
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mohammed Abdullah Alshawsh | + 1865 word(s) | 1865 | 2022-02-24 07:49:26 | | | |
| 2 | Yvaine Wei | Meta information modification | 1865 | 2022-02-28 01:54:58 | | |
Video Upload Options
5-Fluorouracil (5-FU) is a chemotherapeutic medication commonly used to treat colorectal cancer (CRC); however, the drug-associated adverse effects and toxicity have greatly affected its clinical use. Diosmetin, a natural flavonoid, has been shown to inhibit the proliferation of many cancer cells, including CRC cells. The findings suggest that 5-FU/diosmetin combination exhibits synergistic effect against HCT116 cancer cells, and potentially reduces the unfavorable adverse effect of 5-FU while enhancing the anticancer efficacy by inducing apoptosis and interrupting mitosis.
1. Introduction
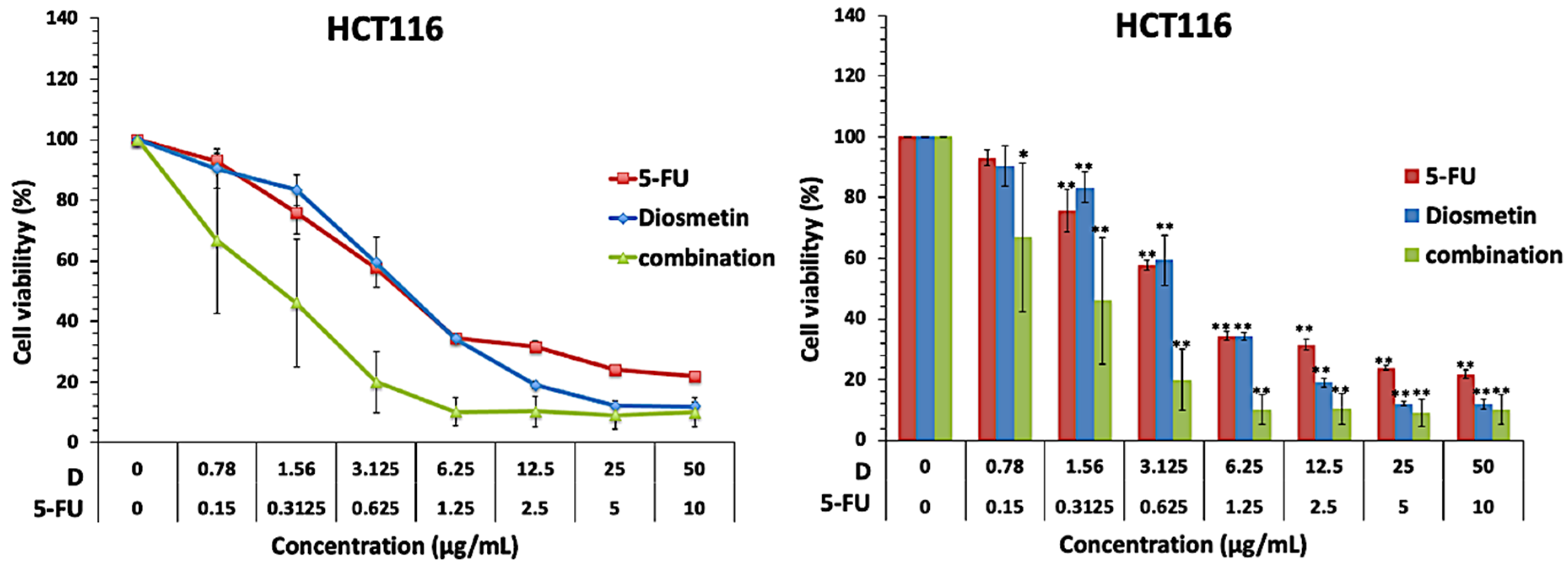
2. Effect of Combination on Cell Proliferation
3. Synergistic Effect of Combination

4. The Synergistic Interactions in Combination Therapy
Combination therapy is based on the positive effects of pharmacodynamic interactions (synergistic or additive) between two or more drugs, with synergistic interactions resulting in more effective treatments. In combination therapy, both compounds are given at lower doses and interact with multiple molecular pathways; therefore, combined treatments based on compounds that exhibit a synergistic or additive effect usually have less toxicity than monotherapy [4]. Combination therapy has shown various advantages over monotherapy, including decreasing drug concentration and toxicity, enhancing the efficacy, targeting several molecular pathways, and sensitizing cells to the treatment [5].
