A cancer-associated fibroblasts (CAFs) are the most important players that modulate tumor aggressiveness. In this entry, researchers aimed to identify CAF-related genes in ovarian serous carcinomas (OSC) that account for the high incidence and mortality of ovarian cancers (OCs) and to develop therapeutic targets for tumor microenvironment modulation. Here, researchers performed a microarray analysis of CAFs isolated from three metastatic and three nonmetastatic OSC tissues and compared their gene expression profiles. Among the genes increased in metastatic CAFs (mCAFs), GLIS1 (Glis Family Zinc Finger 1) showed a significant increase in both the gene mRNA and protein expression levels. Knockdown of GLIS1 in mCAFs significantly inhibited migration, invasion, and wound healing ability of OC cells. In addition, an in vivo study demonstrated that knockdown of GLIS1 in CAFs reduced peritoneal metastasis. Taken together, these results suggest that CAFs support migration and metastasis of OC cells by GLIS1 overexpression. It also indicates GLIS1 in CAFs might be a potential therapeutic target to inhibit OC metastasis.
1. Introduction
The tumor microenvironment (TME) is a milieu in each tumor composed of different cellular and structural factors, including blood vessels, immune cells, stromal cells, and the extracellular matrix, and is involved in either tumor promotion or regression. Cancer-associated fibroblasts (CAFs), among the stromal components, are the most important players involved in modulating the TME and influencing aggressive tumor progression
[1][2][3]. CAFs are reportedly associated with tumor metastases and invasion
[1][2], and evidence has demonstrated that CAFs represent the major players in tumor–stroma crosstalk in the TME and enhance tumor progression by promoting angiogenesis or lymphangiogenesis. The role of CAFs in tumor progression, the regulation of the response to therapies, and the prognostic relevance of markers associated with CAFs in different tumors have been recently studied
[4][5]. In addition, CAFs have phenotypic and functional heterogeneity
[4][5][6], indicating the existence of different subpopulations with distinct functions in the TME.
Based on these findings, many investigators have sought to develop therapeutics targeting CAFs in the TME. Several reports have shown that the downregulation of CAF-mediated genes decreases the metastasis and growth of various human tumors, including lung cancer, breast cancer, and ovarian cancer (OC)
[7][8][9][10].
OC is the most lethal gynecologic malignancy, and its mortality rate has not improved due to frequent recurrence after primary therapy and the lack of treatment after disease relapse
