MicroRNAs (miRNAs) are endogenous small non-coding RNAs that function in the regulation of gene expression and regulate a wide array of biological processes, including carcinogenesis. Several mechanisms are involved in miRNA-associated cancer development, such as amplification or deletion of miRNA genes, abnormal transcriptional control of miRNAs, dysregulated epigenetic changes, and defects in miRNA biogenesis machinery. MiRNA-223 has been found to be a critical miRNA that is involved in a wide range of molecular processes. It is involved in the regulation of inflammatory cytokines, epithelial homeostasis, immune checkpoint signaling pathways, apoptosis, cell cycle, cell proliferation, invasion, and chemosensitivity. Published literature has demonstrated that miRNA-223 expression is associated with cancer development and prevention. Mir-223 functions as either a tumor suppressor or oncogene under certain circumstances, containing multiple targets or specific targets. Hence, miR-223 could be a potential candidate diagnostic biomarker, prognostic biomarker, or therapeutic target of cancer.
1. miR-223 and Cancer
miR-223 is typically repressed in hepatocellular carcinoma and leukemia
[1][2], although higher expression levels of miR-223 are linked to colorectal
[3] and recurrent ovarian cancers
[4]. In some cases, miR-223 downregulation correlates with high tumor burden, disease aggressiveness, and poor patient prognosis. Consequently, understanding the complex role of miR-223 is essential for cancer diagnosis and treatment.
Several miR-223 targets are associated with malignancy: insulin-like growth factor-1 receptor (IGF-1R), monocytic enhancer factor 2C, microtubule destabilizer stathmin 1, and artemin and Forkhead box protein O1A (FOXO1A)
[5]. For example, stathmin 1 plays a key role in cell cycle progression, chromosome segregation, and cell survival. Stathmin 1 overexpression has been observed in malignant hematopoietic cells, and stathmin 1 inhibition reportedly reduced the proliferation of leukemia cell lines. In sum, perturbation of stathmin 1 expression highlights the role of miR-223 in malignant neoplastic diseases
[6]. According to Ma et al., sister chromatid cohesion protein PDS5 homolog B (PDS5B) is a direct target of miR-223 in prostate cancer. Furthermore, overexpression of PDS5B, which acts as a tumor suppressor gene, leads to the reversal of mR-223-mediated tumor progression in prostate cancer cells. Hence, the miR-223/PDS5B complex plays a critical role in the regulation of cell proliferation and invasion in prostate cancer cells
[7]. In general, various hallmarks of cancer are induced by miRNA-223, including cell proliferation, tumorigenesis, and metastasis. In contrast, anti-miR-223 attenuates the invasiveness and proliferation of some cancers. Anti-miR-223 also stimulates apoptosis.
Consequently, miR-223 plays a significant role in cancers, such as gastric, hepatocellular, ovarian, lung, and esophageal cancers
[8][9]. miR-223 downregulation is correlated with the development of chronic lymphocytic leukemia and primary small cell lung cancer (SCLC)
[9][10] while its upregulation is associated with ovarian and colorectal cancers. In particular, miR-223 is regarded as a possible biomarker in recurrent ovarian cancer. For future diagnostic strategies, miR-223 expression can be used to distinguish carcinomas from noncancerous lesions. microRNA-223 can also be considered a therapeutic target for cancer treatment
[10] (
Figure 1).
