Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Dirk Janssen | + 2424 word(s) | 2424 | 2022-02-18 04:27:50 | | | |
| 2 | Catherine Yang | -2 word(s) | 2422 | 2022-02-28 02:29:42 | | | | |
| 3 | Catherine Yang | Meta information modification | 2422 | 2022-02-28 07:53:57 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Janssen, D. ToLCNDV. Encyclopedia. Available online: https://encyclopedia.pub/entry/19910 (accessed on 07 February 2026).
Janssen D. ToLCNDV. Encyclopedia. Available at: https://encyclopedia.pub/entry/19910. Accessed February 07, 2026.
Janssen, Dirk. "ToLCNDV" Encyclopedia, https://encyclopedia.pub/entry/19910 (accessed February 07, 2026).
Janssen, D. (2022, February 25). ToLCNDV. In Encyclopedia. https://encyclopedia.pub/entry/19910
Janssen, Dirk. "ToLCNDV." Encyclopedia. Web. 25 February, 2022.
Copy Citation
The tomato leaf curl New Delhi virus (ToLCNDV) is a bipartite, single-stranded begomovirus that was first identified in India in 1995 affecting solanaceous crops. A different strain, named ToLCNDV-ES, was introduced in Spain in 2012 and causes severe symptoms in zucchini crops. Virus transmission experiments with the whitefly Bemisia tabaci, were used to compare the transmission parameters in zucchini and tomato plants.
Bemisia tabaci
tomato
zucchini
begomovirus
epidemiology
pest control
1. Introduction
The tomato leaf curl New Delhi virus (ToLCNDV) is a bipartite, single-stranded DNA begomovirus (genus Begomovirus, family Geminiviridae) that was first identified in India in 1995 affecting solanaceous crops [1] and thereafter causing major damage to cucurbit crops on the Indian subcontinent [2]. ToLCNDV was first detected in Europe in 2012, affecting zucchini squash, melon, and to a lesser degree tomato, in Spain [3][4]. Subsequently, the virus was reported from Estonia, Greece, Italy, Portugal, Spain, Tunisia, Morocco, and Algeria [5]. The latest studies of ToLCNDV from Spanish isolates provide evidence that it is a new strain, denominated ToLCNDV-ES, that may have evolved by recombination [6][7]. Since the apparent speed by which ToLCNDV spreads to different countries and the extent of several outbreaks in countries such as Spain and Italy [8][9], the virus is listed as a quarantine pest by EPPO [5]. Symptoms that are produced by ToLCNDV-ES in zucchini are curling, chlorosis and vein thickening of leaves, stunted growth, and fruit deformation and abortion, whereas in tomato the symptoms in leaves are reminiscent of tomato yellow leaf curl disease [6]. ToLCNDV-ES genome titers that are detected are significantly lower in tomato than in zucchini plants, which may be related to the dissimilarities in symptom expression, the possibility of detection, and transmission of the virus [10]. Although ToLCNDV transmission through seeds and mechanical transmission has been experimentally shown to be possible, they are expected to be of minor significance under field conditions [11]. Instead, ToLCNDV-ES is reported to be transmitted by Mediterranean-Q1 Bemisia tabaci cryptic species in Spain, and probably by the Med-Q2 species in Italy [12][13].
The Mediterranean country of Spain has over 55,000 ha of tomato, 18,000 ha of muskmelon, and 11,000 ha of zucchini (data from 2020 from https://www.mapa.gob.es). Significant portions of these crop surfaces are very near to one another and are located within the same regions such as the southeast of Spain, where ToLCNDV-ES and its vector, B. tabaci cryptic species Q1 are highly adapted [12]. The presence of a potentially broad host range within the same geographic region jeopardizes economically important crops in the region [14] and could make the control of plant viruses difficult. B. tabaci-transmission of ToLCNDV-ES between tomato and zucchini has been reported [6], but details on the major parameters of the transmission and unknown. Viruses that belong to member species of the genus Begomovirus are considered as being transmitted in a non-propagative, persistent, circulative manner by the whitefly B. tabaci. Circulative, non-propagative viruses do not replicate in vector tissues but traverse the insect gut, hemolymph, and salivary tissue membranes to reach the salivary glands for transmission [15]. Following the acquisition, virions from many begomovirus species can be detected often in the whitefly vector for its entire life [16], yet the efficiency of transmission decreases with an increase in whitefly age and is negatively correlated with the amount of virus that is detectable in the vector.