[3]. Thus, it is important to understand the role of the surrounding tumor microenvironment (TME), especially in CAFs, which regulate tumor progression, to develop better therapeutic strategies for overcoming the tumorigenic effect of the TME in OC.
2. Gene Expression Profile of Primary mCAFs and nmCAFs Isolated from the Cancer Tissues of OSC Patients
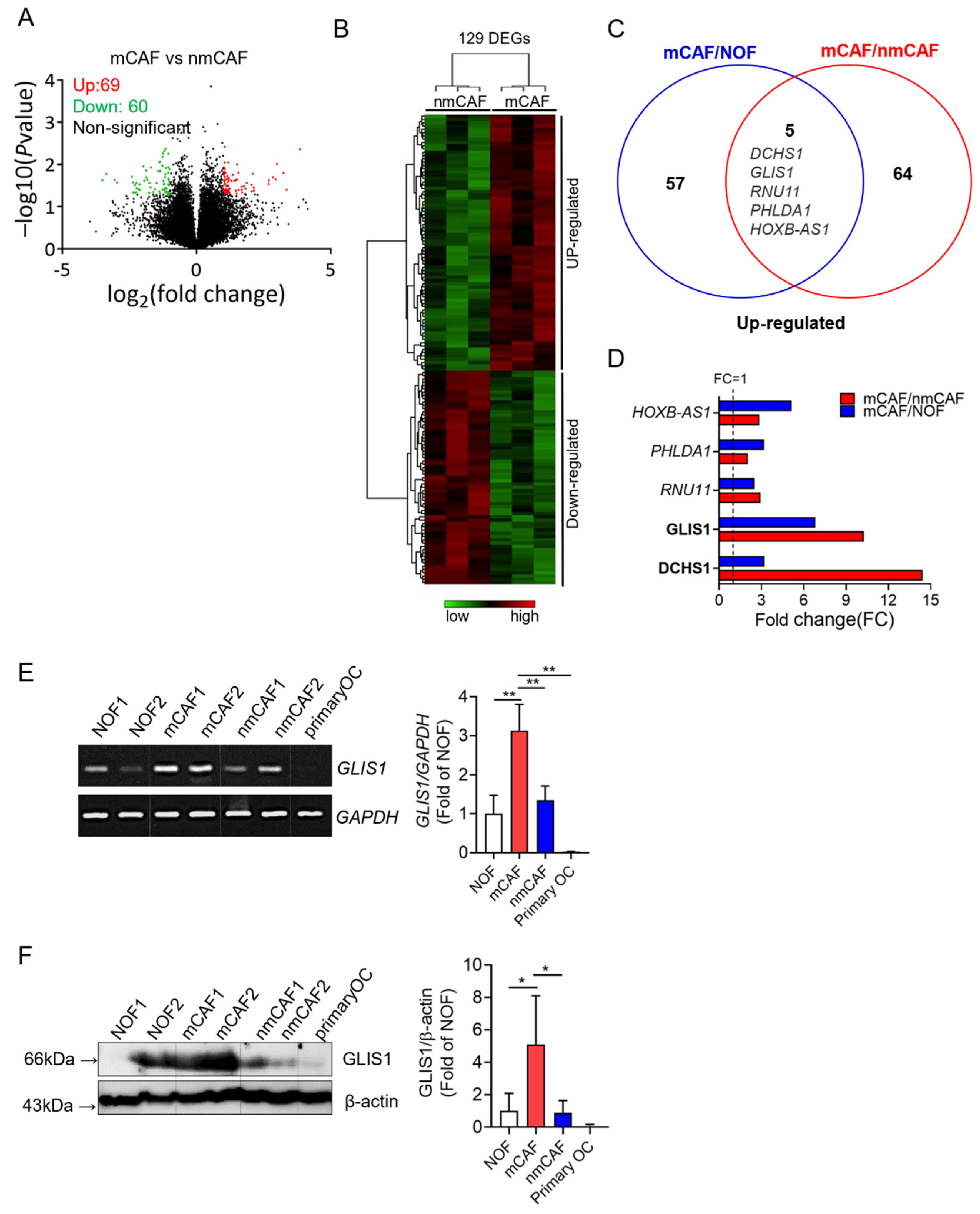
To investigate the gene profiles in normal fibroblasts (NOFs), mCAFs, and nmCAFs from primary OSC tissues, researchers performed a microarray analysis. To define the genes involved in tumor metastases and invasion, they transcriptionally compared sorted mCAFs and nmCAFs. Positive signals were obtained from 22,155 clones hybridized with probes. A total of 129 genes were differentially expressed, of which 69 genes were upregulated and 60 genes were downregulated on mCAFs as compared to nmCAFs (fold change (FC) > 2 or <0.5, p < 0.05) (Figure 1A,B).
Figure 1. Gene expression signature of CAF from OC using microarray analysis. (A) Volcano plot analysis showing significantly altered genes (p value < 0.05, fold-change > 2 or <0.5) between mCAFs and nmCAFs; upregulated genes (red) and downregulated genes (green). (B) The heatmap depicts clustering of 129 differentially expressed genes on mCAFs as compared to nmCAFs (p-value < 0.05 and fold change > 2 or <0.5). Each column represents the expression profiles of individual tumors in each experimental group. Warm color (red) denotes an increase in gene expression, whereas cold color (green) indicates a decrease as compared to the average level of gene expression in nmCAFs. (C) Venn diagram showing the overlap genes significantly upregulated in mCAF vs. NOFs and mCAFs vs. nmCAFs. (D) Significantly upregulated five genes both mCAFs/nmCAFs and mCAF/NOF. (E) Expression of GLIS1 mRNA in NOF, mCAF, nmCAF, and carcinoma cells. Expression levels was examined using RT-PCR. The quantification of relative mRNA levels was normalized to GAPDH. Each experiment was performed in triplicate. Data are represented as the mean ± SD. Statistical analysis was performed using an unpaired t-test (** p < 0.01). (F) Immunoblot analysis of GLIS1 in NOF, mCAF, nmCAF, and carcinoma cells (primary OC). The ratio of the intensity of protein bands relative to that of β-actin was calculated. Bar graph represents the relative protein expression of GLIS1. Each experiment was performed in triplicate. Data are represented as the mean ± SD. Statistical analysis was performed using an unpaired t-test (* p < 0.05). mCAFs: metastatic CAFs, NOFs: normal fibroblasts, nmCAFs: nonmetastatic CAFs.