Conventional chemotherapy medications are usually used in combination for the treatment of different types of cancer including CRC, this combination therapy is associated with serious adverse effects. Therefore, introducing bioactive anticancer natural compounds in combination therapy may promote chemotherapy efficacy and reduce the toxic adverse effects. In addition, natural bioactive compounds have more structural diversity, bioactivity, and complexity than synthetic drugs, and can inhibit some targets previously thought to be undruggable. They also inherently target biologically relevant pathways, because most natural bioactive compounds are secondary metabolites or signaling molecules. In addition, there is a limited overlap between the molecular signaling targeted by natural products and those targeted by synthetic drugs. This property not only indicates the potential for novel therapeutic targets for CRC but can also assist to lower the cost of developing new agents by utilizing compounds that already exist in nature and providing another option for combination therapy [11]. Furthermore, patients receiving FOLFOX regimen, which is the most regularly used chemotherapy regimen for the treatment of CRC, usually suffer from different gastrointestinal, neurological, respiratory and skin adverse effects, including hair loss [12]. Another example of conventional chemotherapy combination is the DCF (docetaxel + cisplatin + 5-FU) regimen, which is linked to stomatitis, diarrhoea, nausea, vomiting, neuropathy and associated with high toxicity [13].
5. The Synergistic Interaction between 5-FU and Diosmetin in HCT116 and HT29 Colorectal Cancer Cells
Since the proliferative ability of cancer cells is crucial for the tumor’s growth [14], the findings revealed that different concentrations of 5-FU and diosmetin dose-dependently inhibited the proliferation of HCT116 and HT29 cells. Moreover, based on interaction analysis using different software, combination therapy showed a synergistic effect in HCT116 cells with CI value less than one and synergy score more than 17. The IC50 of 5-FU reduced by 3-folds form 0.83 µg/ml to 0.27 µg/ml, which is favorable to reduce the sever toxicity and adverse effects associated with 5-FU chemotherapy. Other researchers also demonstrated that diosmetin can interact synergistically with anticancer drugs in other cancer cells, for example, diosmetin combined with paclitaxel synergistically induced apoptosis in non-small cell lung cancer cells via Nrf2 inhibition through disruption of PI3K/Akt/GSK-3β pathway [15]. Additionally, diosmetin generated a synergistic cytotoxic effect in HepG2 cells via cytochrome P450, family 1 (CYP1)-catalyzed metabolism, activation of c-Jun N-terminal kinase (JNK)/extracellular signal-regulated kinase (ERK), and p53/p21 overexpression [16].
On the other hand, the combination therapy induced an additive effect in HT29 cells with CI value equal to one and synergy score of −3.824. Although both cell lines are colorectal cancer cells, HT 29 and HCT-116 represent different extents of mutation and differentiation. HT29 is a p53 mutated type and has an intermediate capacity to differentiate into enterocytes and mucin-expressing lineages, while HCT116 is known to be a highly aggressive wild type cell line that shows no ability to differentiate. Different interactions effects of 5-FU and diosmetin combination on these two cancer cell lines could be attributed to the differences in mutation and differentiation, however the additive effect in HT29 cells still contributes to the beneficial effects of this combination. Another possible reason for the different interaction effect in HCT116 and HT29 cells could be due to the differences in their genetic profiles. Sensitive p53 wild type cancer cells such as HCT116 are usually targeted via p53-mediated apoptosis while mutated or null p53 cells such as HT29 cells can be inhibited by drugs that induce p53-independent cell death pathways [17].