Differences in the transmission of ToLCNDV-ES between different host species could yield epidemiological knowledge that could predict the success of control measures within multicrop horticulture regions. This also refers to the management of infected residues where plants that are removed from greenhouses after the crop harvest has finished are often loaded with B. tabaci immature stages. Traditionally these crop remains are considered to be a source of infection, but the infectivity for ToLCNDV of emerging B. tabaci adults has not been proven. This particular aspect of virus retention in the whitefly is not only important for efficient management of the short-distance spread of the virus, but it also would be relevant to its long-distance spread. In fact, as part of the regulation of the virus under Commission Implementing Regulation (EU) 2019/2072, the main pathway that was identified for the long-distance spread of the virus and of entry in new geographic regions are plants for planting of susceptible hosts and consist of commodities carrying viruliferous B. tabaci.
2. ToLCNDV Accumulation in Zucchini, Tomato, and B. tabaci
The mean relative accumulation of ToLCNDV was 3.08 × 1010 in zucchini and 6.25 × 104 in tomato plants. All plants that were infected with ToLCNDV had detectable amounts of the virus. The viral loads of 10 infected zucchini plants ranged from 6.11 × 109 to 4.39 × 1011, with a mean value of 7.86 × 1010. All 50 B. tabaci adults that were collected from these ToLCNDV-infected zucchini plants were positive for the virus with viral loads ranging from 7.64 × 104 to 2.47 × 1010, with a mean value of 3.49 × 109. A minimum viral load of 105 was found in 98% of these whiteflies. When leaves containing the immature stages of the vector were removed and dried, emerging adults were collected and the ToLCNDV was determined. From 50 emerging adults, 40 (80%) of those that were tested were positive and 10 (20%) were negative. The positive B. tabaci adults had viral loads ranging from 1.82 × 102 to 1.51 × 107. Among the emerging vectors from zucchini leaves, 13 adults had a viral load that was higher than 105, which is equivalent to 26% of all emerging individuals of infected zucchini (Figure 1).
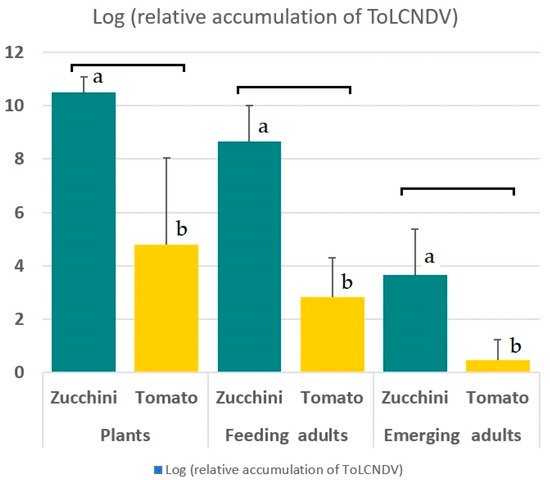
Figure 1. Log-transformed ToLCNDV accumulation in infected zucchini and tomato plants, in B. tabaci adults feeding on infected plants, and in adults emerging from pupae that were collected from dried leaves of infected zucchini and tomato plants. The mean values of 50 replicates in each group; the bars represent S.D. The letters represent the statistically significant difference (p < 0.05) using the Wilcoxon test.
All the tomato plants that were infected with ToLCNDV had detectable amounts of the virus, but the amounts were very variable and ranged from 7.54 × 101 to 1.93 × 109, with a mean value of 8.92 × 108. In contrast to what was observed in the zucchini, approximately 10% of the 50 B. tabaci adults that were collected from the infected tomato tested negative for ToLCNDV by qPCR. The remaining 90% of the insects had detectable amounts of virus, ranging from 1.41 × 101 to 2.31 × 106, and 95% of these positive insects had viral loads below 105. The Wilcoxon test for the comparison of independent populations comparing the accumulation values of ToLCNDV for adults that were collected from infected zucchini (median relative viral load 9.90 × 108) and from tomato (median relative viral load 2.51 × 103) was significantly different (p < 0.05, with a confidence level of 95.0%). From 50 emerging adults, 13 (26%) out of the 50 that emerged from the dried leaves of infected tomato tested negative. The positive B. tabaci had viral loads ranging from 1.02 to 4.68 × 102 (Figure 1).