To exclude the genes upregulated in NOFs, researchers compared mCAFs and NOFs. Sixty-two genes were significantly higher in mCAFs than in NOFs. Among these genes, five genes (DCHS1, GLIS1, RNU11, PHLDA1, and HOXB-AS1) were significantly altered in both comparisons of mCAFs/nmCAFs and mCAFs/NOFs (Figure 1C). In particular, two genes (DCHS1 and GLIS1) were highly overexpressed in mCAFs compared to nmCAFs (Figure 1D).
Therefore, researchers performed RT-PCR and Western blot analysis for DCHS1 (FC:14.44) and GLIS1 (FC:10.25), the two most significantly upregulated genes in mCAFs relative to nmCAF to validate the microarray data. Both mRNA and protein expression levels of GLIS1 were proven to be significantly higher in mCAFs than in NOF and nmCAFs (Figure 1E,F). In addition, primary cancer cells did not express DCHS1 and GLIS1 mRNA and protein. However, the protein expression of DCHS1 was not correlated with its mRNA expression, and the protein expression of DCHS1 in mCAF compared with NOF was not significantly higher, suggesting that post-transcriptional modification might occur in this gene.
They therefore selected GLIS1 for further study to evaluate its functional roles (e.g., migration, invasion, and angiogenesis), which might drive metastatic progression in OSCs.
3. Knockdown of GLIS1 in CAFs Suppresses Migration, Invasion, and Angiogenesis of OC Cells
To elucidate whether CAF-derived GLIS1 plays a role in the metastasis of OC cells, researchers introduced siRNA for GLIS1 in mCAFs. After confirming that mRNA and protein expression of GLIS1 efficiently decreased after GLIS1 siRNA transfection, invasion and migration assays were performed in OC cells.
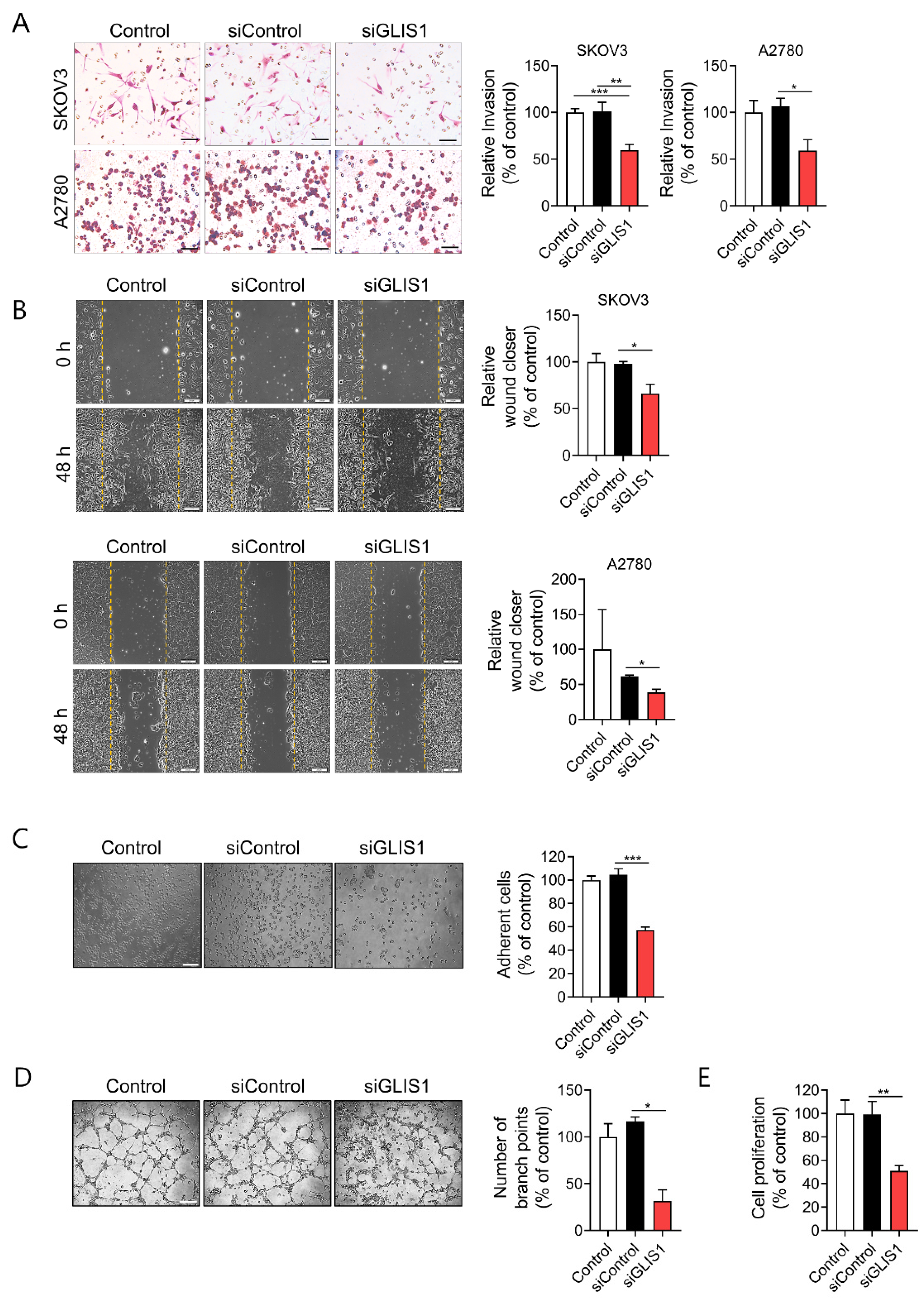
They examined the invasion ability of SKOV3 and A2780 OC cells after OC cells were put into an upper chamber and CAFs were seeded into the lower chamber. Then, OC and CAFs cocultured for 6h. Both SKOV3 and A2780 cells cocultured with GLIS1-knockdown CAFs had significantly reduced invasion ability compared with cells cocultured with siControl CAFs (Figure 2A).
