
Video Upload Options
Copper is a mineral element, which is necessary for the normal growth and development of plants, but high levels of copper will seriously damage plants. Studies have shown that AtGR1 improves the tolerance of Arabidopsis to aluminum and cadmium stress. Researchers identified four genes (named BcGR1.1, BcGR1.2, BcGR2.1 and BcGR2.2, respectively) encoding glutathione reductase (GR) in non-heading Chinese cabbage (Brassica campestris (syn. Brassica rapa) ssp. chinensis), which could be divided into two types based on the subcellular localization. Among them, BcGR1.1, which belonged to the cytoplasmic localization type, was significantly upregulated under copper stress. Compared to WT (the wild type), Arabidopsis thaliana heterologously overexpressed BcGR1.1 had longer roots, higher fresh weight, higher GSH levels and GSH/GSSG (oxidized form of GSH) ratio, and accumulated more superoxide dismutase and peroxidase under copper stress. However, in the AsA-GSH cycle under copper stress, the contents of AsA and AsA/DHA were significantly downregulated, and the contents of DHA and T-AsA (total AsA) were upregulated, in the BcGR1.1-overexpressing Arabidopsis. Therefore, BcGR1.1 could improve the scavenging ability of reactive oxygen species (ROS) by increasing the activity of GR, antioxidant enzymes and the utilization of AsA, and then enhance the copper stress tolerance of plants.
1. Introduction
In addition to the common abiotic stresses in agricultural production, such as drought, flooding and extreme temperatures [1][2], heavy-metal pressure has become another stress threatening crop production [3]. This is mainly due to the unrestricted industrialization and urbanization that has occurred in the last few decades. Heavy-metal pollution has become the focus of global attention due to its wide range, strong toxicity, long-term effects and irreversibility [4][5]. The heavy metals with the greatest influence on plant metabolism are Pb, Cd, Cu and Zn. As an essential heavy-metal element [6], copper plays a key role in many biological processes of plants, including photosynthesis and respiratory electron transfer, cell wall remodeling, superoxide scavenging, lignification and ethylene sensing [7][8][9][10][11][12][13]. If the copper content in plants is insufficient, the growth and development of plant reproductive organs are affected [14]. However, when the concentration of copper exceeds the requirements for plant growth, the structure and function of the plant cell membrane will be damaged, the permeability of plant cell membranes will be affected, and the antioxidant enzyme system and chloroplast structure of plants will be damaged, resulting in inhibition of the growth and development of plants [15][16][17][18][19][20][21].
Under copper stress conditions, the growth of plant primary roots is inhibited [22], which, in turn, affects the growth and development of aerial parts. Under the conditions of copper stress, there will be an excessive accumulation of ROS in plants, which is toxic to plants [23]. In the process of plant evolution, some mechanisms for coping with copper stress have been developed, including adjusting the dynamic balance of copper [24], activating the antioxidant defense response [25], and so on. The defense system includes enzymes that remove ROS, such as superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT) as well as low-molecular-weight antioxidants, such as reduced ascorbic acid (AsA) and reduced glutathione (GSH) [26].
As an important component of the antioxidant system [27], GSH can play an important role in the physiological activity of organisms. It actively participates in the formation of disulfide, sulfide, thiolipid and other sulfides. It also participates in the elimination of excess ROS [28], the reduction in peroxides, the transmission of redox-sensitive signals [29], and the elimination of superoxide free radicals in the process of cell metabolism. It can coordinate with heterologous toxic substances, regulate plant growth and development, and resist various stresses (e.g., temperature, heavy metals, osmotic stress, and pathogen infection) [30][31][32][33][34][35][36][37][38]. GSH can chelate with copper in plants to excrete copper from the plant, thereby significantly reducing the copper content in plant tissues [39]. In addition, GSH is the electron donor of oxidized ascorbic acid (DHA), and its redox state is associated with the AsA-GSH cycle. As the main antioxidant system in plants, the AsA-GSH cycle can inhibit the production of ROS. This plays an important role in plant anti-aging and stress adaptation [40].
In general, glutathione mainly exists in two completely different forms: the reduced form (GSH) and the oxidized form (GSSG). Glutathione in plants is mostly reduced [41]. Therefore, glutathione is commonly referred to as the reduced form (GSH). In the face of stress, the activity of GR in an organism is improved, and the content of GSH is increased by the catalytic reduction in GSSG to increase plants’ ability to resist environmental stress [42][43][44][45]. Therefore, high GR activity is necessary for plants to maintain high levels of GSH, especially under stress conditions.
In recent years, arable land area is decreasing. Thus, how to safely use the heavy-metal-contaminated arable land has become a major problem. However, since plants are immovable, they lack the ability to avoid the polluted environment. Therefore, the only chance for them to survive under adverse conditions is the mobilization of defense mechanisms and the evolution of a more tolerant genotype [46]. In Arabidopsis thaliana, overexpression of AtGR1 results in high levels of GSH and GSH/GSSG ratios that help suppress ROS and lipid-peroxide-derived reactive carbonyl species (RCS) damage to plants, and enhance the dual detoxification function of plants, thereby enhancing the aluminum tolerance of Arabidopsis thaliana [47]. However, unlike aluminum stress, copper, as one of the components of heavy-metal pollution, is itself an essential trace element for plants. Therefore, it is particularly important to improve the plants’ ability to cope with copper stress. The copper content in the soil should be not too much or too little. Therefore, it is particularly important to study the coordinated evolution of copper in plants and soils.