3. Research and Findings
The whitefly B. tabaci transmits more than 100 species of viruses to plants. The mode of the transmission is related to the taxonomical status of the viruses and is characterized as semipersistent (criniviruses, ipomoviruses, and torradoviruses) and persistent (begomoviruses) [17]. These viruses generally differ in the values of the transmission parameters, such as the probability of viruliferous insects to infect a plant, the time it takes a vector to ingest virus and to inoculated the virus into plants by feeding, and in the time the whiteflies remain infective following acquisition of the virus. The parameters of transmission of the bipartite begomovirus ToLCNDV-ES by the vector, B. tabaci Med-Q1, in two different crop species, tomato and zucchini have been compared.
A single insect is able to acquire monopartite begomovirus TYLCV and transmit it to tomato plants. The reported minimum AAP and IAP of TYLCV by B. tabaci biotype B (MEAM1) varies from 15 to 60 min and from 15 to 30 min, respectively [18][19][20]. Similar values were reported for other monopartite geminiviruses infecting tomato such as TYLCV Sardinia virus (TYLCSV) from Italy [21]. The mean infectivity of infected B. tabaci adults was retained during 7 to 14 days. In general, begomoviruses are retained in the vector for almost its entire life. Adults of MEAM1 B. tabaci that acquired tomato severe rugose virus (ToSRV) during an AAP of 24 h on infected tomato remained viruliferous for 25 days, the maximum period that the insects survived when kept on cabbage plants that are immune to the virus [22]. Tomato leaf curl Sinaloa virus (TOLSCI) was detected in adults of B. tabaci for up to nine days [23]. However, the squash leaf curl virus (SLCV) was retained in the insect for 26 days [24].
Thus, the maximum retention of 14 days has been measured after a six-hour acquisition period [25]. The retention values of 20 days have also been reported for Chino del tomato virus (CdTV) and tomato yellow vein streak virus (ToYVSV) [26][27], and life-long retention in the vector was reported for tomato yellow leaf curl Thailand virus (TYLCTHV) [28]. Comparisons of the transmission of ToLCNDV-ES and other begomoviruses should be done with caution. Despite differences in vector species, plant host species, and in the experimental circumstances, the values for IAP, AAP, and the persistence of ToLCNDV-ES were similar to those of monopartite and other bipartite begomoviruses. Transmission efficiencies, however, were different, and in other begomovirus pathosystems, this type of difference has been shown to be associated with feeding habits and preferences on the plant hosts that were used for acquisition and transmission [29][30][31][32]. The efficiency is further complicated by differences in the amount and distribution of begomoviruses in the different plant hosts being studied [33]. Also, the presence of selected endosymbionts (ex. Hamiltonella spp.) has been shown to affect the transmission efficiency of begomoviruses [34][35]. Finally, ToLCNDV-ES in Mediterranean countries occurs in cucurbitaceous crops that are potentially co-infected with B. tabaci-transmitted cucurbit yellow stunting disorder crinivirus (CYSDV) and cucumber vein yellowing ipomovirus (CVYV) [36]. These semipersistant transmitted viruses have no latent period after ingestion and the retention in the vector lasts from hours to days, depending on the species [37]. There is no evidence of interference between CYSDV and CVYV in the transmission by B. tabaci [38][39], but the effect of coinfecting plant viruses on vector-transmission has been suggested in other combinations of mixed infections, i.e., of CYSDV and aphid-transmitted watermelon mosaic virus (WMV) [40]. Therefore, the effect of criniviruses and ipomoviruses on the transmission of ToLCNDV-ES requires further investigation.
The efficiency of ToLCNDV-ES transmission by single insects was low in tomato and very high in zucchini. These differences in the inoculation efficiency were used to explain the predominance of different begomoviruses that are acquired at similar rates by the same vector species in the same crop species, such as tomato severe rugose virus (ToSRV) versus tomato golden vein virus (ToSRV) in Brazilian tomato fields [41][42]. But transmission efficiencies may also reflect host plant resistance and the ability of a virus to replicate inside the host plant [43]. The higher the host plant's resistance, the lower the transmission rate, and the lower is the amount of virus that is detected in the host plant. This has been shown for TYLCV where viral DNA accumulation was shown to be lower in the resistant source plants compared with the susceptible plants [44].
