
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | ALEXANDROS GEORGAKILAS | + 2641 word(s) | 2641 | 2022-02-24 09:31:30 | | | |
| 2 | Lindsay Dong | -27 word(s) | 2614 | 2022-02-25 03:56:44 | | |
Video Upload Options
The Australian Synchrotron (AS) and the European Synchrotron Radiation Facility (ESRF) are best configured for a wide range of biomedical research involving animals and future cancer patients. Due to ultra-high dose rates, treatment doses can be delivered within milliseconds, abiding by FLASH radiotherapy principles. In addition, a homogeneous radiation field can be spatially fractionated into a geometric pattern called microbeam radiotherapy (MRT); a coplanar array of thin beams of microscopic dimensions. Both are clinically promising radiotherapy modalities because they trigger a cascade of biological effects that improve tumor control, while increasing normal tissue tolerance compared to conventional radiation. Synchrotrons can deliver high doses to a very small volume with low beam divergence, thus facilitating the study of non-targeted effects of these novel radiation modalities in both in-vitro and in-vivo models. Non-targeted radiation effects studied at the AS and ESRF include monitoring cell–cell communication after partial irradiation of a cell population (radiation-induced bystander effect, RIBE), the response of tissues outside the irradiated field (radiation-induced abscopal effect, RIAE), and the influence of irradiated animals on non-irradiated ones in close proximity (inter-animal RIBE).
1. Introduction
2. Characteristics of Synchrotron X-rays at AS and ESRF
-
The high brightness, or brilliance, which describes synchrotron radiation power. Brilliance measures the source quality and implicates the number of photons produced per second. The higher the brilliance value, the stronger the emitted beam.
-
The low divergence of the synchrotron beam enables the irradiation of the target with collimated parallel microbeams, in contrast to conventional RT. The low divergence results from the fact that the target is several meters away from the permanent magnet wiggler which generates the X-ray beam. At the AS, the distance between the wiggler and the target is about 32 m, while at the ESRF it is 40 m.
-
The beam current, which is the basic quantity of the beam. In this regard, the ESRF’s characteristics are superior to those of the AS. The ESRF has a significantly longer periphery of 844 m, while the AS has 200 m. The brilliance values are 8 × 1020 and 4.6 × 1018 photons/(s × 0.1% bandwidth × mrad2) for the ESRF and AS, respectively [4]. Both have a beam current of 200 mA; however, the maximum electron energy is 6 GeV for ESRF and 3 GeV for AS.
-
The intense flux (dose rate) of photons allows samples to be irradiated very quickly in the range of seconds and milliseconds. Dose rates in the AS range from 30–1000 Gy/s, while at the ESRF can be up to 16,000 Gy/s.
-
The energy of the synchrotron beam is in the KeV range, which has the advantage of allowing for low secondary electron (Compton) scattering. The energy range at both the AS and ESRF is “tunable”, meaning, the energy spectrum can be filtered to remove low energy photons and use a poly energetic X-ray beam typically between 30 and 120 KeV.
3. Synchrotron-Generated Novel RT Modalities
3.1. Microbeam Radiation Therapy (MRT)
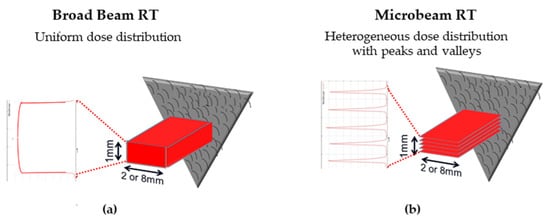
-
The main clinical research focus at the IMBL, AS, are RT and imaging. Imaging can be performed in an energy range of 15–150 KeV, while radiotherapy can be performed in an energy range of 30–120 KeV and with dose rates close to 1000 Gy/s [7]. The radiation source of MRT is a wiggler with a peak magnetic field of 4.2 T [8]. Starting from a storage ring current of 200 mA, a standard MRT spectral configuration can be delivered in 300 Gy/s by setting the wiggler to 3 T, which produces a spectrum with an average energy of 94 KeV and a peak energy of 87 KeV. Another MRT configuration can deliver 991.7 Gy/s by setting the wiggler to 4 T, with similar spectra as above (93 KeV on average) [9].
-
The ESRF’s beamline ID17 is also intended for medical imaging and RT studies. The radiation used for MRT comes from a wiggler of 1.5 m in length and a magnetic field of 1.6 T at a gap of 24.8 mm. The X-ray beam produced by the wiggler then continues for 37 m until it is attenuated to produce one of three different spectral configurations: (i) the standard MRT configuration used for rodent experiments for many years has an average energy of 104.2 KeV and a peak energy of 87.7 keV, (ii) the preclinical MRT configuration developed for veterinary trials and has a mean energy of 119 KeV and a peak energy of 102.1 KeV, and/or (iii) the clinical MRT configuration for future clinical applications with a mean energy of 122.8 KeV and a peak energy of 108.2 KeV [3][10].