Imbalance between proliferation and apoptosis is a major element in the initiation of cancer. Apoptosis serves as a key function in correcting normal tissue stability [18]. Therefore, therapy options that target apoptosis may be effective in preventing the progression of CRC. Apoptosis is characterized by cell shrinkage, chromatin, nuclear condensation, and plasma membrane blebbing [19]. AO/PI staining and Annexin V-FITC were conducted to detect whether the suppression of HCT116 cells proliferation induced by combination therapy was associated with apoptosis. The treated cells exhibited apoptotic characteristics, including membrane blebbing in early apoptosis and chromatin condensation in late apoptosis. Before the cellular membrane disintegrates during early apoptosis, phospholipid asymmetry occurs [20][21]. Phosphatidylserine (PS) translocates to the outer plasma membrane, where it is exposed to the exterior surface. As a result, PS translocation can be used to investigate apoptosis. Annexin V is a calcium-dependent phospholipid-binding protein with a high affinity for PS, and it is frequently used in conjunction with PI (fluorescent dye) to identify apoptotic and necrotic cells [14]. To further quantify the apoptotic HCT116 cells following the treatment with monotherapy and combination, cells were exposed to Annexin V/PI staining and subjected to flow cytometry. Combination therapy significantly increased apoptotic cells to 45%, compared with 5-FU-treated cells, which showed only 24.6% of apoptosis. There were more necrotic cells (34.4%) in HCT116 cells treated with 5-FU than combination-treated cells (19.1%). It has been reported that chemotherapeutic drugs not only trigger apoptosis, but also other types of cell suicide, such as necrosis, which triggers further inflammation. Thus, it is not a preferred pathway for cancer treatment [22]. Therefore, the combination of 5-FU and diosmetin has the advantage to act through activating the apoptosis pathway with less impact on the necrosis pathway compared with 5-FU.
6. Combination Therapy in Anti-Cancer Management
Several dysregulated signaling pathways have been linked to cancer development. Conventional chemotherapy agents have toxicity and severe adverse effects. Therefore, finding new multi-targeted treatment to reduce cancer’s dysregulated signaling is critical [23]. Diosmetin was reported to have a potential effect on signaling pathways involved in colorectal cancer. These pathways include apoptosis, TGF-β/BMP, NF-kB [24], PI3K/AKT [25], and Notch signaling pathways [26]. A combination of diosmetin with chemotherapeutic drug (5-FU) could target multiple signaling pathways and produce a higher response rate against colorectal cancer. Combination therapy has shown to be significantly effective in terms of anti-cancer management. Its superiority arises from its capacity to target many pathways, and reducing drug resistance to a minimum. Pathway dysregulation in cancer cells, as well as the alteration of homeostatic settings, all contribute to the unregulated proliferation. For example, mutations in tumor suppressor genes such as p53, which normally activates cell cycle arrest when DNA is damaged, resulting in the accumulation of damaged DNA and the inhibition of cell cycle arrest, contribute to an increase in the rate of cell proliferation. Additionally, in cancer cells, upregulated autocrine growth factor production or an upregulated autocrine loop can contribute to tumor cell growth [27]. In colon cancer cells, a similar effect can be seen [28]. When it comes to autocrine growth factors, if VEGF is upregulated it can lead to metastasis, which can make the prognosis for survival worse [27][29]. Therefore, targeting several pathways with a multiple-agent combination can enhance the treatment while lowering the risk of cancer cells becoming more aggressive and incurable. In addition, the doses of each drug/compound in combination therapy can be lowered, resulting in fewer adverse effects compared with monotherapy [30]. Another benefit of combination therapy is that different drugs can target the heterogeneous character of tumors, boosting the chances of killing cancer cells, including the cancer stem cell population, which has been linked to drug resistance and cancer recurrence following remission [31][32][33].
7. Conclusions
References
- Ciardiello, D.; Vitiello, P.P.; Cardone, C.; Martini, G.; Troiani, T.; Martinelli, E.; Ciardiello, F. Immunotherapy of colorectal cancer: Challenges for therapeutic efficacy. Cancer Treat. Rev. 2019, 76, 22–32.
- Li, Q.; Wei, L.; Lin, S.; Chen, Y.; Lin, J.; Peng, J. Synergistic effect of kaempferol and 5-fluorouracil on the growth of colorectal cancer cells by regulating the PI3K/Akt signaling pathway. Mol. Med. Rep. 2019, 20, 728–734.
