Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Szőri Milán | + 1808 word(s) | 1808 | 2022-02-23 09:42:27 | | | |
| 2 | Catherine Yang | -21 word(s) | 1787 | 2022-02-28 03:00:15 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Milán, S. Isocyanoaminoanthracene. Encyclopedia. Available online: https://encyclopedia.pub/entry/19942 (accessed on 07 February 2026).
Milán S. Isocyanoaminoanthracene. Encyclopedia. Available at: https://encyclopedia.pub/entry/19942. Accessed February 07, 2026.
Milán, Szőri. "Isocyanoaminoanthracene" Encyclopedia, https://encyclopedia.pub/entry/19942 (accessed February 07, 2026).
Milán, S. (2022, February 26). Isocyanoaminoanthracene. In Encyclopedia. https://encyclopedia.pub/entry/19942
Milán, Szőri. "Isocyanoaminoanthracene." Encyclopedia. Web. 26 February, 2022.
Copy Citation
Isocyanoaminoarenes (ICAAr-s) are a novel and versatile group of solvatochromic fluorophores. Despite their versatile applicability, such as antifungals, cancer drugs and analytical probes, they still represent a mostly unchartered territory among intramolecular charge-transfer (ICT) dyes.
anthracene
fluorescence
1. Introduction
Polycyclic aromatic hydrocarbon (PAH) moieties such as pyrene and anthracene are important building blocks of smart electronic and fluorescent materials [1][2][3]. Owing to their rigid planar structure and easy substitutability with reactive or bulky functional groups, they can be incorporated into more complex structures, such as graphene nanoribbons [4] and ribbon-like pyrene-fused pyrazaacenes (PPAs) [5]. The unprecedented optoelectronic properties of these complex structures can be utilized in many optoelectronic applications, for example, in dye sensitized solar cells (DSSCs) [6] among all. In addition, anthracene moiety makes up the core of many important fluorescent probes used for the detection of transition metal ions such as Zn2+ [7]. The formation of at least two amino groups on PAHs in symmetric positions offers an easy way to incorporate the aromatic moiety into more complex structures through alkylation, acylation or diazotation reactions. In addition, by varying the number and position of N atoms and the substitution on the aromatic core along the π-framework, it is possible to modulate the electronic structure, stability, solubility and supramolecular organization [8][9]. In this context, the molecular organization in π-conjugated systems could be further controlled by virtue of the cooperative effect of stronger non-covalent interactions. Among them, hydrogen bonding represents an appropriate tool [10][11][12], as it is evidenced by biological systems in which the combination of π-stacking and hydrogen bonds determines the macrostructure of proteins or nucleic acids, just to mention well-known examples. One of the most important members of diamino PAHs is 1,5-diaminoanthracene, which is used to construct organic semiconductors [13], dinuclear nickel complexes for highly active ethylene dimerization [14], self-assembled small-molecule-based hole-transporting material for inverted perovskite solar cells [15], photoactivated healable vitrimeric copolymers [16], amorphous porous organic polymers for highly efficient iodine capture [17], bimetallic aluminum complexes for ring-opening polymerization of lactide [18], Luminescent Supramolecular Lanthanide Complexes [19], Squaraine dyes [20] and others. Despite its widespread application, the literature on the optical properties of 1,5-diaminoanthracene is very limited.
The optical properties may be further enhanced (while preserving the important H-bonding ability) by converting one of the amino groups into isocyanide using dichlorocarbene [21]. Only a few members of the resulting isocyanoaminoarene substance family have been prepared and studied until recently, despite their simple structure and preparation. Isocyanoaminoarenes are typically built up from an electron-donating (amino, D) and an electron-withdrawing (isocyano, A) group, connected through an aromatic π-linker moiety. These so-called D-π-A type solvatochromic fluorophores are therefore based on the shift of the electron density from the donor group to the acceptor moiety through the π-system upon excitation; hence, an intramolecular charge-transfer (ICT) takes place [22][23], which may result in an increase in the excited state dipole moment with respect to that of the ground state. It is believed that the presence of ICT, in the absence of any specific interaction, i.e., hydrogen-bonding, between the fluorophore and the solvent is the primary reason of solvatochromism. Fluorophores, whose fluorescence emissions are particularly sensitive to the polarity of their microenvironment and hence they alter the emitted light color upon the effect of polarity change, are called solvatochromic fluorophores [23].
