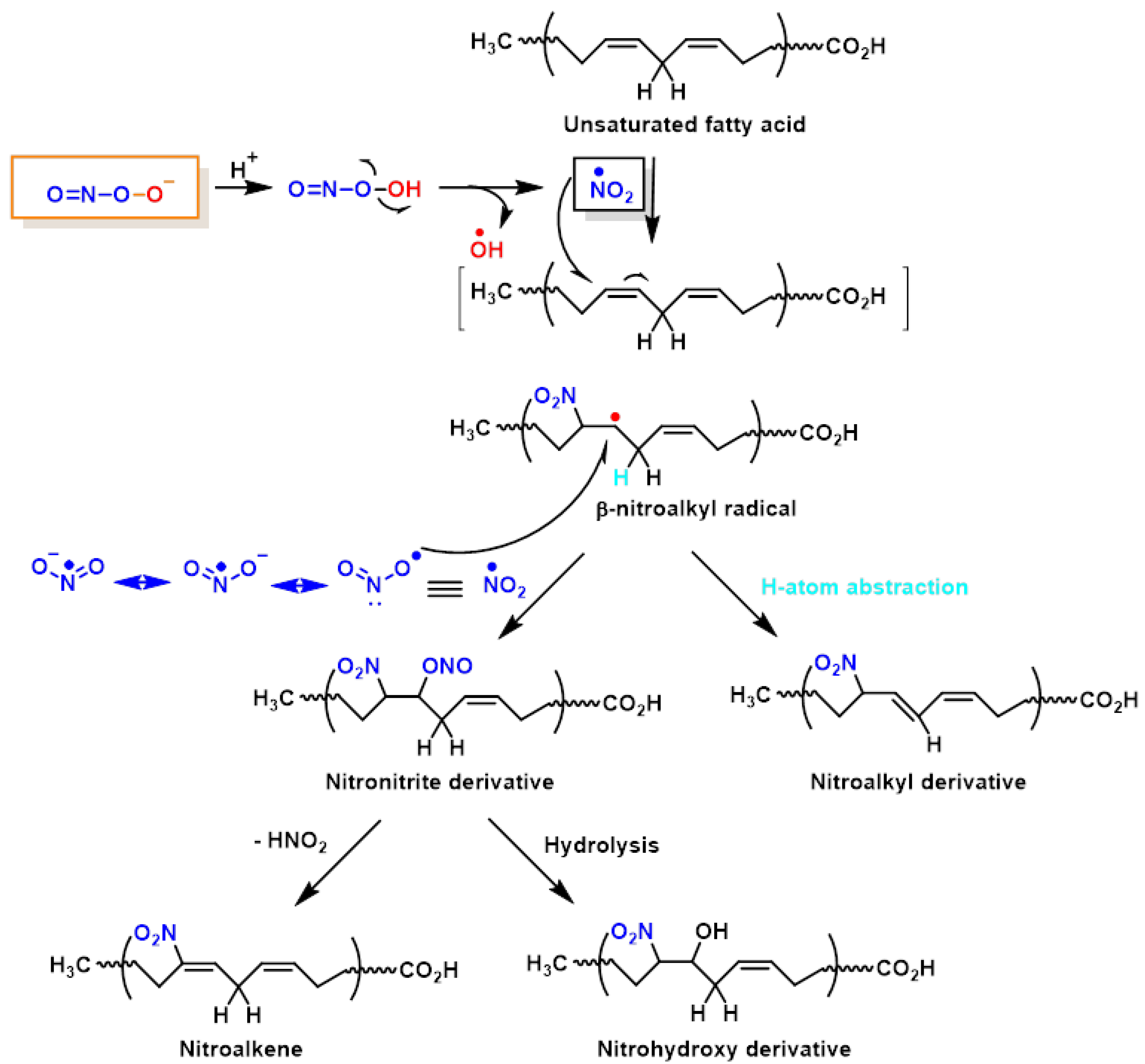
2. Lipid Nitration
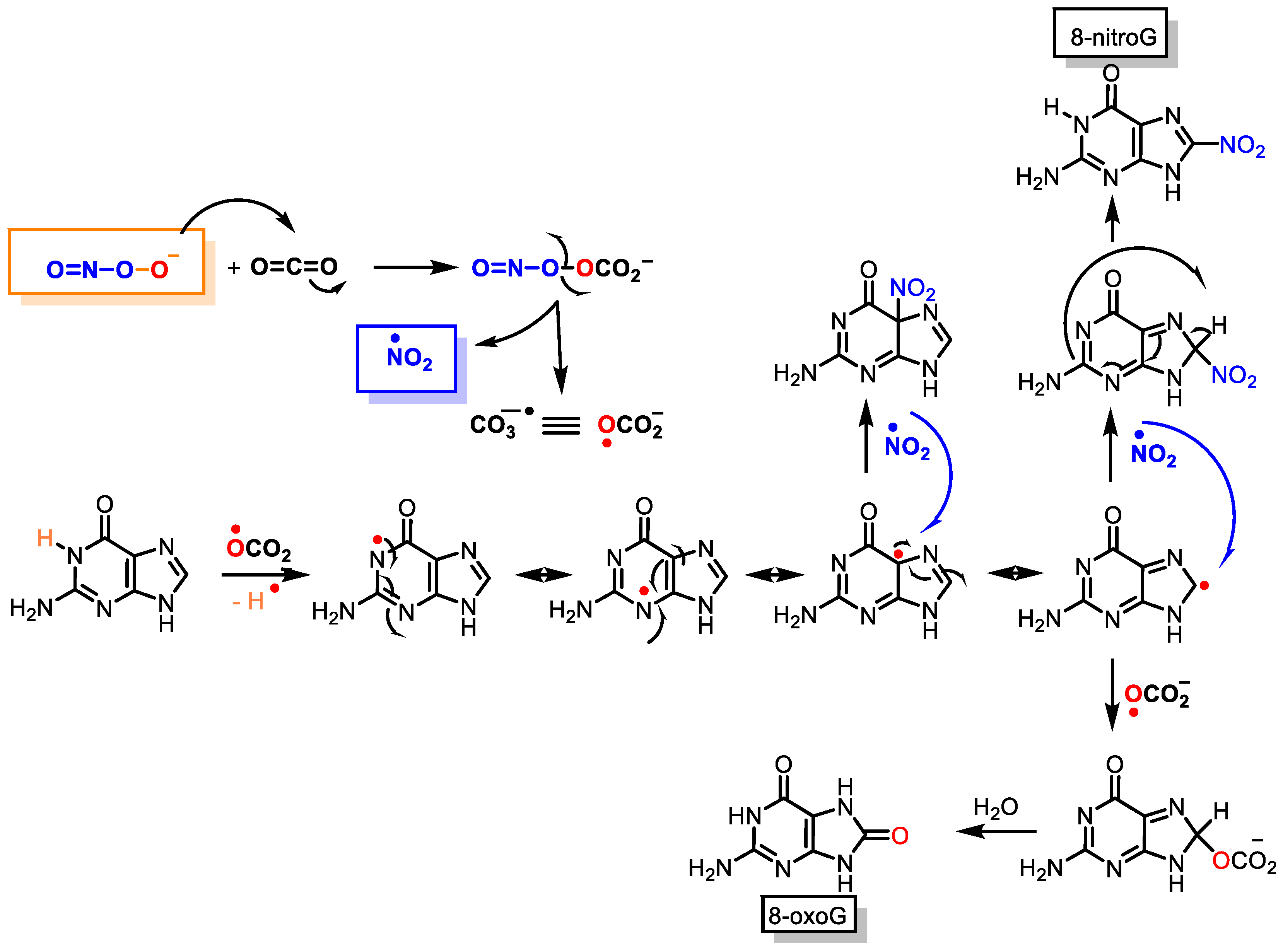
3. DNA Nitration
4. Role of •NO as a Potential Treatment against COVID-19
References
- Michalska, P.; León, R. When It Comes to an End: Oxidative Stress Crosstalk with Protein Aggregation and Neuroinflammation Induce Neurodegeneration. Antioxidants 2020, 9, 740.