- Ţigu, A.B.; Toma, V.A.; Mot, A.C.; Jurj, A.; Moldovan, C.S.; Fischer-Fodor, E.; Berindan-Neagoe, I.; Pârvu, M. The synergistic antitumor effect of 5-fluorouracil combined with allicin against lung and colorectal carcinoma cells. Molecules 2020, 25, 1947.
- Milczarek, M.; Pogorzelska, A.; Wiktorska, K. Synergistic interaction between 5-fu and an analog of sulforaphane—2-oxohexyl isothiocyanate—in an in vitro colon cancer model. Molecules 2021, 26, 3019.
- Chen, S.J.; Chung, Y.C.; Chang, H.L.; Chang, H.P.; Chou, J.L.; Lin, C.C.; Chen, C.H.; Hsu, C.P. Synergistic Effect of Combined Treatment with Longan Flower Extract and 5-Fluorouracil on Colorectal Cancer Cells. Nutr. Cancer 2020, 72, 209–217.
- Dehghan, R.; Bahreini, F.; Najafi, R.; Saidijam, M.; Amini, R. The Combination of Zerumbone and 5-FU: A Significant Therapeutic Strategy in Sensitizing Colorectal Cancer Cells to Treatment. BioMed Res. Int. 2021, 2021, 6635874.
- Ghosh, S.; Pal, A.; Ray, M. Methylglyoxal in combination with 5-Fluorouracil elicits improved chemosensitivity in breast cancer through apoptosis and cell cycle inhibition. Biomed. Pharmacother. 2019, 114, 108855.
- Xie, Y.H.; Chen, Y.X.; Fang, J.Y. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct. Target. Ther. 2020, 5, 22.
- He, L.; Zhu, H.; Zhou, S.; Wu, T.; Wu, H.; Yang, H.; Mao, H.; SekharKathera, C.; Janardhan, A.; Edick, A.M.; et al. Wnt pathway is involved in 5-FU drug resistance of colorectal cancer cells. Exp. Mol. Med. 2018, 50, 101.
- Chou, T.C. Drug Combination Studies and Their Synergy Quantification Using the Chou-Talalay Method. Cancer Res. 2010, 70, 440–447.
- Chamberlin, S.R.; Blucher, A.; Wu, G.; Shinto, L.; Choonoo, G.; Kulesz-Martin, M.; McWeeney, S. Natural product target network reveals potential for cancer combination therapies. Front. Pharmacol. 2019, 10, 557.
- Kogan, L.G.; Davis, S.L.; Brooks, G.A. Treatment delays during FOLFOX chemotherapy in patients with colorectal cancer: A multicenter retrospective analysis. J. Gastrointest. Oncol. 2019, 10, 841–846.
- Salehifar, E.; Avan, R.; Janbabaei, G.; Mousavi, S.K.; Faramarzi, F. Comparison the incidence and severity of side effects profile of folfox and dcf regimens in gastric cancer patients. Iran. J. Pharm. Res. 2019, 18, 1032–1039.
- Chen, H.M.; Lai, Z.Q.; Liao, H.J.; Xie, J.H.; Xian, Y.F.; Chen, Y.L.; Ip, S.P.; Lin, Z.X.; Su, Z.R. Synergistic antitumor effect of brusatol combined with cisplatin on colorectal cancer cells. Int. J. Mol. Med. 2018, 41, 1447–1454.
- Chen, X.; Wu, Q.; Chen, Y.; Zhang, J.; Li, H.; Yang, Z.; Yang, Y.; Deng, Y.; Zhang, L.; Liu, B. Diosmetin induces apoptosis and enhances the chemotherapeutic efficacy of paclitaxel in non-small cell lung cancer cells via Nrf2 inhibition. Br. J. Pharmacol. 2019, 176, 2079–2094.
- Androutsopoulos, V.P.; Spandidos, D.A. The flavonoids diosmetin and luteolin exert synergistic cytostatic effects in human hepatoma HepG2 cells via CYP1A-catalyzed metabolism, activation of JNK and ERK and P53/P21 up-regulation. J. Nutr. Biochem. 2013, 24, 496–504.