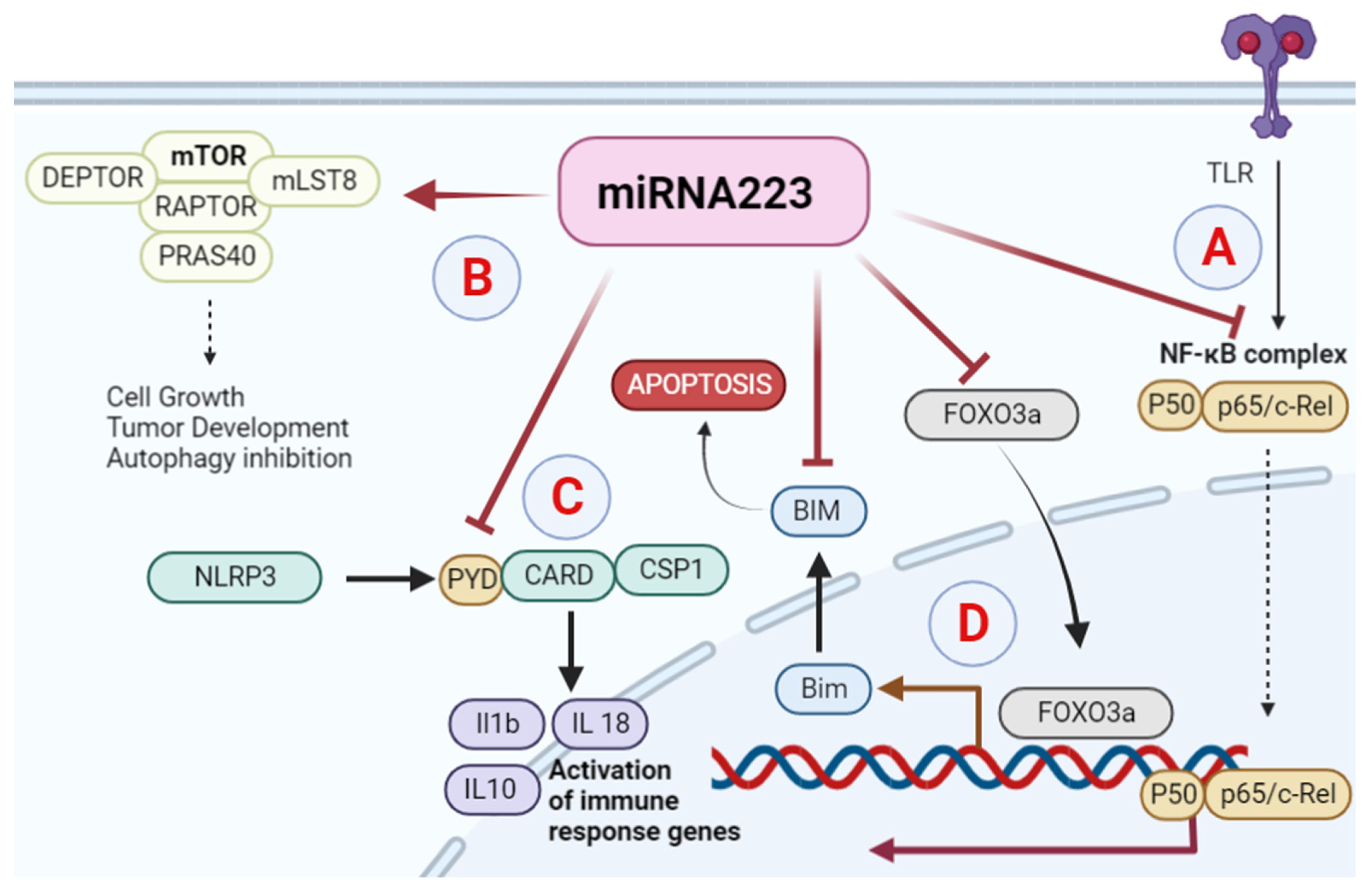
Figure 1. Role of miRNA223 in different cancers: (A) activated miR-223 negatively regulates the TLR4/MyD88-NF-κB signaling pathway. (B) miR-223 plays a role as a tumor suppressor in hepatocellular carcinoma by apoptosis through mTOR pathway activation. (C) miR-223 inhibits NLRP3 and its downstream factor CARD-PYD in breast cancer. (D) In colon cancer, miR-223 downregulates the transcription factor FoxO3a and BAM.
1.1. Gastric Cancer
Gastric cancer is a multifactorial disease with a complex interplay between genetics, lifestyle, and environment
[11][12][13][14].