2. Phylogeny and Conserved Domains Analysis of GRs
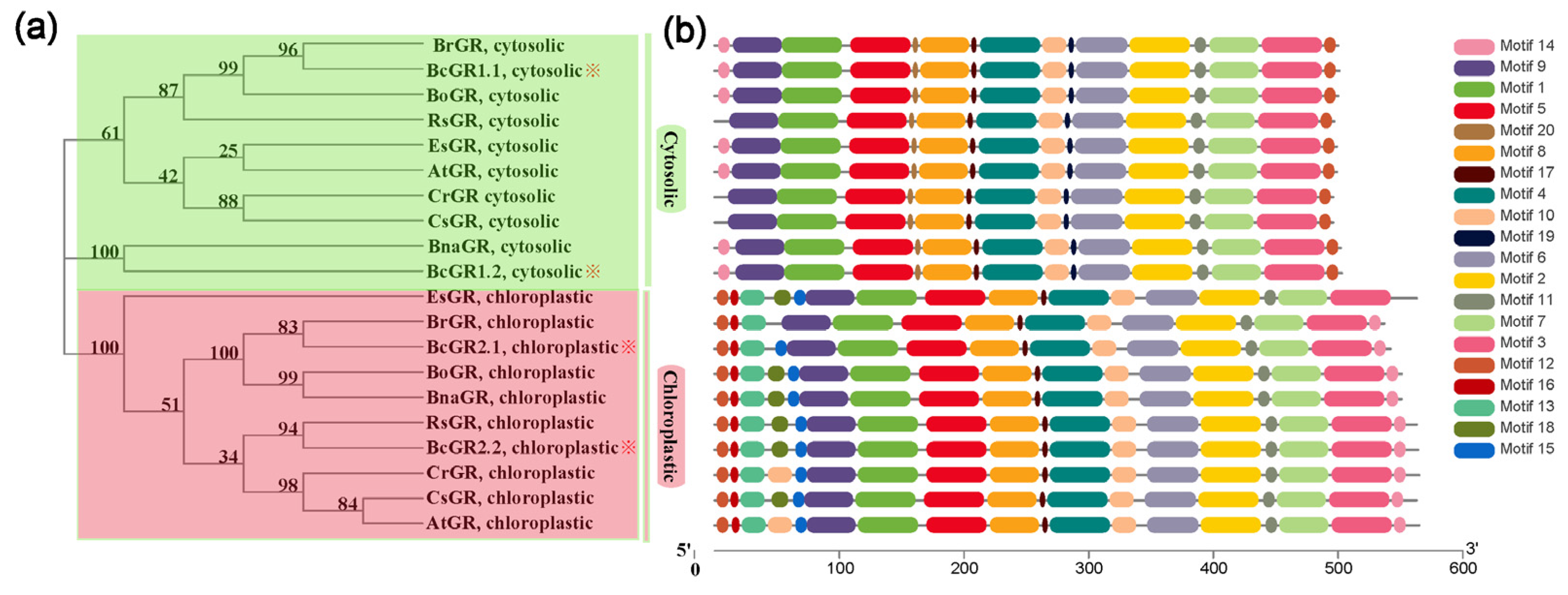
Four GR genes in non-heading Chinese cabbage were identified, and named BcGR1.1, BcGR1.2, BcGR2.1 and BcGR2.2. The results of phylogenetic tree analysis and the differential distribution of conserved motifs indicate that the GRs of non-heading Chinese cabbage are similar to other varieties, with two subcellular localization types: cytoplasm (Figure 1a, green box) and chloroplast (Figure 1a, pink box). Phylogenetic tree analysis showed that there is a close evolutionary relationship among non-heading Chinese cabbage, Brassica rapa, Brassica napus and Raphanus sativus (Figure 1a). Motif analysis showed that the motifs of BcGRs are overwhelmingly the same as those of other species (Figure 1b). For the cytoplasmic localized GR, there was only a difference in the first motif, motif 13, which was only identified in several other species, except RsGR, CrGR, and CsGR. Chloroplast-localized GR, according to the difference between the fourth and fifth conserved motifs, can be divided into four categories. EsGR, BoGR, BnaGR, RsGR, BcGR2.2, CsGR have exactly the same motifs and belong to the same class; compared with this class, BrGR lacks motif 18 and motif 15, and BcGR2.1 does not contain motif 18, The fourth motif of CrGR and AtGR is motif 10 instead of motif 18.
3. Subcellular Localization of BcGRs
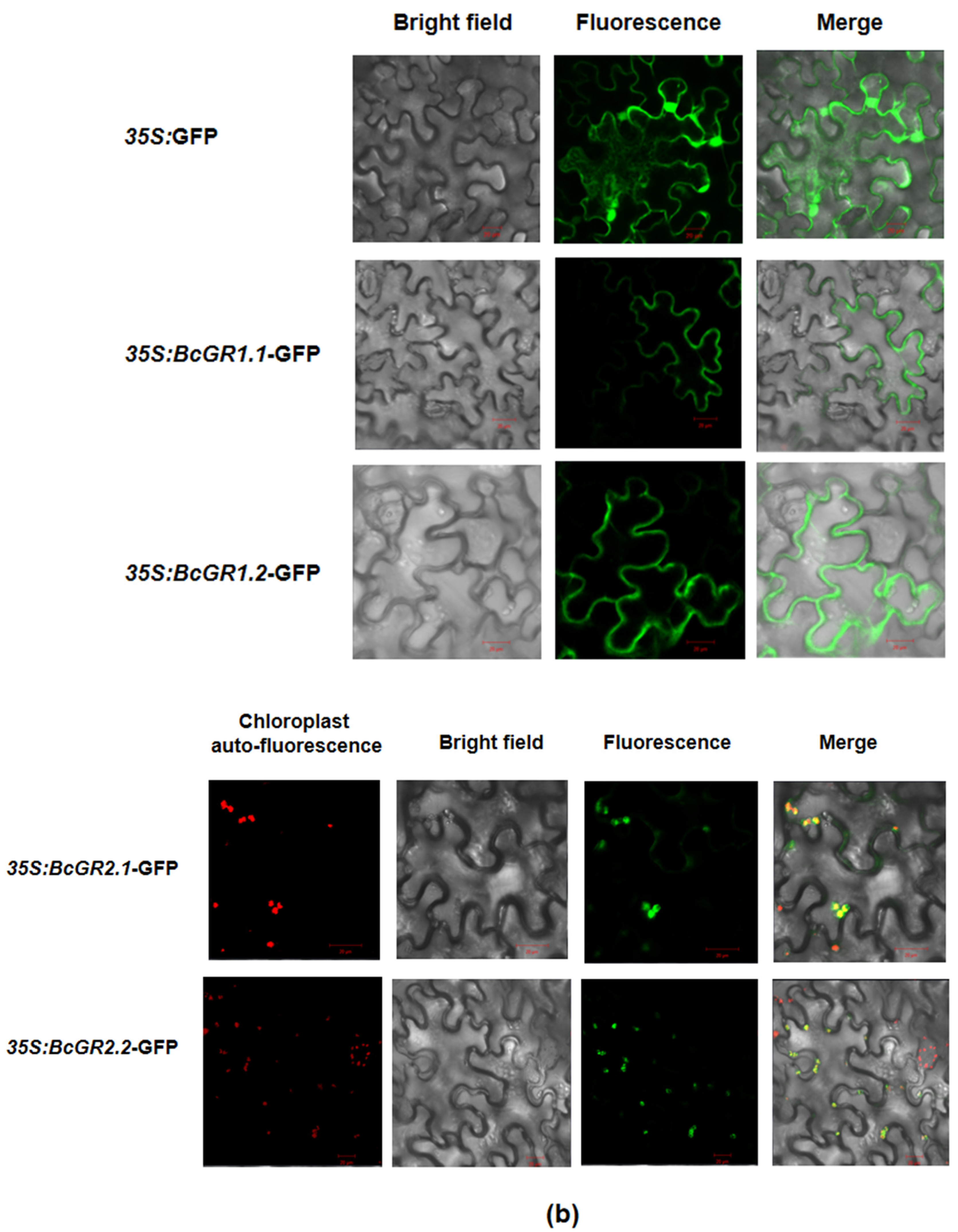
To further determine the specific location of BcGR proteins in cells, subcellular localization was performed. Researchers constructed a vector (Figure 2a), fused BcGRs with GFP, and detected the localization of BcGRs protein in cells by observing GFP. Results show that GFP protein was expressed in the whole cell, while BcGR1.1-GFP and BcGR1.2-GFP fusion proteins were mainly expressed in the cell membrane and cytoplasm, BcGR2.1-GFP and BcGR2.2-GFP fusion proteins were mainly expressed in the chloroplast (Figure 2b). This result indicated that the non-heading Chinese cabbage had two types of GR proteins: cytosolic localization and chloroplastic localization, which is consistent with the results of evolutionary analysis.