- Radi, R. Protein Tyrosine Nitration: Biochemical Mechanisms and Structural Basis of Functional Effects. Acc. Chem. Res. 2013, 46, 550–559.
- Lee, J.R.; Kim, J.K.; Lee, S.J.; Kim, K.P. Role of protein tyrosine nitration in neurodegenerative diseases and atherosclerosis. Arch. Pharmacal Res. 2009, 32, 1109–1118.
- Surmeli, N.B.; Litterman, N.K.; Miller, A.-F.; Groves, J.T. Peroxynitrite Mediates Active Site Tyrosine Nitration in Manganese Superoxide Dismutase. Evidence of a Role for the Carbonate Radical Anion. J. Am. Chem. Soc. 2010, 132, 17174–17185.
- Rubbo, H.; Trostchansky, A.; O’Donnell, V.B. Peroxynitrite-mediated lipid oxidation and nitration: Mechanisms and consequences. Arch. Biochem. Biophys. 2009, 484, 167–172.
- Rubbo, H. Nitro-fatty acids: Novel anti-inflammatory lipid mediators. Braz. J. Med Biol. Res. 2013, 46, 728–734.
- Ríos, N.; Prolo, C.; Álvarez, M.N.; Piacenza, L.; Radi, R. Peroxynitrite Formation and Detection in Living Cells. In Nitric Oxide; Elsevier: Amsterdam, The Netherlands, 2017; pp. 271–288.
- Siddiqi, H.K.; Libby, P.; Ridker, P.M. COVID-19—A vascular disease. Trends Cardiovasc. Med. 2021, 31, 1–5.
- Adusumilli, N.C.; Zhang, D.; Friedman, J.M.; Friedman, A.J. Harnessing nitric oxide for preventing, limiting and treating the severe pulmonary consequences of COVID-19. Nitric Oxide 2020, 103, 4–8.
- Åkerström, S.; Gunalan, V.; Keng, C.T.; Tan, Y.-J.; Mirazimi, A. Dual effect of nitric oxide on SARS-CoV replication: Viral RNA production and palmitoylation of the S protein are affected. Virology 2009, 395, 1–9.
- Fang, W.; Jiang, J.; Su, L.; Shu, T.; Liu, H.; Lai, S.; Ghiladi, R.A.; Wang, J. The role of NO in COVID-19 and potential therapeutic strategies. Free Radic. Biol. Med. 2021, 163, 153–162.
- Griffiths, H.R.; Gao, D.; Pararasa, C. Redox regulation in metabolic programming and inflammation. Redox Biol. 2017, 12, 50–57.
- Tejero, J.; Shiva, S.; Gladwin, M.T. Sources of Vascular Nitric Oxide and Reactive Oxygen Species and Their Regulation. Physiol. Rev. 2019, 99, 311–379.
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric Oxide and Peroxynitrite in Health and Disease. Physiol. Rev. 2007, 87, 315–424.
- Schulz, E.; Gori, T.; Münzel, T. Oxidative stress and endothelial dysfunction in hypertension. Hypertens. Res. 2011, 34, 665–673.
- Hayden, M.R.; Tyagi, S.C. Myocardial redox stress and remodeling in metabolic syndrome, type 2 diabetes mellitus, and congestive heart failure. Med. Sci. Monit. 2003, 9, SR35–SR52.
- Rees, C.A.; Rostad, C.A.; Mantus, G.; Anderson, E.J.; Chahroudi, A.; Jaggi, P.; Wrammert, J.; Ochoa, J.B.; Ochoa, A.; Basu, R.K. Altered amino acid profile in patients with SARS-CoV-2 infection. Proc. Natl. Acad. Sci. USA 2021, 118, e2101708118.
- Kobayashi, J. Lifestyle-mediated nitric oxide boost to prevent SARS-CoV-2 infection: A perspective. Nitric Oxide 2021, 115, 55–61.