Helicobacter pylori infection is the most common chronic bacterial infection and affects over 50% of the world’s population. It is a major cause of gastritis and gastric ulcers, and gastric cancer
[15][16][17]. Recent gene profiling studies identified a link between miRNAs and gastric-related disease. Compared to healthy individuals, miRNA expression was significantly different in patients with chronic gastritis and gastric cancer
[18]. Gastric cell lines with elevated miR-223 levels displayed a significant reduction in apoptosis. miR-223 overexpression also activated gastric cancer cell proliferation and invasion. Overall, miR-223 may play a crucial role in tumor progression and the survival of gastric cancer cells. As a result, miR-223 is proposed as an oncogene for gastric cancer
[10]. As such, miR-223 expression can assist in the diagnosis and treatment of gastric cancer
[10].
The mechanism responsible for increased gastric carcinogenesis was reported by Li et al., 2011. Their work demonstrated that miR-223 binds to FBXW7/hCdc4 at the 3′-UTR region of FBXW7/hCdc4 mRNA. The FBXW7/hCdc4 gene is a well-established p53-dependent tumor suppressor gene that encodes an ubiquitin ligase involved in chromosome stability. FBXW7 is a commonly deregulated ubiquitin-proteasome system protein in human cancers. As follows, miRNA-223 downregulation of FBXW7/hCdc4 is involved in gastric tumorigenesis due to increased genetic instability. In summary, miR-223 exerts its carcinogenic role by downregulating FBXW7/hCdc4 expression. This outcome rationalizes micrRNA-223 as a novel therapeutic target for gastric cancer
[18][10].
1.2. Hepatocellular Carcinoma
Hepatocellular carcinoma (HCC) is the third leading cause of cancer mortality worldwide, and the number of cases and deaths is predicted to increase
[19]. HCC patients showed significantly higher miR-223 levels compared to healthy individuals. As a result, miR-223 is proposed as a potential diagnostic biomarker for HCC. However, tissue injury, more than carcinogenesis, is implicated in miR-223 overexpression for hepatocellular disorders. Hepatitis B patients with HCC-negative results showed higher serum levels of miR-223 compared to HCC and healthy subjects. Chronic hepatitis patients displayed greater hepatocyte damage compared to HCC patients, and hepatitis (tissue injury) likely leads to elevated miRNA-223 serum levels rather than HCC
[20].
1.3. Breast Cancer
Breast cancer is a major cause of mortality among women in developed and developing countries (IARC, 2008)
[21][22]. Its incidence is continuing to increase, likely due to early screening and its public health emphasis, along with other factors
[23][24]. According to Gong et al., breast cancer cells (MDA-MB-231) express abnormally low levels of miR-223 and abnormally high levels of Caprin-1. Caprin-1 overexpression promotes proliferation and invasion of breast cancer cells. Conversely, miR-223 expression inhibits breast cancer cell proliferation and invasion in MDA-MB-231 cell lines
[25]. miR-223 targets 3′-UTR of Caprin-1 mRNA and downregulates Caprin-1 expression. A decrease in miR-223 biogenesis likely reduces Caprin-1 regulation. Caprin-1 deregulation then results in greater carcinogenesis in breast cancer cell lines, such as MDA-MB-231
[25]. In summary, miR-223 may suppress proliferation and invasion of breast cancer cells by directly targeting Caprin-1. As a result, miR-223 and Caprin-1 expression can be used predictively in cancer diagnosis and prognosis
[25].
1.4. Lung Cancer
Lung cancer is an aggressive disease, particularly when considering its high incidence and mortality rates
[26]. Consequently, novel therapeutic biomarkers are needed to improve disease outcomes
[27]. Disease progression displays a negative correlation between miR-223 expression and lung cancer development and metastasis
[28][29][30]. According to Nian et al., miR-223 functions as a potent tumor suppressor by targeting IGF-1 receptor and cyclin-dependent kinase 2. Low miR-223 serum levels are associated with high cancer-related death. As a result, miR-223 can be used as an early diagnostic and therapeutic biomarker to improve the treatment of lung cancer
[5].