4. Expression patterns of BcGRs
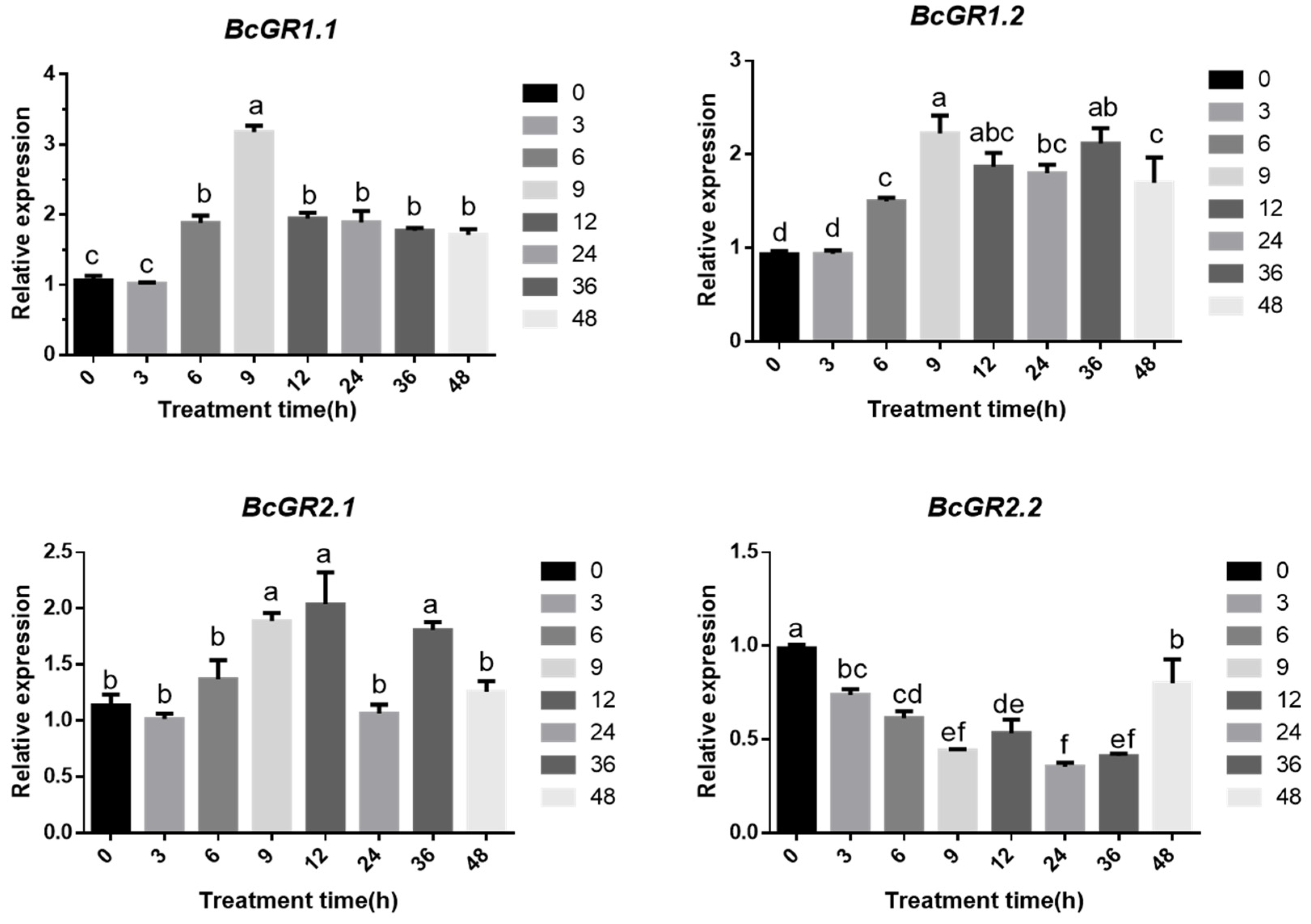
Here, researchers used one-month-old, non-heading Chinese cabbage for copper treatment. The qRT-PCR showed that the four GR homologous genes in non-heading Chinese cabbage have different responses under copper stress (Figure 3). BcGR1.1, BcGR1.2 and BcGR2.1 were upregulated, especially BcGR1.1. The expression rapidly increased 6 h after copper stress, reaching a peak at 9 h and then slowly decreasing. However, the expression pattern of BcGR2.2 was completely different from the other three BcGRs. BcGR2.2 was downregulated, and showed the lowest expression at 9 h. The results showed that, among the four GR homologous genes, BcGR1.1 may play a major role in copper stress.
5. Heterologous Overexpression of BcGR1.1 in Arabidopsis thaliana
To further study the function of BcGR1.1, researchers transformed Arabidopsis thaliana by dipping flowers to obtain BcGR1.1-overexpressing plants. For each generation of transgenic plants, DNA level detection (Figure 4b), GUS detection (Figure 4c) and expression analysis (Figure 4d) were performed, respectively. After obtaining the transgenic lines, researchers tested the GR activity in the WT and transgenic lines. The results showed that the expression levels of BcGR1.1-0E3, BcGR1.1-0E7, and BcGR1.1-0E8 were increased to 274, 228, and 255 times of the WT, and the GR activity were significantly higher than that of the WT, which were 4.3, 3.1, and 3.3 times that of the WT, respectively (Figure 4e).