ToLCNDV-ES has been found as natural and experimental infections in cucurbit and solanaceous species [4][8]. However, relative incidences of the virus are very different among crops in the same agronomic region of the southeast of Spain. Among commercial crops, the percentages of plants that are infected with ToLCNDV varied from 95% in zucchini, 80% in melon, 50% in cucumber, 0% in watermelon, and 15% in tomato [8]. Viral loads in tomato that were experimentally infected with ToLCNDV-ES were also reduced when compared with zucchini (Figure 1). This conforms to previously published comparisons of the viral loads of ToLCNDV-ES in zucchini and in tomato [10]. As such, both the natural incidences of ToLCNDV-ES in zucchini and tomato, the viral loads in this paper, and those that were published before suggest that these are in line with the significantly different efficiencies of transmission in both crop species. Since the transmission efficiency in zucchini is almost 100% for single insects, and because the disease can be fatal in this crop species, control of ToLCNDV in zucchini is a big challenge and requires efficient physical vector exclusion using greenhouses and careful planning and application of biological and integrated pest management [45][46]. Moreover, the success of these control strategies could change whenever evidence of differences in the ToLCNDV-ES transmission by co-infecting criniviruses or ipomoviruses is obtained, following the future research as suggested above.
Natural incidences of ToLCNDV-ES are lower in commercial tomato crops [8], which is consistent with the reduced transmission efficiency in this host plant. However, crop protection against B. tabaci and ToLCNDV should also be applied with care in tomato because these plants may often be co-infected with other begomoviruses, such as monopartite TYLCV species [10], which may compromise the interpretation of the observed symptoms.
The retention of ToLCNDV in adult vectors to be around between 7 and 14 days was established, but here the researchers also determined a different aspect of virus retention: the researchers showed that 20% of adults that emerged from pupae on drying zucchini plant leaves were actually infective. This ratio is comparable to TYLCV in tomato where 28% of emerging adults were found to be infective [26]. The researchers also established that the amounts of the virus in emerging adults (ranging between approx. 102 and 107), were generally lower than the amounts in the plants (between approx. 109 and 1011). Since the 20% higher virus levels in adults from the dried zucchini leaves were approx. 105 or more, this value may well represent the minimum viral load in vectors that is necessary to achieve a successful infection. In comparison, all single adults that were collected from infected zucchini plants had values above 7 × 104, and 96% of single adults that successfully infected zucchini. In contrast, none of the adults emerging from pupae on the infected dried tomato leaves were able to infect zucchini plants, and 95% of these B. tabaci had viral loads below 105.
The efficiency of transmission and the viral loads in adults that were emerging from pupae may explain why biological control using predators of B. tabaci eggs and immature stages can significantly reduce the short-distance spread of the virus in greenhouses [45][46]. However, it may be also significant in the long-distance control of ToLCNDV-ES, i.e., in the trade and transport of infected plant material, when no B. tabaci adults are spotted, these commodities may contain small-sized immature stages that could well be a source of infection [47]. On the other hand, the infectivity of emerging adults from infected dried plant materials provides evidence that plants that are removed from the crop either during the growing and harvesting stage, or after the crops have finished, should be carefully covered, in sealed boxes, containers, etc., and carefully treated or destroyed, but not exposed or transported as such as that would permit the spread of emerging viruliferous vectors and, consequently, the spread of the virus. So, ToLCNDV-ES management of crops not only should involve hygiene, vector exclusion, and control during the nursery, production, and harvesting stages but also once the production and harvesting have finished and the plants are removed from the field or the greenhouse because even as dried materials, they can contain immature vector stages and produce viruliferous emerging insects.
References
- Padidam, M.; Beachy, R.N.; Fauquet, C.M. Tomato leaf curl geminivirus from India has a bipartite genome and coat protein is not essential for infectivity. J. Gen. Virol. 1995, 76, 25–35.