2. Fluorescence Quantum Yield of ICAA Derivatives in Different Solvents
An essential property, which also determines the practical applicability of any fluorophore, is their quantum yield (√f), i.e., the ratio of the emitted and absorbed photons. The fluorescence quantum yield of ICAAs is strongly influenced by the polarity of the solvent. The starting anthracene-diamine is highly fluorescent in every solvent (√f = 12–53%); however, in water, the quantum yield drops to only 3%. In contrast, √f rarely exceeds 10% in nonpolar solvents for the isocyano derivatives ICAA, MICAA and DIMICAA, and they are practically nonfluorescent in solvents more polar than dioxane. In polar solvents, the quantum yields of ICAA-DIMICAA are close to only 1%; moreover, it drops to only √f = 0.1–0.2% in water. The fluorescence quantum yield of DAA, ICAA, MICAA and DIMICAA in different solvents was correlated with the empirical Dimroth polarity parameters ET(30) of the solvents (Figure 1). While no clear correlation was obtained for the diamine (DAA), the isocyano derivatives (ICAA-DIMICAA) show almost identical behavior, i.e., a sharp decrease in √f between ET(30) = 30–40 (kcal/mol), followed by a constant minimum range above ET(30) > 40 (kcal/mol). A very similar behavior was described for 1,8-naphthalimides [24][25]. This phenomenon implies the potential application of ICAAs to probe the polarity of the medium. The very low quantum yield in water is favorable in practical applications, such as cell-staining, resulting in a reduced background fluorescence as we have shown previously for the ICAN dyes [26]. The reduced fluorescence in polar solvents can also be practical for the selective staining of different nonpolar cell compartments such as cell membrane and/or nucleus.
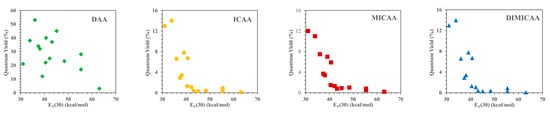
Figure 1. Dependence of quantum fluorescence yields of the 1,5-disubstituted anthracene dyes on the empirical parameter of solvent polarity ET(30).
Two of the most common ways to quantify the solvatochromic effect in solvents of different polarity is to plot the fluorescence emission maxima (νEm) as a function of the empirical solvent polarity parameter ET(30) [27] and the Lippert–Mataga (LM) plot [28][29], which are presented in Figure 2.
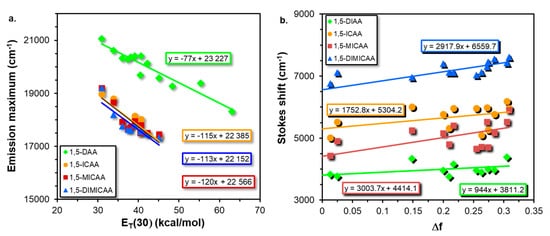
Figure 2. Variation of the fluorescence emission maximum with the empirical solvent polarity parameter ET(30) (a) and the Lippert–Mataga (LM) (b) plots for the 1,5-disubstituted anthracene dyes.
Interestingly, two groups can be identified: one belonging to the diamine (DAA) and the other to the isocyano (ICAA-DIMICAA) dyes (Figure 2a). In both cases, the correlation is linear between the fluorescence emission maximum and ET(30) for all the anthracene fluorophores. It can be surmised from the corresponding slopes that ICAA, MICAA and DIMICAA have almost the same solvatochromic shift (113–120 kcal−1cm−1mol), while this value is significantly lower for the 1,5-DAA (77 kcal−1cm−1mol) supporting the ICT character of the emission state of ICAA-DIMICAA, which is in good agreement with the computational findings. It should be noted, however, that because of their strong H-bond donating character (i.e., the solvatochromic response is strongly modulated by specific interactions), protic solvents (iPrOH, MeOH and H2O) were not included in the plot.
Lippert–Mataga’s Equation (1), which is based on the correlation of energy difference between the ground and excited states (Stokes’ shift) with the solvent orientation polarizability (Δf), can be used to investigate the change in dipole moment between the excited singlet state (µe) and ground state (µg).
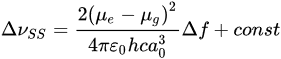 where ΔνSS (in cm−1) is the Stokes shift, ε0, h and c are the vacuum permittivity (8.8541 × 10−12 C⋅V−1m−1), Planck constant (h = 6.626 × 10−34 Js), speed of light (c = 299,792,458 m s−1), respectively, and the dipole moment is given in Debye. The Onsager cavity radius (a0), which closely reflects to the radius of a spherical cavity the fluorophore molecule occupies, is either obtained from quantum chemical calculations or by using Suppan’s Equation (2) [30],
where ΔνSS (in cm−1) is the Stokes shift, ε0, h and c are the vacuum permittivity (8.8541 × 10−12 C⋅V−1m−1), Planck constant (h = 6.626 × 10−34 Js), speed of light (c = 299,792,458 m s−1), respectively, and the dipole moment is given in Debye. The Onsager cavity radius (a0), which closely reflects to the radius of a spherical cavity the fluorophore molecule occupies, is either obtained from quantum chemical calculations or by using Suppan’s Equation (2) [30],
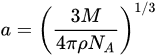 where M is the molecular weight of the fluorophore, NA is the Avogadro’s constant and ρ is the density. Δf stands for the orientation polarizability defined as:
where M is the molecular weight of the fluorophore, NA is the Avogadro’s constant and ρ is the density. Δf stands for the orientation polarizability defined as:
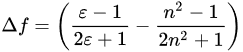 where ε and n are the dielectric constant and the refractive index of the solvent, respectively.