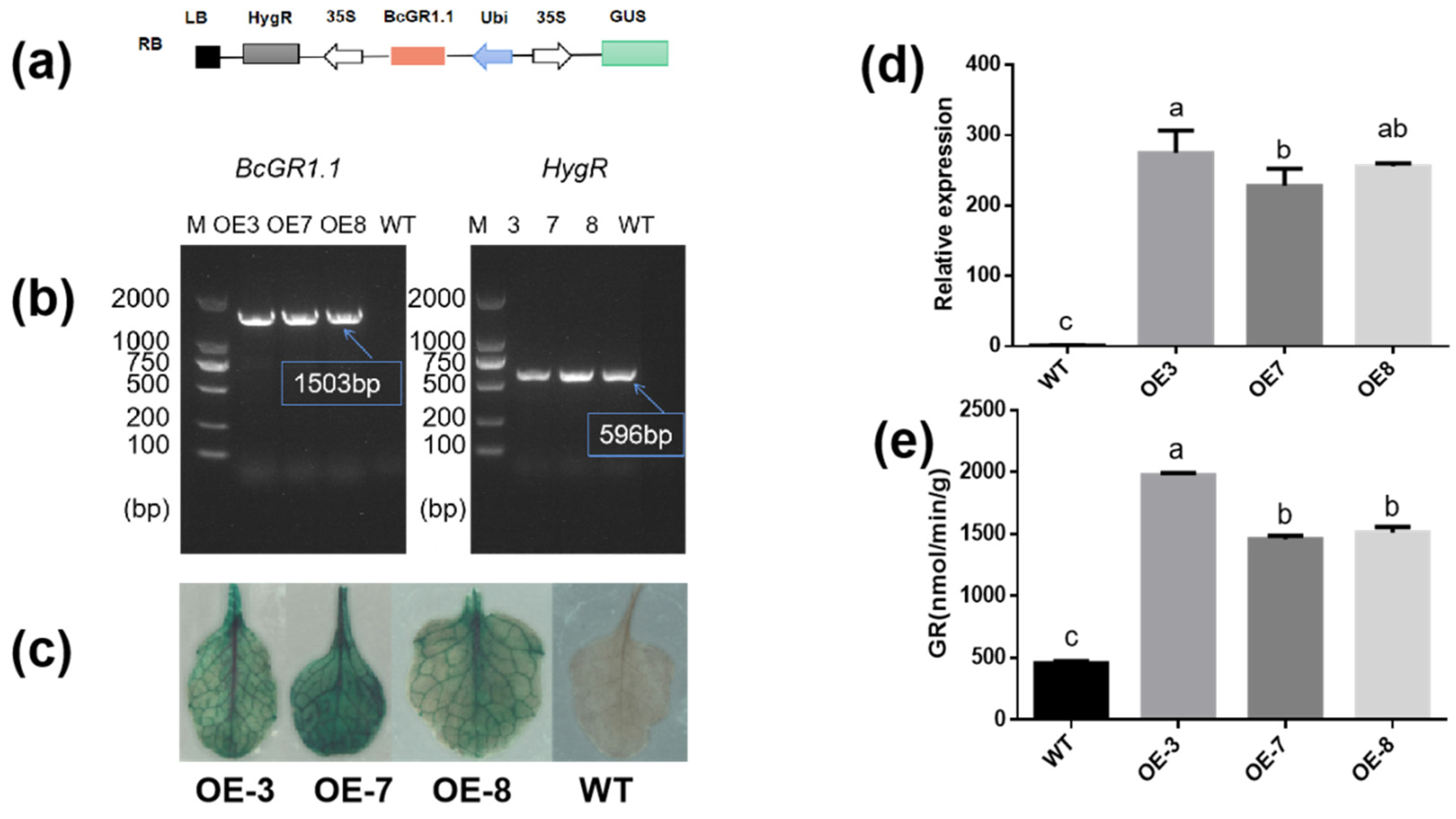
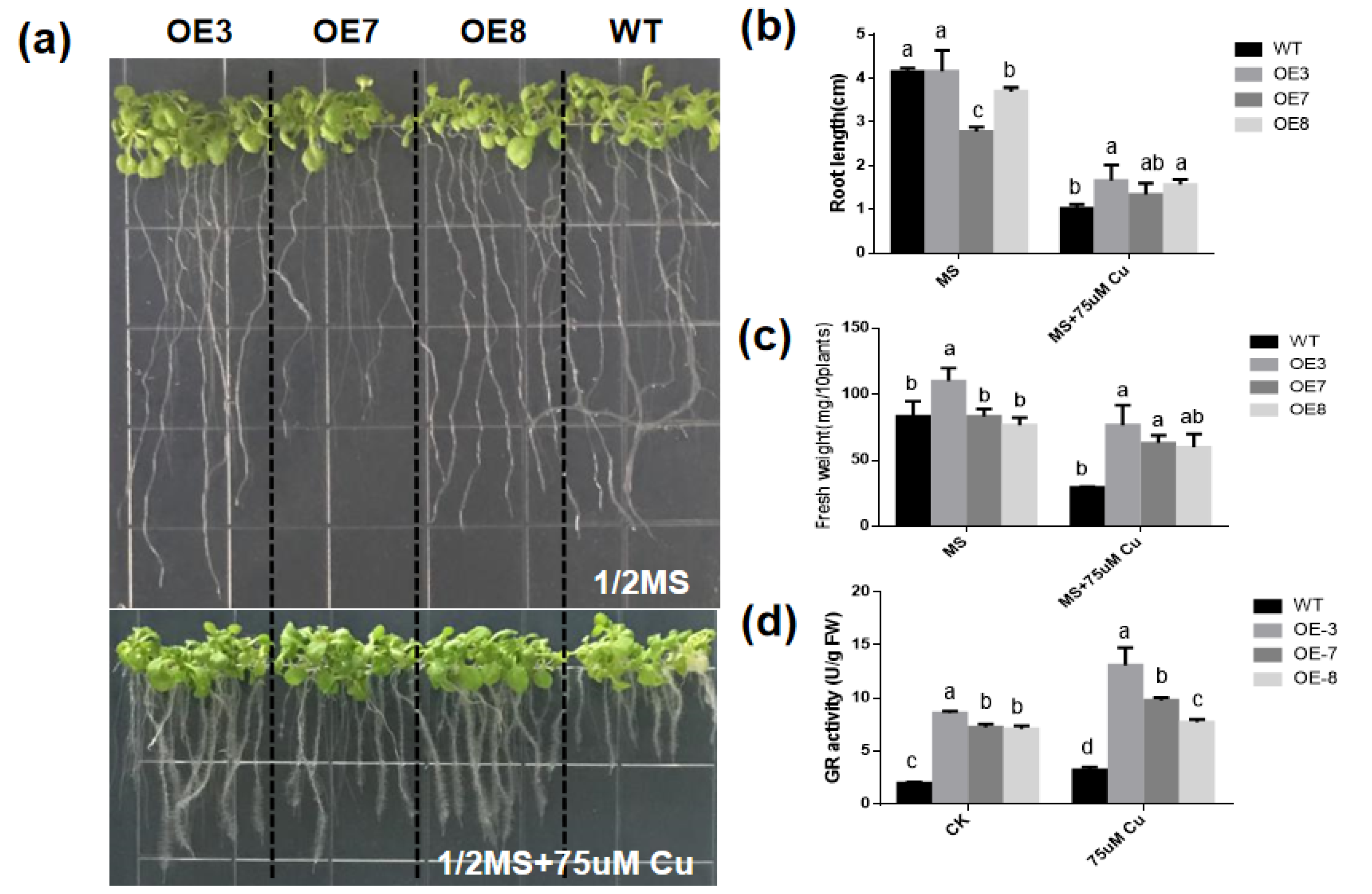
6. Overexpression of BcGR1.1 Improved Copper Stress Tolerance in Arabidopsis thaliana
The expression of BcGR1.1 was significantly upregulated under copper stress, and its heterologous overexpression in Arabidopsis obviously increased the GR activity. Therefore, researchers further studied the role of BcGR1.1 in the response of plant to copper-stress . The GR, root length and fresh weight of WT and BcGR1.1-OE plants were measured under normal conditions (CK) and copper stress. Regardless of the presence of copper stress, the activity of GR in BcGR1.1-OE plants was always higher than that of WT. The GR activities of BcGR1.1-OE and WT were both increased under copper stress (Figure 5d). Under normal growth conditions, the roots of WT were longer than that of transgenic lines. Under copper treatment, root growth of WT and transgenic plant lines was significantly inhibited, and the root growth of WT plants was shorter than that of BcGR1.1-OE plants (the inhibition rates were 75% and 56%, respectively) (Figure 5b). There was no significant difference between the WT and BcGR1.1-OE plants under normal growth conditions, except OE3. However, the fresh weight of the transgenic lines was significantly higher than that of WT under copper treatment (Figure 5c). The results showed that the BcGR1.1-OE plants grew better under copper stress due to the longer roots and increased biomass.