- Zaidi, S.S.; Martin, D.P.; Amin, I.; Farooq, M.; Mansoor, S. Tomato leaf curl New Delhi virus: A widespread bipartite begomovirus in the territory of monopartite begomoviruses. Mol. Plant Pathol. 2017, 18, 901–911.
- Juárez, M.; Tovar, R.; Fiallo-Olivé, E.; Aranda, M.A.; Gosálvez, B.; Castillo, P.; Moriones, E.; Navas-Castillo, J. First detection of Tomato leaf curl New Delhi virus infecting zucchini in Spain. Plant Dis. 2014, 98, 857.
- Ruiz, M.L.; Simón, A.; Velasco, L.; García, M.C.; Janssen, D. First report of Tomato leaf curl New Delhi virus infecting tomato in Spain. Plant Dis. 2015, 98, 894.
- EPPO (2021) EPPO Global Database Tomato Leaf Curl New Delhi Virus (ToLCNDV). Available online: https://gd.eppo.int/taxon/TOLCND (accessed on 20 December 2021).
- Ruiz, L.; Simon, A.; Velasco, L.; Janssen, D. Biological characterization of Tomato leaf curl New Delhi virus from Spain. Plant Pathol. 2017, 66, 376–382.
- Fortes, I.M.; Sanchez-Campos, S.; Fiallo-Olive, E.; Diaz-Pendon, J.A.; Navas-Castillo, J.; Moriones, E. A novel strain of tomato leaf curl new delhi virus has spread to the mediterranean basin. Viruses 2016, 8, 307.
- Juárez, M.; Rabadan, M.P.; Martinez, L.D.; Tayahi, M.; Grande-Perez, A.; Gomez, P. Natural hosts and genetic diversity of the emerging tomato leaf curl New Delhi virus in Spain. Front. Microbiol. 2019, 10, 140.
- Panno, S.; Caruso, A.G.; Troiano, E.; Luigi, M.; Manglli, A.; Vatrano, T.; Iacono, G.; Marchione, S.; Bertin, S.; Tomassoli, L.; et al. Emergence of tomato leaf curl New Delhi virus in Italy: Estimation of incidence and genetic diversity. Plant Pathol. 2019, 68, 601–608.
- Simón, A.; Ruiz, L.; Velasco, L.; Janssen, D. Absolute Quantification of Tomato leaf curl New Delhi virus Spain strain, ToLCNDV-ES: Virus Accumulation in a Host-Specific Manner. Plant Dis 2018, 102, 165–171.
- Kil, E.-J.; Vo, T.T.B.; Fadhila, C.; Ho, P.T.; Lal, A.; Troiano, E.; Parrella, G.; Lee, S. Seed Transmission of Tomato Leaf Curl New Delhi Virus from Zucchini Squash in Italy. Plants 2020, 9, 563.
- Janssen, D.; Simon, A.; Crespo, O.; Ruiz, L. Genetic population structure of Bemisia tabaci in Spain associated with Tomato leaf curl New Delhi virus. Plant Protect. Sci. 2017, 53, 25–31.
- Bertin, S.; Luigi, M.; Parrella, G.; Giorgini, M.; Davino, S.; Tomassoli, L. Survey of the distribution of Bemisia tabaci (Hemiptera: Aleyrodidae) in Lazio region (Central Italy): A threat for the northward expansion of Tomato leaf curl New Delhi virus (Begomovirus: Geminiviridae) infection. Phytoparasitica 2018, 46, 171–182.
- Moriones, E.; Praveen, S.; Chakraborty, S. Tomato leaf curl New Delhi Virus: An emerging virus complex threatening vegetable and fiber crops. Viruses 2017, 9, 264.
- Ghosh, S.; Ghanim, M. Factors Determining Transmission of Persistent Viruses by Bemisia tabaci and Emergence of New Virus-Vector Relationships. Viruses 2021, 13, 1808.
- Rubinstein, G.; Czosnek, H. Long-term association of tomato yellow leaf curl virus with its whitefly vector Bemisia tabaci: Effect on the insect transmission capacity, longevity and fecundity. J. Gen. Virol. 1997, 78, 2683–2689.
- Navas-Castillo, J.; Fiallo-Olivé, E.; Sánchez-Campos, S. Emerging Virus Diseases Transmitted by Whiteflies. Annu Rev. Phytopathol 2011, 49, 219–248.