Figure 2. GLIS1 knockdown in CAFs inhibits OC cells migration and invasion. (A) Transwell Matrigel invasion assay was performed by counting migrated SKOV3 and A2780 cells co-cultured with siControl- or siGLIS1-transfected CAFs. After 6 h, the invaded cells on the basal side of the membrane were dyed and counted. Left: representative images of invaded OC cells. Right: Quantification of invasion. Percentage of number of invaded cells of each group relative to the number of the Control group. Scale bar, 50 μm. (B) Wound healing assays were performed to evaluate cell migration ability after 48 h. Left: representative images of scratched and recovering of wounded areas on confluence monolayers of SKOV3 and A2780 cells with CAF-CM. Yellow dotted lines indicate the wound borders at the beginning of the assay. Right: Quantification of wound closure. Relative wound closure was determined by percentage of the area of migrated cells of each group compared with that of the Control. Scale bar, 200 μm. (C) SKOV3 cells cultured with CAF-CM for 24 h were reseeded on plates. After 20 min, non adherent cells were removed with PBS washing. Adhesion assay was performed by counting the number of adherent SKOV3 cells. Left: representative images of adherent SKOV3 cells. Right: Quantification of adhesion. Percentage of the number of adhesive cells relative to the number of the Control group. Scale bar, 100 μm. (D) Tube formation abilities of human endothelial cells affected by GLIS1 silencing. HUVECs were cocultured with CAF CM and siGLIS1- or siControl-transfected CAF cells. The graph represents the relative number of branch points of HUVECs in each group compared with that of the control. Scale bar, 500 μm. (E) Proliferative ability, measured using a CCK-8 assay, of SKOV3 cells cultured with CAF-CM for 6 days. Percentage of OD value of each group relative to that of the Control group. Each experiment was performed in triplicate. Data are represented as the mean ± SD. Statistical analysis was performed using an unpaired t-test (* p < 0.05, ** p < 0.01 and *** p < 0.001).