7. Effects of BcGR1.1 Overexpression on the Status and Content of glutathione and AsA in A. thaliana
In the cycle of ASA-GSH (Figure 6a) [48], ASA and GSH are both important reducing substances in plants. Their oxidation/reduction status is closely related to the stress tolerance ability of plants. Therefore, researchers analyzed the changes in the status and content of these two antioxidant substances under copper stress.
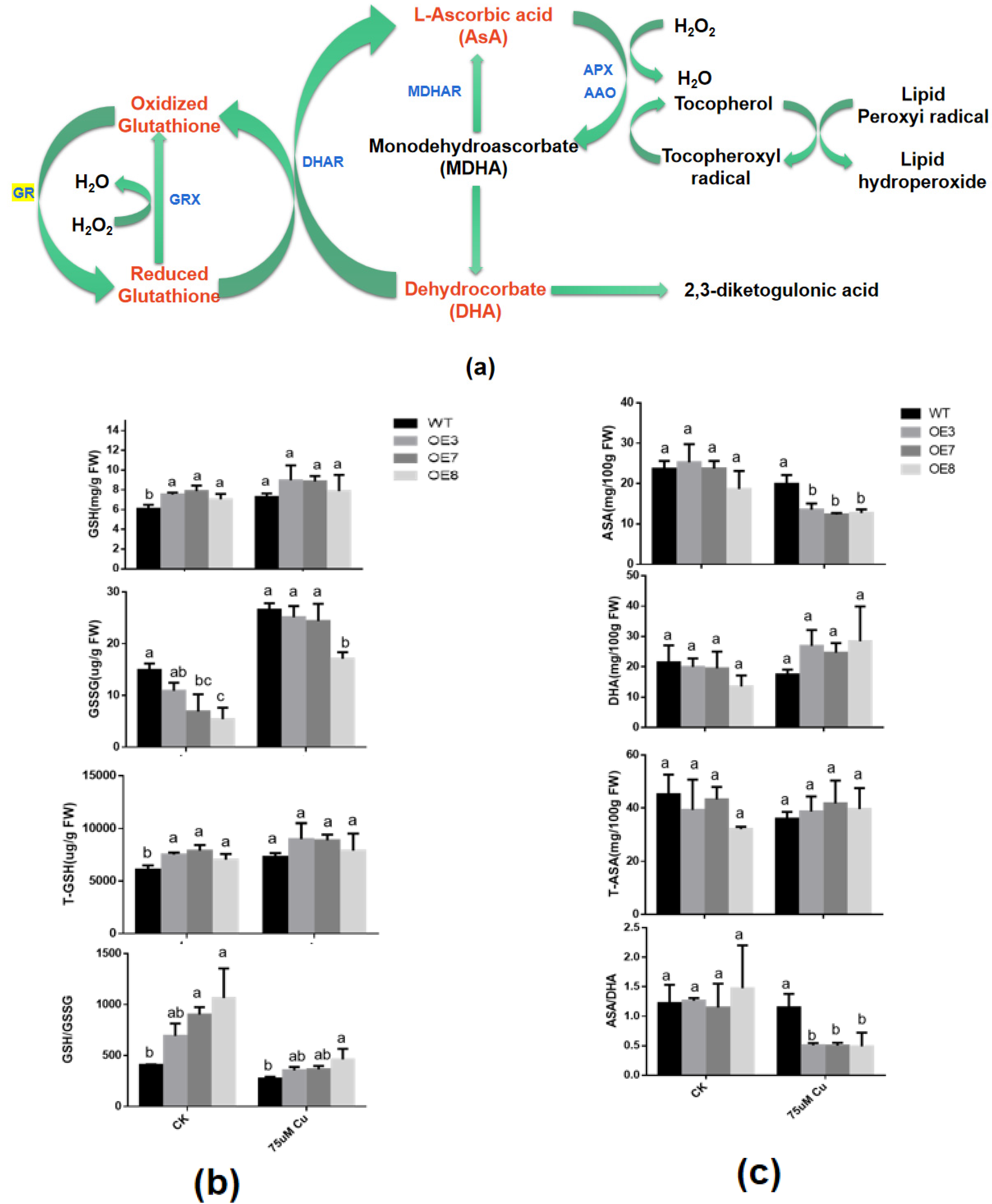
As far as glutathione is concerned, the GR enzyme activity in transgenic lines are significantly increased under normal growth conditions and under copper treatment (Figure 5d), which may result in a higher GSH content and lower GSSG and total glutathione (T-GSH) content. However, this difference is significant under normal growth conditions, but not under copper stress. This may be the result of the significant upregulation of GR in WT under copper stress. GSH/GSSG in both wild-type and transgenic plants decreased under copper stress. However, in transgenic lines, GSH/GSSG is still higher than WT (Figure 6b). These results indicated that higher levels of GSH may be beneficial for plants to cope with copper stress.
As for AsA, there is no significant difference in AsA, DHA, T-AsA and AsA/DHA content in WT and transgenic lines under normal conditions. Under copper stress, the AsA content in the transgenic line was significantly lower than that of the WT, while the content of DHA was the opposite. However, there was no significant difference between T-AsA. This resulted in a significant reduction in AsA/DHA in transgenic lines, to about 50% of the WT plants (Figure 6c). These results suggest that the heterologous overexpression of BcGR1.1 in Arabidopsis may improve the efficiency of transforming AsA into DHA, thereby efficiently clearing ROS and improving plant tolerance to copper stress.