- Cohen, S.; Harpaz, I. Periodic, rather than continual acquisition of a new tomato virus by its vector, the tobacco whitefly (Bemisia tabaci Gennadius). Entomol. Exp. Appl. 1964, 7, 155–166.
- Mansour, A.; Al-Musa, A. Tomato yellow leaf curl virus: Host range and virus-vector relationships. Plant Pathol. 1992, 41, 122–125.
- Mehta, P.; Wyman, J.A.; Nakhla, M.K.; Maxwell, S.P. Transmission of tomato yellow leaf curl geminivirus by Bemisia tabaci (Homoptera: Aleyrodidae). J. Econ. Entomol. 1994, 87, 1291–1297.
- Caciagli, P.; Bosco, D. Quantitation over time of tomato yellow leaf curl geminivirus DNA in its whitefly vector. Phytopathology 1997, 87, 610–613.
- Toloy, R.S.; Mituti, T.; Freitas, D.M.S.; Maluta, N.K.P.; Silva, T.N.Z.; Lopes, J.R.S.; Fereres, A.; Marques, J.A. Features of the relationship between Tomato severe rugose begomovirus and Bemisa tabaci MEAM1 reveal that the virus is acquired during a probe lasting only one minute. Eur. J. Plant Pathol. 2018, 151, 541–547.
- Idris, A.M.; Brown, J.K. Sinaloa tomato leaf curl virus geminivirus: Biological and molecular evidence for a new sub-group III virus. Phytopathology 1998, 88, 648–657.
- Cohen, S.; Duffus, J.E.; Larsen, R.C.; Liu, H.; Flock, R.A. Purification, serology and vector relationships of squash leaf curl virus, a whitefly-transmitted geminivirus. Phytopathology 1983, 73, 1669–1673.
- Cohen, S.; Nitzany, F.E. Transmission and host range of Tomato yellow leaf curl virus. Phytopathology 1966, 56, 1127–1131.
- Costa, A.S.; Bennett, C.W. Whitefly-transmitted mosaic of Euphorbia prunifolia. Phytopathology 1950, 40, 266–283.
- Firmino, A.C.; Yuki, V.A.; Moreira, A.G.; Rezende, J.A.M. Tomato yellow vein streak virus: Relationship with Bemisia tabaci biotype B and host range. Sci. Agric. 2009, 66, 793–799.
- Weng, S.H.; Tsai, W.S.; Kenyon, L.; Tsai, C.W. Different transmission efficiencies may drive displacement of tomato begomoviruses in the fields in Taiwan. Ann. Appl. Biol. 2015, 166, 321–330.
- Bedford, I.D.; Briddon, R.W.; Brown, J.K.; Rosell, R.C.; Markham, P.G. Geminivirus transmission and biological characterization of whitefly Bemisia tabaci biotypes from different geographic regions. Ann. Appl. Biol. 1994, 125, 311–325.
- Jiang, Y.X.; de Blas, C.; Barrios, L.; Fereres, A. Correlation between whitefly (Homoptera: Aleyrodidae) feeding behavior and transmission of tomato yellow leaf curl virus. Ann. Entomol. Soc. Am. 2000, 93, 573–579.
- Jiang, Y.X.; de Blas, C.; Bedford, I.D.; Nombela, G.; Muñiz, M. Effect of Bemisia tabaci biotype in the transmission of Tomato yellow leaf curl Sardinia virus (TYLCSV-ES) between tomato and common weeds. Span. J. Agric. Res. 2004, 2, 115–119.
- Sanchez-Campos, S.; Navas-Castillo, J.; Camero, R.; Soria, C.; Diaz, J.A.; Moriones, E. Displacement of tomato yellow leaf curl virus (TYLCV)-Sr by TYLCV-Is in tomato epidemics in Spain. Phytopathology 1999, 89, 1038–1043.
- Azzam, O.; Frazer, J.; de La Rosa, D.; Beaver, J.S.; Alquist, P.; Maxwell, D.P. Transmission and efficient ssDNA accumulation of bean golden mosaic geminivirus require functional coat protein. Virology 1994, 204, 289–296.