where ε and n are the dielectric constant and the refractive index of the solvent, respectively.
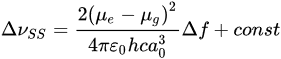
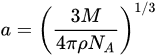
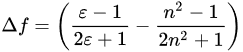
After plotting the Stoke’s shift values versus Δf , the dipole moment change can be calculated from the slope of the plot as:
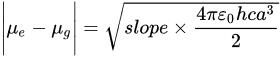
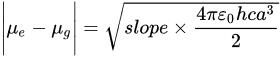
MICAA has the highest slope (3003 cm−1) obtained from the LM plot, which is closely followed by DIMICAA (2917 cm−1). The smallest slope belongs to DAA (944 cm−1), which is almost twice as small as the corresponding values for ICAA (1752 cm−1). In addition, the Stokes shifts at ΔfLM = 0, i.e., the intercepts of the lines determined from the LM plots, decrease in the order of DIMICAA > ICAA > MICAA > DAA. The difference between the excited and the ground state dipole moments, i.e., Δμ = μE − μG, have been calculated according to Equation (4) and by using the DFT results. To calculate Δμ, first, a has to be determined, and instead of using Equation (2), it has been associated with the half distance between the amino and isocyano groups of the corresponding optimized geometries.
However, contrary to the expectations and DFT calculations, DAA also has a significant dipole moment change (Δμ = 2.22 D). Amongst the molecules studied, only the structure of DIA is planar, and the isocyano groups linearly attached to the ring structure resulted in a Cs symmetric molecule. On the other hand, the structure of DAA has unique structural features in such a way that the aromatic rings are slightly bent which come from the interaction of amine nitrogens with the ring structure. One of the hydrogens in each amine group is almost in the rings’ plane while the other one sticks out of the plane (Figure 3). Since two amine groups are attached to this structure, these hydrogens can be on one side of the rings or on opposite sides. Since the latter conformer has only a permanent dipole moment, we have considered it.
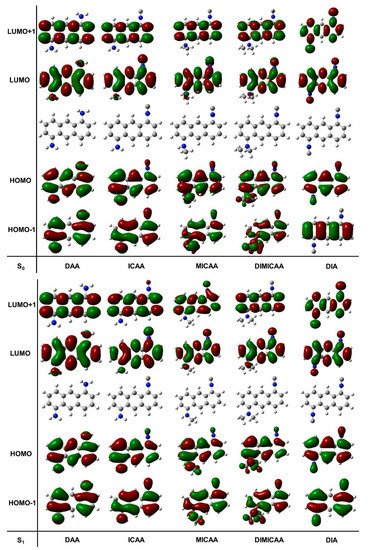
Figure 3. Representation of the relevant molecular orbitals (MO) for the relaxed ground (S0(solv, s0)) and excited states (S1(solv, s1)) of the 1,5-disubstituted anthracene dye structures in dioxane (isovalue for electron density was set to 0.000400 a.u.).
Interestingly, the Δμ values calculated from the Lippert–Mataga equation are half than those obtained by DFT. However, the change of the values from the LM plots are in good agreement with the fully DFT-based results. That is, Δμ changes in the order of 0 D = DIA < DAA < ICAA < MICAA ≅ DIMICAA. In addition, Δμ values are lower for ICAA, MICAA and DIMICAA, while in the case of DAA, Δμ is higher compared to the results in water.
TD-DFT calculations were performed to obtain a deeper understanding of the electronic behavior of the excited states of the studied structures (Figure 3). Optimizations have been carried out on the ground and excited state geometries. The HOMO, LUMO, HOMO-1, LUMO+1 molecular orbitals for all the dye structures in this study are depicted in Figure 3. There are no significant differences between the corresponding ground and excited state molecular orbitals.