- Kosakowska-Cholody, T.; Cholody, W.; Hariprakasha, H.; Meyer, C.; Michejda, C. Gene expression profiles in HCT116 and HT29 cells exposed to RTA 502 lead to insights into the mechanism of action. Exp. Mol. Ther. 2007, 67, 4895.
- Kroemer, G.; Reed, J.C. Mitochondrial control of cell death. Nat. Med. 2000, 6, 513–519.
- Reed, J.C. Warner-Lambert/Parke Davis award lecture: Mechanisms of apoptosis. Am. J. Pathol. 2000, 157, 1415–1430.
- Fadok, V.A.; De Cathelineau, A.; Daleke, D.L.; Henson, P.M.; Bratton, D.L. Loss of phospholipid asymmetry and surface exposure of phosphatidylserine is required for phagocytosis of apoptotic cells by macrophages and fibroblasts. J. Biol. Chem. 2001, 276, 1071–1077.
- Vermes, I.; Haanen, C.; Steffens-Nakken, H.; Reutellingsperger, C. A novel assay for apoptosis Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J. Immunol. Methods 1995, 184, 39–51.
- Ricci, M.S.; Zong, W.-X. Chemotherapeutic Approaches for Targeting Cell Death Pathways. Oncologist 2006, 11, 342–357.
- Nouri, Z.; Fakhri, S.; Nouri, K.; Wallace, C.E.; Farzaei, M.H.; Bishayee, A. Targeting multiple signaling pathways in cancer: The rutin therapeutic approach. Cancers 2020, 12, 2276.
- Koosha, S.; Mohamed, Z.; Sinniah, A.; Alshawsh, M.A. Investigation into the Molecular Mechanisms underlying the Anti-proliferative and Anti-tumorigenesis activities of Diosmetin against HCT-116 Human Colorectal Cancer. Sci. Rep. 2019, 9, 5148.
- Zhang, T.; Ma, Y.; Fang, J.; Liu, C.; Chen, L. A Deregulated PI3K-AKT Signaling Pathway in Patients with Colorectal Cancer. J. Gastrointest. Cancer 2019, 50, 35–41.
- Qiao, J.; Liu, J.; Jia, K.; Li, N.; Liu, B.; Zhang, Q.; Zhu, R. Diosmetin triggers cell apoptosis by activation of the p53/Bcl-2 pathway and inactivation of the notch3/Nf-κB pathway in HepG2 cells. Oncol. Lett. 2016, 12, 5122–5128.
- Morfoisse, F.; Kuchnio, A.; Frainay, C.; Gomez-Brouchet, A.; Delisle, M.B.; Marzi, S.; Helfer, A.C.; Hantelys, F.; Pujol, F.; Guillermet-Guibert, J.; et al. Hypoxia induces VEGF-C expression in metastatic tumor cells via a HIF-1α-independent translation-mediated mechanism. Cell Rep. 2014, 6, 155–167.
- Ruan, W.J.; Lai, M.D. Autocrine stimulation in colorectal carcinoma (CRC): Positive autocrine loops in human colorectal carcinoma and applicable significance of blocking the loops. Med. Oncol. 2004, 21, 1–8.
- Xia, H.; Shen, J.; Chen, S.; Huang, H.; Xu, Y.; Ma, H. Overexpression of VEGF-C correlates with a poor prognosis in esophageal cancer patients. Cancer Biomarkers 2016, 17, 165–170.
- Mokhtari, R.B.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination therapy in combating cancer. Oncotarget 2017, 8, 38022–38043.
- Wang, T.; Narayanaswamy, R.; Ren, H.; Torchilin, V.P. Combination therapy targeting both cancer stem-like cells and bulk tumor cells for improved efficacy of breast cancer treatment. Cancer Biol. Ther. 2016, 17, 698–707.
- Yuan, S.; Wang, F.; Chen, G.; Zhang, H.; Feng, L.; Wang, L.; Colman, H.; Keating, M.J.; Li, X.; Xu, R.H.; et al. Effective elimination of cancer stem cells by a novel drug combination strategy. Stem Cells 2013, 31, 23–34.
- Eyler, C.E.; Rich, J.N. Survival of the fittest: Cancer stem cells in therapeutic resistance and angiogenesis. J. Clin. Oncol. 2008, 26, 2839–2845.