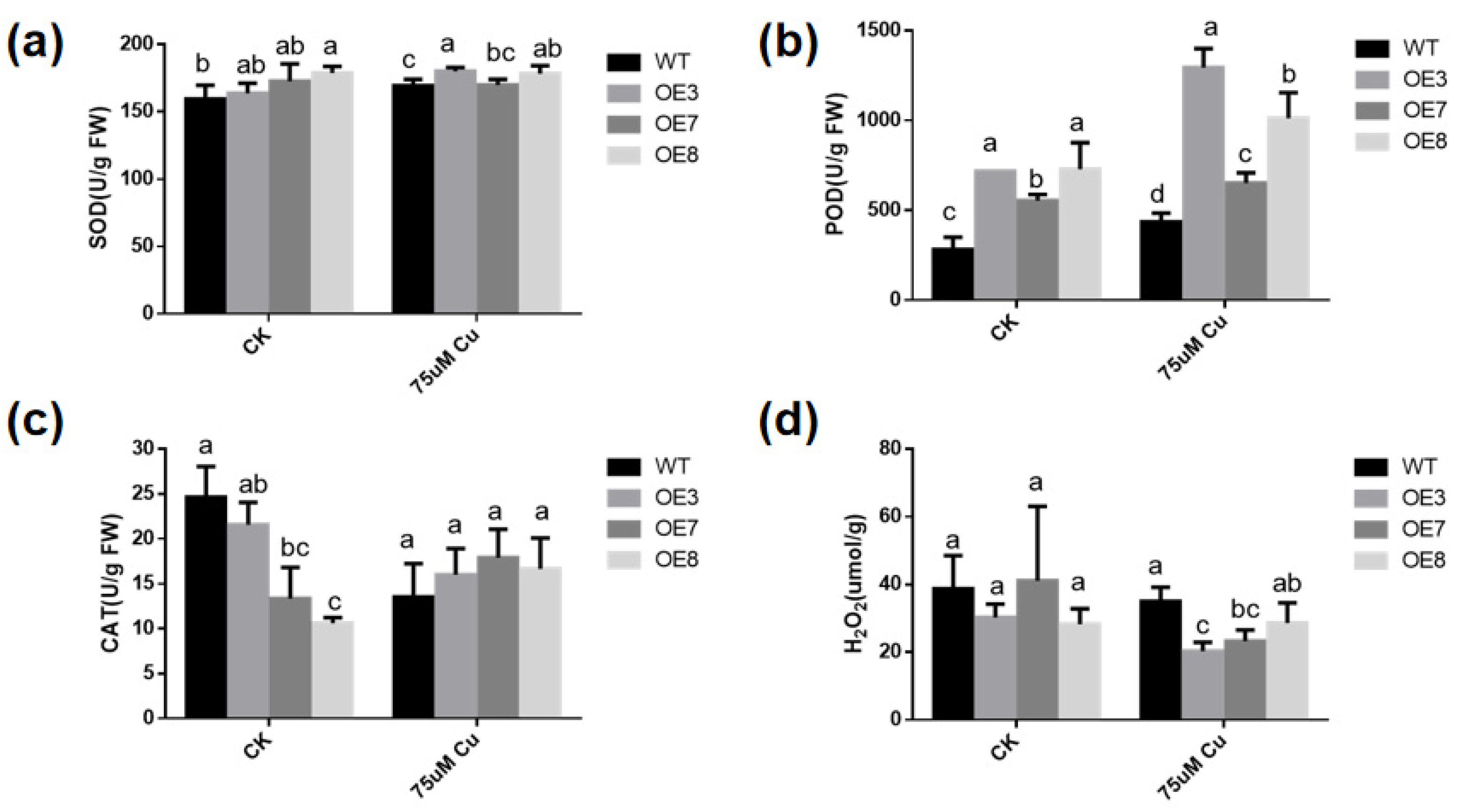
8. Antioxidant Enzyme Activities Are Altered in Transgenic A. thaliana
To determine whether the increase in copper-stress tolerance of transgenic plants is related to the change in antioxidant activity, the activities of SOD (Figure 7a), POD (Figure 7b), CAT (Figure 7c) and the content of H2O2 were measured in WT and transgenic plants with or without excessive copper treatment. With or without copper stress, the SOD and POD activities in BcGR1.1-OE plants were higher than those in WT. Under copper-stress treatment, SOD activity did not significantly change in WT and BcGR1.1-OE lines, but POD enzyme activity significantly increased. Under copper stress, the POD activities of OE3 and OE8 were 2.9 times and 2.3 times higher than that of WT, respectively. The CAT activity of BcGR1.1-OE line was lower than that of WT plants under normal conditions, but showed no difference under copper stress treatment. Upregulation of POD activity levels in transgenic lines under copper stress resulted in significantly lower H2O2 content in transgenic lines than in WT (Figure 7d). The results indicated that the heterologous overexpression of BcGR1.1 in Arabidopsis can regulate the activity of certain antioxidant enzymes, reduce the ROS damage to plants, and improve the tolerance of plants to copper stress.
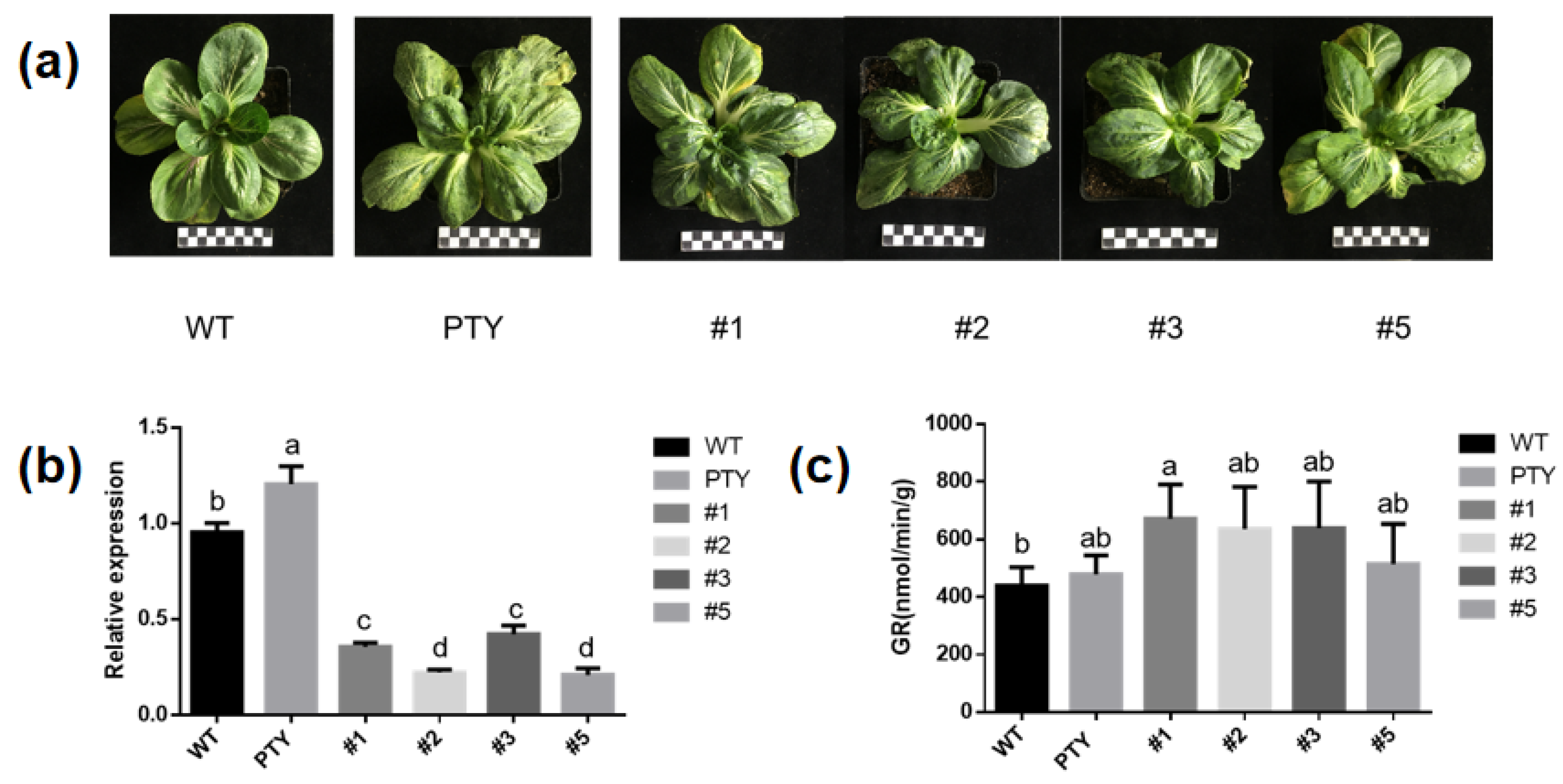
9. Virus-Induced BcGR1.1 Silencing in Non-Heading Chinese Cabbage
Researchers used VIGS experiment to further study the function of BcGR1.1 in non-heading Chinese cabbage. Two weeks after virus inoculation, PTY-BcGR1.1 and PTY showed a mosaic leaf phenotype (Figure 8a). Plants with potential BcGR1.1 function loss were sampled, and qRT-PCR was used to evaluate the silencing efficiency of BcGR1.1. As shown in Figure 8b, compared with PTY plants, BcGR1.1 expression was significantly reduced by 70.4%, 81.4%, 64.9% and 82.6% in pTY-BcGR1.1#1, #2, #3, and #5 seedlings, respectively. There was no significant difference in GR activity between PTY-BcGR1.1 and PTY plants (Figure 8c), which may be the result of functional complementarity between BcGR homologous genes.