Molecular electrostatic potential (MESP) isosurfaces have also been created for the relaxed ground and excited states as shown in Figure 4. There is no visible difference in the MESPs of the S0 and S1 in the case of the diamino and diisocyano structures, DAA and DIA, respectively (Figure 4). However, a slight difference between the ground and excited state MESPs of ICAA, MICAA and DIMICAA can be seen. However, these cannot be associated with significant changes in the geometries. The largest difference between the S0 and S1 MESPs occurs on the isocyano group in case of MICAA, where a rotation of the methylated amino group is also experienced.
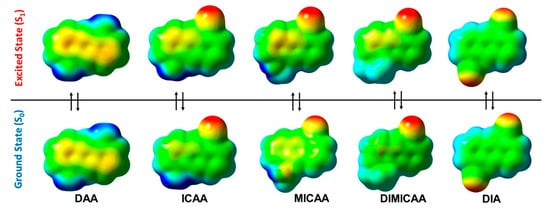
Figure 4. Molecular electrostatic potential (MESP) isosurfaces (isovalue = 0.02 a.u.) for the relaxed ground (S0(solv, s0)) and excited states (S1(solv, s1)) of the 1,5-disubstituted anthracene dye structures in dioxane (blue color corresponds to +0.045. a.u. (ca. +120 kJ/mol) potential while red represents −0.045 a.u. (ca. −120 kJ/mol)).
References
- Bendikov, M.; Wudl, F.; Perepichka, D.F. Tetrathiafulvalenes, Oligoacenenes and Their Buckminsterfullerene Derivatives: The Brick and Mortar of Organic Electronics. Chem. Rev. 2004, 104, 4891–4946.
- Anthony, J.E. Functionalized Acenes and Heteroacenes for Organic Electronics. Chem. Rev. 2006, 106, 5028–5048.
- Anthony, J.E. The Larger Acenes: Versatile Organic Semiconductors. Angew. Chem. Int. Ed. 2008, 47, 452–483.
- Chen, L.; Hernandez, Y.; Feng, X.; Müllen, K. From Nanographene and Graphene Nanoribbons to Graphene Sheets: Chemical Synthesis. Angew. Chem. Int. Ed. 2012, 51, 7640–7654.
- Mateo-Alonso, A. Pyrene-fused pyrazaacenes: From small molecules to nanoribbons. Chem. Soc. Rev. 2014, 43, 6311–6324.
- Lin, Y.-Z.; Huang, C.H.; Chang, Y.J.; Yeh, C.-W.; Chin, T.-M.; Chi, K.-M.; Chou, P.-T.; Watanabe, M.; Chow, T.J. Anthracene based organic dipolar compounds for sensitized solar cells. Tetrahedron 2014, 70, 262–269.
- Nguyen, M.-H.; Nguyen, T.-N.; Do, D.-Q.; Nguyen, H.-H.; Phung, Q.-M.; Thirumalaivasan, N.; Wu, S.-P.; Dinh, T.-H. A highly selective fluorescent anthracene-based chemosensor for imaging Zn2+ in living cells and zebrafish. Inorg. Chem. Commun. 2020, 115, 107882.
- Gómez, P.; Cerdá, J.; Más-Montoya, M.; Georgakopoulos, S.; da Silva, I.; García, A.; Ortí, E.; Aragó, J.; Curiel, D. Effect of molecular geometry and extended conjugation on the performance of hydrogen-bonded semiconductors in organic thin-film field-effect transistors. J. Mater. Chem. C 2021, 9, 10819–10829.
- Matsumoto, H.; Nishimura, Y.; Arai, T. Excited-state intermolecular proton transfer dependent on the substitution pattern of anthracene–diurea compounds involved in fluorescent ON1–OFF–ON2 response by the addition of acetate ions. Org. Biomol. Chem. 2017, 15, 6575–6583.
- Black, H.T.; Lin, H.; Bélanger-Gariépy, F.; Perepichka, D.F. Supramolecular control of organic p/n-heterojunctions by complementary hydrogen bonding. Faraday Discuss. 2014, 174, 297–312.
- Głowacki, E.D.; Irimia-Vladu, M.; Bauer, S.; Sariciftci, N.S. Hydrogen-bonds in molecular solids–from biological systems to organic electronics. J. Mater. Chem. B 2013, 1, 3742–3753.
- Irimia-Vladu, M.; Kanbur, Y.; Camaioni, F.; Coppola, M.E.; Yumusak, C.; Irimia, C.V.; Vlad, A.; Operamolla, A.; Farinola, G.M.; Suranna, G.P.; et al. Stability of Selected Hydrogen Bonded Semiconductors in Organic Electronic Devices. Chem. Mater. 2019, 31, 6315–6346.