Next, researchers examined the effects of GLIS1 derived from CAFs on cell migration by wound healing assay. They prepared conditioned medium (CM) of cultured CAFs that were transfected with siControl or siGLIS1. Both SKOV3 and A2780 cells cultured with CM of siGLIS1-CAFs for 48 h had significantly reduced wound healing ability compared with siControl CAF (SKOV3: 66.2% vs. 98.1%,
p < 0.05, A2780: 35.5% vs. 62.8%,
p < 0.05) (
Figure 2B). The siGLIS1 CM also significantly reduced adhesion of SKOV3 cells on the culture plate (57.3% vs. 104.6%,
p < 0.001) as compared to the siControl (
Figure 2C). Cell-to-cell interactions facilitating tumor cell adhesion are essential steps in the metastatic cascade
[11]. Cancer cells, especially with the highly metastatic potential, are believed to have enhanced adhesion ability that facilitates the migration of the cells to a new site to establish new tumors in the body. Therefore, the cell adhesion assay is often used to evaluate the metastatic ability of cancer cells. These data suggest that GLIS1 from CAFs regulates OC cell invasion, migration, and adhesion which are related to the initial stage of metastasis.
To determine whether GLIS1 in CAFs regulates tumor angiogenesis, researchers examined its effect on tube formation ability of HUVECs cultured with CAF-CM. HUVECs cultured for 4 h with CM from siGLIS1-CAFs had significantly fewer capillary-like branch points than those treated with CM from siControl-CAFs (31.6% vs. 116.6%, p < 0.05) (Figure 2D). These data indicate that the downregulation of GLIS1 in CAFs reduces angiogenesis.
They also examined cancer cell proliferation using the CCK-8 assay to determine whether GLIS1 in CAF is involved in cancer cell proliferation. The proliferation of SKOV3 cells was significantly inhibited siGLIS1-CM (51.3%) as compared to the siControl-CM (Figure 2E). These data suggest that GLIS1 in CAFs plays a role in cancer cell proliferation.
4. GLIS1 Silencing Reduces the Peritoneal Spread of OC Cells In Vivo
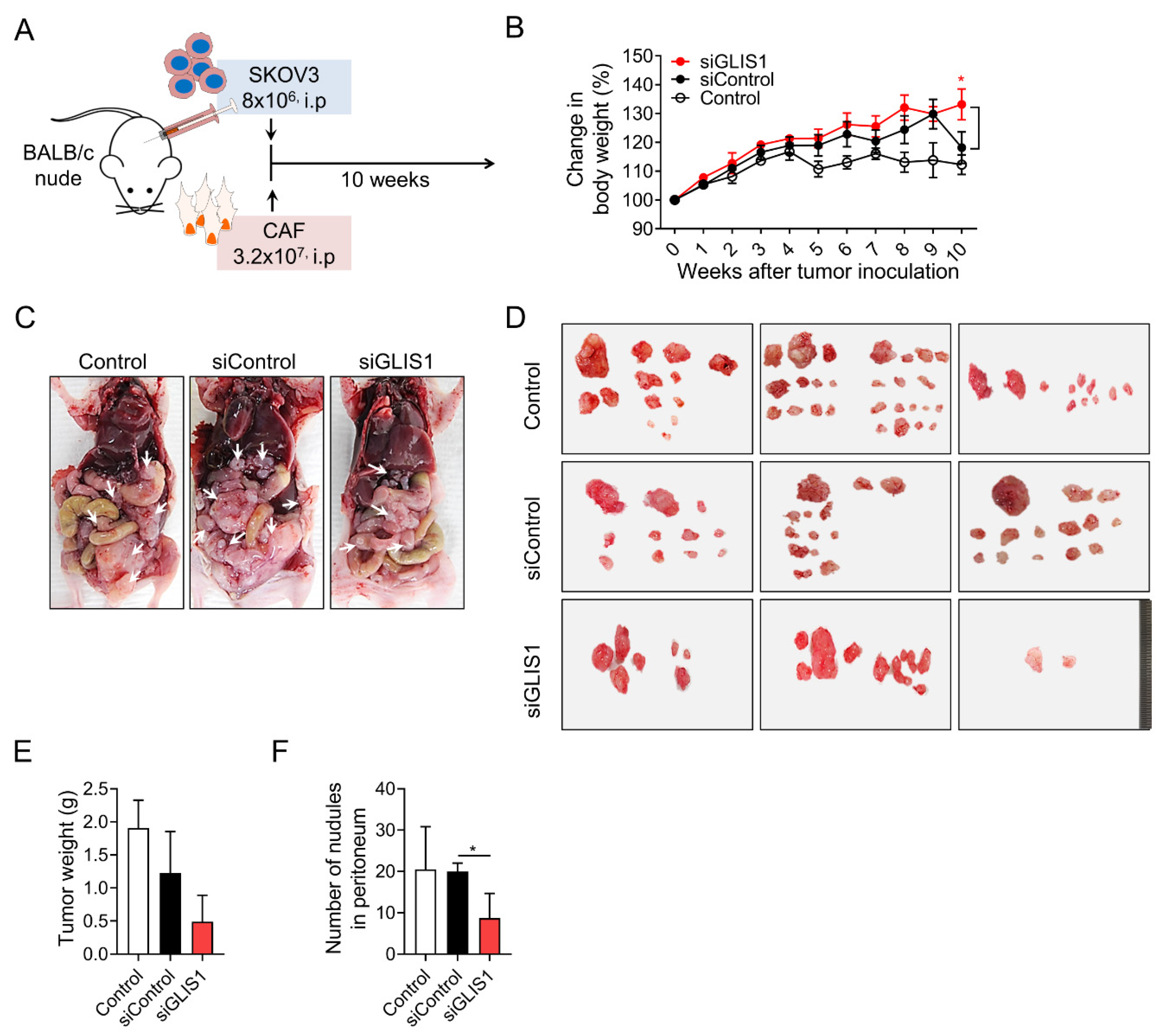