1.5. Ovarian Cancer
Ovarian cancer is a heterogeneous disease that encompasses a number of different cellular subtypes. The most common subtype is high-grade serous ovarian cancer (HGSOC). HGSOC is also the most lethal of all gynecologic malignancies diagnosed in the United States
[31]. Recent advances have been unable to significantly improve patient outcomes. In the last 20 years, patient survival at 5 years post-diagnosis has marginally improved to 46% from about 35–38%
[32].
miR-223-3p expression is upregulated in ovarian cancer. Therefore, miR-223 decreases sex-determining region Y-box 11 (SOX11) expression. Inhibiting the
sox11 gene negatively regulates cell proliferation, migration, and invasion. Accordingly, a therapeutic target of miR-223-3p can potentially regulate ovarian cancer by targeting SOX11 expression
[32].
1.6. Osteosarcoma
Osteosarcoma is a bone tumor malignancy in adolescents and young adults
[33]. miR-223 is downregulated in osteosarcoma
[34][35]. Xu et al. reported that upregulation and downregulation of miRNA-223 lead to significantly diminished and enhanced cell proliferation and cell cycle progression in osteosarcoma, respectively. Thus, miR-223 is proposed as a therapeutic biomarker for osteosarcoma
[35].
1.7. Other Cancers
miRNA dysregulation is frequently observed in colon cancer
[36]. Previous studies found that the miR-223 oncogene is upregulated in colon cancer
[37][38]. In 2017, Liu et al. proposed that miR-223 promotes colon cancer invasion and metastasis by downregulating p120, which reduces intercellular adhesion, promotes RhoA activity, and activates β-catenin signaling
[37][39]. As a result, miR-223 is a potential diagnostic and therapeutic target for anti-colon cancer treatment. It was reported that miR-223 functions as an oncomiR in T cell acute lymphoblastic leukemia, whereas in acute myeloid leukemia (AML), it functions as a tumor suppressor
[40][41][42]. According to Gao et al., low expression of miR-223 has been found in various human hematologic malignancies and solid tumors, where it acts as a tumor suppressor, and has a significant role in leukemia and lymphomas. miR-223 significantly inhibits the proliferation, growth rate, and colony formation of cells in vitro, and tumor formation in vivo through targeting IGF-1R and its downstream PI3K/Akt/mTOR/p70S6K pathway in leukemia
[41][43][44]. Compared with normal tissues, miR-223 is also upregulated within the cancerous tissues of pancreatic, gastric, and prostate cancer biopsies
[39][45]. In a study by Wei et al., miR-223 promoted prostate tumor development and malignancy by decreasing cell apoptosis and increasing cell migration. These effects were reversed by upregulating SEPT6. As a gene target of miR-223, SEPT6 is downregulated by miR-223 overexpression. Consequently, SEPT6 can serve as a potential therapeutic target for prostate cancer
[46].
2. miR-223 Application as a Diagnostic and Therapeutic Biomarker for Cancers
Cancer treatment is challenging for many reasons, including clinical and tumor heterogeneity and the lack of disease-specific diagnostic biomarkers
[47][48][49][50]. Certain microRNAs have tumor suppressor or pro-oncogenic functions, making them attractive targets for cancer therapy
[51]. Significant advances have been made in miRNA-based therapy; however, many challenges must be overcome before clinical usage
[52]. Regardless, several studies reported miR-223 expression as a potential diagnostic and therapeutic target
[20][52][53][54].
2.1. Hepatocellular Carcinoma and Liver Damage
Hepatocellular carcinoma (HCC) displays a high recurrence rate. This common reoccurrence requires further investigation into diagnostic and therapeutic targets for HCC treatment
[55]. miR-223 overexpression was reported in patients with either HCC or chronic hepatitis B. The usefulness of miR-223 as a diagnostic biomarker for HCC is limited by its shared miR-223 profile. Chronic hepatitis B also displays elevated miR-223 levels; however, this overexpression is caused by inflammation and liver injury, not the presence of hepatocellular carcinoma
[20][56][57].
According to Karakatsanis et al., miR-223 downregulation inhibits HCC cell growth, suggesting that miR-223 plays an important role in HCC progression and metastasis
[57] Moreover, it was reported that miR-223-3p could be used as a promising prognostic biomarker as circulating miR-223-3p significantly differentiates HCC from the non-HCC groups
[58]. Consequently, further investigation of miR-223 is essential.