- Gómez, P.; Georgakopoulos, S.; Más-Montoya, M.; Cerdá, J.; Pérez, J.; Ortí, E.; Aragó, J.; Curiel, D. Improving the Robustness of Organic Semiconductors through Hydrogen Bonding. ACS Appl. Mater. Interfaces 2021, 13, 8620–8630.
- Feng, C.; Zhou, S.; Wang, D.; Zhao, Y.; Liu, S.; Li, Z.; Braunstein, P. Cooperativity in Highly Active Ethylene Dimerization by Dinuclear Nickel Complexes Bearing a Bifunctional PN Ligand. Organometallics 2021, 40, 184–193.
- Más-Montoya, M.; Gómez, P.; Curiel, D.; Da Silva, I.; Wang, J.; Janssen, R.A.J. A Self-Assembled Small-Molecule-Based Hole-Transporting Material for Inverted Perovskite Solar Cells. Chem. A Eur. J. 2020, 26, 10276–10282.
- Wright, T.; Tomkovic, T.; Hatzikiriakos, S.G.; Wolf, M.O. Photoactivated Healable Vitrimeric Copolymers. Macromolecules 2018, 52, 36–42.
- Guo, Z.; Sun, P.; Zhang, X.; Lin, J.; Shi, T.; Liu, S.; Sun, A.; Li, Z. Amorphous Porous Organic Polymers Based on Schiff-Base Chemistry for Highly Efficient Iodine Capture. Chem. Asian J. 2018, 13, 2046–2053.
- Shi, T.; Zheng, Q.-D.; Zuo, W.-W.; Liu, S.-F.; Li, Z.-B. Bimetallic aluminum complexes supported by bis(salicylaldimine) ligand: Synthesis, characterization and ring-opening polymerization of lactide. Chin. J. Polym. Sci. 2017, 36, 149–156.
- Li, X.-Z.; Zhou, L.-P.; Yan, L.-L.; Yuan, D.-Q.; Lin, C.-S.; Sun, Q.-F. Evolution of Luminescent Supramolecular Lanthanide M2nL3n Complexes from Helicates and Tetrahedra to Cubes. J. Am. Chem. Soc. 2017, 139, 8237–8244.
- Kuster, S.; Geiger, T. Coupled π-conjugated chromophores: Squaraine dye dimers as two connected pendulums. Dye. Pigment. 2015, 113, 110–116.
- Rácz, D.; Nagy, M.; Mándi, A.; Zsuga, M.; Kéki, S. Solvatochromic properties of a new isocyanonaphthalene based fluorophore. J. Photochem. Photobiol. A Chem. 2013, 270, 19–27.
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer: New York, NY, USA, 2006.
- Marini, A.; Muñoz-Losa, A.; Biancardi, A.; Mennucci, B. What is Solvatochromism? J. Phys. Chem. B 2010, 114, 17128–17135.
- Poteau, X.; Brown, A.I.; Brown, R.G.; Holmes, C.; Matthew, D. Fluorescence switching in 4-amino-1,8-naphthalimides: “on–off–on” operation controlled by solvent and cations. Dye. Pigment. 2000, 47, 91–105.
- Staneva, D.; Vasileva-Tonkova, E.; Grabchev, I. Chemical modification of cotton fabric with 1,8-naphthalimide for use as heterogeneous sensor and antibacterial textile. J. Photochem. Photobiol. A Chem. 2019, 382, 111924.
- Nagy, M.; Rácz, D.; Nagy, Z.L.; Fehér, P.P.; Kalmár, J.; Fábián, I.; Kiss, A.; Zsuga, M.; Kéki, S. Solvatochromic isocyanonaphthalene dyes as ligands for silver(I) complexes, their applicability in silver(I) detection and background reduction in biolabelling. Sens. Actuators B Chem. 2018, 255, 2555–2567.
- Reichardt, C. Solvatochromic Dyes as Solvent Polarity Indicators. Chem. Rev. 1994, 94, 2319–2358.
- Lippert, E. Dipolmoment und Elektronenstruktur von angeregten Molekülen. Z. Für Nat. A 1955, 10, 541–545.
- Mataga, N.; Kaifu, Y.; Koizumi, M. The Solvent Effect on Fluorescence Spectrum, Change of Solute-Solvent Interaction during the Lifetime of Excited Solute Molecule. Bull. Chem. Soc. Jpn. 1955, 28, 690–691.
- Suppan, P. Excited-state dipole moments from absorption/fluorescence solvatochromic ratios. Chem. Phys. Lett. 1983, 94, 272–275.
More
Information
Subjects:
Spectroscopy
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
690
Revisions:
2 times
(View History)
Update Date:
28 Feb 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