- Gottlieb, Y.; Zchori-Fein, E.; Mozes-Daube, N.; Kontsedalov, S.; Skaljac, M.; Brumin, M.; Sobol, I.; Czosnek, H.; Vavre, F.; Fleury, F.; et al. The transmission efficiency of tomato yellow leaf curl virus by the whitefly Bemisia tabaci is correlated with the presence of a specific symbiotic bacterium species. J. Virol. 2010, 84, 9310–9317.
- Su, Q.; Pan, H.; Liu, B.; Chu, D.; Xie, W.; Wu, Q.; Wang, S.; Xu, B.; Zhang, Y. Insect symbiont facilitates vector acquisition, retention, and transmission of plant virus. Sci. Rep. 2013, 3, 1367.
- Velasco, L.; Ruiz, L.; Galipienso, L.; Rubio, L.; Janssen, D. A Historical Account of Viruses in Intensive Horticultural Crops in the Spanish Mediterranean Arc: New Challenges for a Sustainable Agriculture. Agronomy 2020, 10, 860.
- Fiallo-Olivé, E.; Pan, L.L.; Liu, S.S.; Navas-Castillo, J. Transmission of Begomoviruses and Other Whitefly-Borne Viruses: Dependence on the Vector Species. Phytopathology 2020, 110, 10–17.
- Ruiz, L.; Janssen, D.; Martin, G.; Velasco, L.; Segundo, E.; Cuadrado, I.M. Analysis of the temporal and spatial disease progress of Bemisia tabaci-transmitted Cucurbit yellow stunting disorder virus and Cucumber vein yellowing virus in cucumber. Plant Pathol. 2006, 55, 264–275.
- Gil-Salas, F.M.; Morris, J.; Colyer, A.; Budge, G.; Boonham, N.; Cuadrado, I.M.; Janssen, D. Development of real-time RT-PCR assays for the detection of Cucumber vein yellowing virus (CVYV) and Cucurbit yellow stunting disorder virus (CYSDV) in the whitefly vector Bemisia tabaci. J. Virol. Meth. 2007, 146, 45–51.
- Domingo-Calap, M.L.; Moreno, A.B.; Díaz Pendón, J.A.; Moreno, A.; Fereres, A.; López-Moya, J.J. Assessing the Impact on Virus Transmission and Insect Vector Behavior of a Viral Mixed Infection in Melon. Phytopathology 2020, 110, 174–186.
- Macedo, M.A.; Michereff Filho, M.; Navas-Castillo, J.; Inoue-Nagata, A.K. Host range and whitefly transmission efficiency of Tomato severe rugose virus and tomato golden vein virus in tomato plants. Trop. Plant Pathol. 2015, 40, 405–409.
- Fernandes, F.R.; Albuquerque, L.C.; Giordano, L.B.; Boiteux, L.C.; Ávila, A.C.; Inoue-Nagata, A.K. Diversity and prevalence of Brazilian bipartite begomovirus species associated to tomatoes. Virus Genes 2008, 36, 251–258.
- Martin, R.R.; Keese, P.K.; Young, M.J.; Waterhouse, P.M.; Gerlach, W.L. Evolution and molecular biology of luteoviruses. Annu. Rev. Phytopathol. 1990, 28, 341–363.
- Lapidot, M.; Friedmann, M.; Pilowsky, M.; Ben-Joseph, R.; Cohen, S. Effect of Host Plant Resistance to Tomato yellow leaf curl virus (TYLCV) on Virus Acquisition and Transmission by Its Whitefly Vector. Phytopathology 2001, 91, 1209–1213.
- Tellez, M.M.; Simon, A.; Rodriguez, E.; Janssen, D. Control of Tomato leaf curl New Delhi virus in zucchini using the predatory mite Amblyseius swirskii. Biol. Control 2017, 114, 106–113.
- Rodríguez, E.; Téllez, M.M.; Janssen, D. Whitefly control strategies against tomato leaf curl New Delhi virus in greenhouse zucchini. Int. J. Environ. Res. Public Health 2019, 16, 2673.
- Pasquali, S.; Gilioli, G.; Janssen, D.; Winter, S. Optimal strategies for interception, detection, and eradication in plant biosecurity. Risk Anal. 2015, 35, 1663–1673.
More
Information
Subjects:
Agronomy; Plant Sciences; Virology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.7K
Revisions:
3 times
(View History)
Update Date:
28 Feb 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