References
- Thao, N.P.; Tran, L.S. Potentials toward genetic engineering of drought-tolerant soybean. Crit. Rev. Biotechnol. 2012, 32, 349–362.
- Xia, X.J.; Zhou, Y.H.; Shi, K.; Zhou, J.; Foyer, C.H.; Yu, J.Q. Interplay between reactive oxygen species and hormones in the control of plant development and stress tolerance. J. Exp. Bot. 2015, 66, 2839–2856.
- Ahmad, P.; Sarwat, M.; Bhat, N.A.; Wani, M.R.; Kazi, A.G.; Tran, L.S. Alleviation of cadmium toxicity in Brassica juncea L. (Czern. & Coss.) by calcium application involves various physiological and biochemical strategies. PLoS ONE 2015, 10, e0114571.
- Vardhan, K.H.; Kumar, P.S.; Panda, R.C. A review on heavy metal pollution, toxicity and remedial measures: Current trends and future perspectives. J. Mol. Liq. 2019, 290, 111197.
- Bo, B.; Anderberg, S.; Lohm, U. Accumulated environmental impact: The case of cadmium in sweden. Sci. Total Environ. 1994, 145, 13–28.
- Ghre, V.; Paszkowski, U. Contribution of the arbuscular mycorrhizal symbiosis to heavy metal phytoremediation. Planta 2006, 223, 1115–1122.
- Yruela, I. Copper in plants: Acquisition, transport and interactions. Funct. Plant Biol. 2009, 36, 409–430.
- Yruela, I. Copper in plants. Braz. J. Plant Physiol. 2005, 17, 145–156.
- Küpper, H.; Götz, B.; Mijovilovich, A.; Meyer-Klaucke, F. Complexation and toxicity of copper in higher plants. i. characterization of copper accumulation, speciation, and toxicity in Crassula helmsii as a new copper accumulator. Plant Physiol. 2009, 151, 702–714.
- Ravet, K.; Pilon, M. Copper and iron homeostasis in plants: The challenges of oxidative stress. Antioxid. Redox Signal. 2013, 19, 919–932.
- Pilon, M.; Abdel-Ghany, S.E.; Cohu, C.M.; Gogolin, K.A.; Ye, H. Copper cofactor delivery in plant cells. Curr. Opin. Plant Biol. 2006, 9, 256–263.
- Burkhead, J.L.; Gogolin Reynolds, K.A.; Abdel-Ghany, S.E.; Cohu, C.M.; Pilon, M. Copper homeostasis. New Phytol. 2009, 182, 799–816.
- Bernal, M.; Casero, D.; Singh, V.; Wilson, G.T.; Grande, A.; Yang, H.; Dodani, S.C.; Pellegrini, M.; Huijser, P.; Connolly, E.L.; et al. Transcriptome sequencing identifies SPL7-regulated copper acquisition genes FRO4/FRO5 and the copper dependence of iron homeostasis in Arabidopsis. Plant Cell 2012, 24, 738–761.
- Song, Y.; Zhou, L.; Yang, S.; Wang, C.; Zhang, T.; Wang, J. Dose-dependent sensitivity of Arabidopsis thaliana seedling root to copper is regulated by auxin homeostasis. Environ. Exp. Bot. 2017, 139, 23–30.
- Liu, J.; Wang, J.; Lee, S.; Wen, R. Copper-caused oxidative stress triggers the activation of antioxidant enzymes via ZmMPK3 in maize leaves. PLoS ONE 2018, 13, e0203612.
- Moravcová, Š.; Tůma, J.; Dučaiová, Z.K.; Waligórski, P.; Kula, M.; Saja, D.; Słomka, A.; Bąba, W.; Libik-Konieczny, M. Influence of salicylic acid pretreatment on seeds germination and some defence mechanisms of Zea mays plants under copper stress. Plant Physiol. Biochem. 2018, 122, 19–30.
- Pätsikkä, E.; Kairavuo, M.; Sersen, F.; Aro, E.M.; Tyystjärvi, E. Excess copper predisposes photosystem II to photoinhibition in vivo by outcompeting iron and causing decrease in leaf chlorophyll. Plant Physiol. 2002, 129, 1359–1367.
- Nielsen, H.D.; Brownlee, C.; Coelho, S.M.; Brown, M.T. Inter-population differences in inherited copper tolerance involve photosynthetic adaptation and exclusion mechanisms in Fucus serratus. New Phytol. 2003, 160, 157–165.
- Demirevska-Kepova, K.; Simova-Stoilova, L.; Stoyanova, Z.; Hölzer, R.; Feller, U. Biochemical changes in barley plants after excessive supply of copper and manganese. Environ. Exp. Bot. 2004, 52, 253–266.
- Drazkiewicz, M.; Skórzyńska-Polit, E.; Krupa, Z. Copper-induced oxidative stress and antioxidant defence in Arabidopsis thaliana. Biometals 2004, 17, 379–387.
- Wang, P.; De Schamphelaere, K.A.; Kopittke, P.M.; Zhou, D.M.; Peijnenburg, W.J.; Lock, K. Development of an electrostatic model predicting copper toxicity to plants. J. Exp. Bot. 2012, 63, 659–668.
- Navari-Izzo, F.; Cestone, B.; Cavallini, A.; Natali, L.; Giordani, T.; Quartacci, M.F. Copper excess triggers phospholipase D activity in wheat roots. Phytochemistry 2006, 67, 1232–1242.
- Zhan, E.; Zhou, H.; Li, S.; Liu, L.; Tan, T.; Lin, H. OTS1-dependent deSUMOylation increases tolerance to high copper levels in Arabidopsis. J. Integr. Plant Biol. 2018, 60, 310–322.