To investigate the effects of CAF-derived GLIS1 on intraperitoneal tumor metastasis in vivo, researchers established xenograft tumor models through inoculation of a mixture of SKOV3 cells and CAFs transfected with siGLIS1 or Control siRNA at a 1:4 ratio in the peritoneal cavity of nude mice (Figure 3A). Whereas the body weight of siGLIS1 groups was steadily increased, the body weight of the mice of siControl group increased up to 9 weeks but drastically reduced in the 10th week (siControl vs. siGLIS1.; 113% vs. 133%, p < 0.05) (Figure 3B). They cannot exactly explain the reason why the body weight of siControl group reduced drastically at the 9th and 10th weeks, however researchers guess that increasing tumor burden presumably contributed to the increase in body weight to some extent until 9th week in siControl group. After 9 weeks, the mice with high tumor burden seemed to be struggling to endure them and turned out to show the loss of weight. At the end of experiment, mice were euthanized, and the tumor nodules were immediately removed and weighed (Figure 3C). The total weight of tumor nodules in the siGLIS1-CAF group was less than that of those in the siControl or Control group (39.9% and 25.6%, respectively) (Figure 3E), although it was not statistically significant. The number of tumor nodules was significantly lower in the siGLIS1 group than in the siControl group (siControl vs. siGLIS1; 20 vs. 8.6, p <0.05) (Figure 3F). These results indicate that GLIS1 overexpression in CAFs might be related to the peritoneal spread of OC cells and that the downregulation of GLIS1 in CAFs might inhibit peritoneal tumor spread in OC in vivo.
Figure 3. In vivo effect of GLIS1 silencing in CAFs on peritoneal tumor formation. (A) Schematic experimental design. SKOV3 cells mixed with CAFs transfected with siGLIS1 or Control siRNA (siControl) were intraperitoneally inoculated into nude mice. The weights and numbers of tumor nodules were observed after 10 weeks (n = 5). (B). The graph represents the changes in body weight from each group. (C) Representative image of peritoneal tumor nodules from the control, siControl, and siGLIS1 groups. Arrows indicate disseminated tumors. (D) Images of tumor nodules isolated from each group. Tumor weight (E) and number of nodules (F) were measured. Data are represented as the mean ± SD. Statistical analysis was performed using an unpaired t-test (* p < 0.05).