2.2. Breast Cancer
Escobar et al. highlighted the effects of macrophages and miRNA on breast cancer cells. miR-223 overexpression, coupled with high levels of macrophage infiltration, results in an aggressive cancer phenotype
[54]. In 2018, Yang et al. showed that miR-223 inhibits the proliferation and invasion of breast cancer by targeting STIM1
[59]. According to Chen et al., high expression of miR-223 acts as a tumor suppressor, which is correlated with prolonged survival in triple-negative breast cancer patients. Hence, miR-223 could be an independent prognostic biomarker in breast cancer
[60]. As reported, miR-223 can serve as a potential diagnostic biomarker for breast cancer
[54][59].
2.3. Esophageal Cancer
Esophageal cancer is a leading cause of cancer-related deaths worldwide. Most physicians recommend surgical resection for esophageal carcinomas at their early stages
[61]. Due to the limited treatment options, further research is needed to identify biomolecular pathways involved in esophageal cancer malignancy
[53][61][62].
Artemin (ARTN) is a growth factor associated with the tumorigenesis and progression of human cancers. ARTN also promotes cell migration, invasion, and metastasis
[62][63][64]. After chemotherapy, artemin expression is strongly correlated with relapse and death in carcinoma patients
[65][66].
According to Wu et al., miR-223 directly binds the ARTN 3′-UTR and regulates ARTN protein expression
[65]. ARTN function is suppressed by miR-223 overexpression, which reduces esophageal cancer cell migration and invasion. In contrast, ARTN upregulation via miR-223 silencing promotes cell migration and invasion
[65]. These observations rationalize microRNA-223 and artemin as therapeutic targets for esophageal cancer treatment.
2.4. Lung Cancer
In lung cancer, like all cancers, early diagnosis promotes favorable patient outcomes
[66]. As a result, the identification of novel early diagnostic biomarkers related to tumorigenesis is vital for future advancements in cancer diagnosis and treatment
[27].
miR-223 targets IGF-1R, a transmembrane receptor tyrosine kinase related to lung cancer oncogenesis, tumor growth, and cancer cell survival
[66]. IGF-1R upregulation is involved in the initial dysregulation that shifts previously healthy cells towards tumorigenesis and malignancy. In contrast, IGF-1R downregulation sensitizes lung cancer cells to chemotherapy and radiation by inhibiting cell proliferation. As reported, miR-223 overexpression leads to tumor suppression in lung cancer
[66]. According to Antona et al., miR-223 could be an early-stage serum biomarker for non-small cell lung cancer (NSCLC). Moreover, miR-223 is considered as an effective and reproducible serum biomarker
[67]. Overall, miR-223 targets IGF-1R and its downstream signaling pathway for downregulation, leading to inhibited cell growth and proliferation
[51]. Targeting miR-223 for overexpression could be a good strategy for lung cancer treatment.
3. Concluding Remarks
Herein highlights the importance of miR-223 in cancer biology. miR-223 expression potentially plays a major role in cancer diagnosis, progression, and treatment. Data characterizes miR-223 as emerging triple-negative breast, gastric, lung, and ovarian cancer biomarkers. Several genes and gene products were identified as targets of miR-223, including NLRP3, IL18, IL1β, DNA methyltransferase 1, and checkpoint kinase-1, signifying its potential use as a cancer-specific diagnostic biomarker and therapy. However, further investigation is needed to better understand the mechanisms of miR-223 involved in tumor activation or suppression. Nonetheless, miR-223 regulation is a promising tool for controlling key cancer motifs: increased cell growth, proliferation, and invasiveness in addition to decreased apoptosis rates.
Abbreviations
| ARTN |
Artemin |
| Caprin-1 |
cytoplasmic activation/proliferation-associated protein-1 |
| FOXO |
Forkhead box protein O |
| HCC |
Hepatocellular carcinoma |
| HGSOC |
high-grade serous ovarian cancer |
| IGF-1R |
Insulin-like growth factor 1 receptor |
| IARC |
International agency for research on cancer |
| miRNAs |
microRNAs |
| mRNA |
messenger RNA |
| miR-223 |
microRNA-223 |
| SOX |
sex determining region Y-box 11 |
| UTR |
Untranslated region |