- Clemens, S. Molecular mechanisms of plant metal tolerance and homeostasis. Planta 2001, 212, 475–486.
- Sandalio, L.M.; Dalurzo, H.C.; Gómez, M.; Romero-Puertas, M.C.; del Río, L.A. Cadmium-induced changes in the growth and oxidative metabolism of pea plants. J. Exp. Bot. 2001, 52, 2115–2126.
- Li, M.; Xu, G.; Xia, X.; Wang, M.; Yin, X.; Zhang, B.; Zhang, X.; Cui, Y. Deciphering the physiological and molecular mechanisms for copper tolerance in autotetraploid Arabidopsis. Plant Cell Rep. 2017, 36, 1585–1597.
- García-Giménez, J.L.; Markovic, J.; Dasí, F.; Queval, G.; Schnaubelt, D.; Foyer, C.H.; Pallardó, F.V. Nuclear glutathione. Biochim. Biophys. Acta 2013, 1830, 3304–3316.
- Zetterström, R.C. Eijkman (1858–1930) and Sir F.G. Hopkins (1861–1947): The dawn of vitamins and other essential nutritional growth factors. Acta Paediatr. 2006, 95, 1331–1333.
- Meyer, A.J. The integration of glutathione homeostasis and redox signaling. J. Plant Physiol. 2008, 165, 1390–1403.
- Xiang, C.; Werner, B.L.; Christensen, E.M.; Oliver, D.J. The biological functions of glutathione revisited in arabidopsis transgenic plants with altered glutathione levels. Plant Physiol. 2001, 126, 564–574.
- Kocsy, G.; Pál, M.; Soltész, A.; Szalai, G.; Boldizsár, Á.; Kovács, V.; Janda, T. Low temperature and oxidative stress in cereals. Acta Agron. Hung. 2011, 59, 169–189.
- Ding, S.; Lei, M.; Lu, Q.; Zhang, A.; Yin, Y.; Wen, X.; Zhang, L.; Lu, C. Enhanced sensitivity and characterization of photosystem II in transgenic tobacco plants with decreased chloroplast glutathione reductase under chilling stress. Biochim. Biophys. Acta 2012, 1817, 1979–1991.
- Hasanuzzaman, M.; Bhuyan, M.; Anee, T.I.; Parvin, K.; Nahar, K.; Mahmud, J.A.; Fujita, M. Regulation of ascorbate-glutathione pathway in mitigating oxidative damage in plants under abiotic stress. Antioxidants 2019, 8, 384.
- Most, P.; Papenbrock, J. Possible roles of plant sulfurtransferases in detoxification of cyanide, reactive oxygen species, selected heavy metals and arsenate. Molecules 2015, 20, 1410–1423.
- Borgohain, P.; Saha, B.; Agrahari, R.; Chowardhara, B.; Sahoo, S.; van der Vyver, C.; Panda, S.K. SlNAC2 overexpression in Arabidopsis results in enhanced abiotic stress tolerance with alteration in glutathione metabolism. Protoplasma 2019, 256, 1065–1077.
- Song, F.M.; Ge, X.C.; Zheng, Z. Changes of glutathione contents in cotton seedlings infected by Fusarium oxysporum f. sp. vasinfectum and its relationship to disease resistance. J. Zhejiang Univ.-Agric. Life Sci. 2001, 27, 615–618.
- Mullineaux, P.M.; Rausch, T. Glutathione, photosynthesis and the redox regulation of stress-responsive gene expression. Photosynth. Res. 2005, 86, 459–474.
- Yang, J.; Gao, M.X.; Hu, H.; Ding, X.M.; Lin, H.W.; Wang, L.; Xu, J.M.; Mao, C.Z.; Zhao, F.J.; Wu, Z.C. OsCLT1, a CRT-like transporter 1, is required for glutathione homeostasis and arsenic tolerance in rice. New Phytol. 2016, 211, 658–670.
- Grill, E.; Löffler, S.; Winnacker, E.L.; Zenk, M.H. Phytochelatins, the heavy-metal-binding peptides of plants, are synthesized from glutathione by a specific gamma-glutamylcysteine dipeptidyl transpeptidase (phytochelatin synthase). Proc. Natl. Acad. Sci. USA 1989, 86, 6838–6842.
- Wang, P.; Yin, L.; Liang, D.; Li, C.; Ma, F.; Yue, Z. Delayed senescence of apple leaves by exogenous melatonin treatment: Toward regulating the ascorbate-glutathione cycle. J. Pineal Res. 2012, 53, 11–20.
- Musgrave, W.B.; Yi, H.; Kline, D.; Cameron, J.C.; Wignes, J.; Dey, S.; Pakrasi, H.B.; Jez, J.M. Probing the origins of glutathione biosynthesis through biochemical analysis of glutamate-cysteine ligase and glutathione synthetase from a model photosynthetic prokaryote. Biochem. J. 2013, 450, 63–72.
- Lin, T.H.; Rao, M.Y.; Lu, H.W.; Chiou, C.W.; Lin, S.T.; Chao, H.W.; Zheng, Z.L.; Cheng, H.C.; Lee, T.M. A role for glutathione reductase and glutathione in the tolerance of Chlamydomonas reinhardtii to photo-oxidative stress. Physiol. Plant. 2018, 162, 35–48.
- Pilon-Smits, E.A.H.; Zhu, Y.L.; Sears, T.; Terry, N. Overexpression of glutathione reductase in Brassica juncea: Effects on cadmium accumulation and tolerance. Physiol. Plant. 2010, 110, 455–460.
- Kouřil, R.; Lazar, D.; Lee, H.; Jo, J.; Nauš, J. Moderately elevated temperature eliminates resistance of rice plants with enhanced expression of glutathione reductase to intensive photooxidative stress. Photosynthetica 2003, 41, 571–578.
- Chen, Y.P.; Xing, L.P.; Wu, G.J.; Wang, H.Z.; Wang, X.E.; Cao, A.Z.; Chen, P.D. Plastidial glutathione reductase from Haynaldia villosa is an enhancer of powdery mildew resistance in wheat (Triticum aestivum). Plant Cell Physiol. 2007, 48, 1702–1712.
- Chmielowska-Bąk, J.; Gzyl, J.; Rucińska-Sobkowiak, R.; Arasimowicz-Jelonek, M.; Deckert, J. The new insights into cadmium sensing. Front. Plant Sci. 2014, 5, 245.
- Yin, L.; Mano, J.; Tanaka, K.; Wang, S.; Zhang, M.; Deng, X.; Zhang, S. High level of reduced glutathione contributes to detoxification of lipid peroxide-derived reactive carbonyl species in transgenic Arabidopsis overexpressing glutathione reductase under aluminum stress. Physiol. Plant. 2017, 161, 211–223.
- Cruz-Rus, E.; Amaya, I.; Valpuesta, V. The challenge of increasing vitamin C content in plant foods. Biotechnol. J. 2012, 7, 1110–1121.