3.2. Ultra-High Dose Rate Radiotherapy (FLASH-RT)
3.3. MRT Delivered in a FLASH Mode
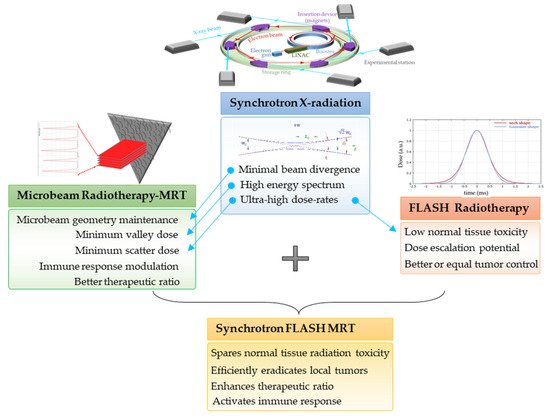
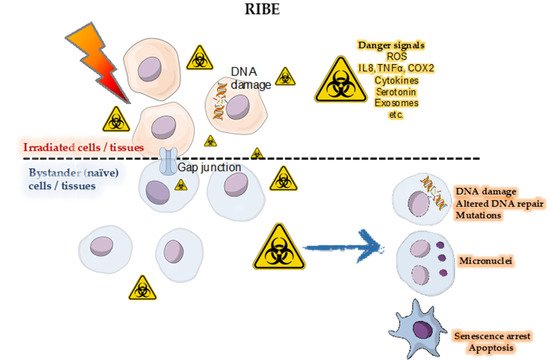
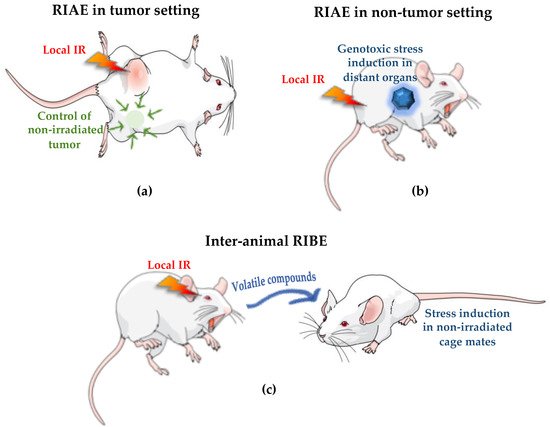
4. Radiation-Induced Bystander and Abscopal Effects
4.1. Studies of RIBE and RIAE and Their Mediators
4.2. First RIBE Studies at Synchrotrons
5. Conclusions
References
- Pełka, J. Synchrotron radiation in biology and medicine. Acta Phys. Pol. A 2008, 2, 309–329.
- Stevenson, A.W.; Crosbie, J.C.; Hall, C.J.; Hausermann, D.; Livingstone, J.; Lye, J.E. Quantitative characterization of the X-ray beam at the Australian Synchrotron Imaging and Medical Beamline (IMBL). J. Synchrotron Radiat. 2017, 24, 110–141.
- Pellicioli, P.; Donzelli, M.; Davis, J.A.; Esteve, F.; Hugtenburg, R.; Guatelli, S.; Petasecca, M.; Lerch, M.L.F.; Brauer-Krisch, E.; Krisch, M. Study of the X-ray radiation interaction with a multislit collimator for the creation of microbeams in radiation therapy. J. Synchrotron Radiat. 2021, 28, 392–403.
- Bharti, A.; Goyal, N. Fundamental of Synchrotron Radiations. In Synchrotron Radiation-Useful and Interesting Applications; IntechOpen: London, UK, 2019.
- Laissue, J.A.; Geiser, G.; Spanne, P.O.; Dilmanian, F.A.; Gebbers, J.O.; Geiser, M.; Wu, X.Y.; Makar, M.S.; Micca, P.L.; Nawrocky, M.M.; et al. Neuropathology of ablation of rat gliosarcomas and contiguous brain tissues using a microplanar beam of synchrotron-wiggler-generated X rays. Int. J. Cancer 1998, 78, 654–660.
- Sprung, C.N.; Cholewa, M.; Usami, N.; Kobayashi, K.; Crosbie, J.C. DNA damage and repair kinetics after microbeam radiation therapy emulation in living cells using monoenergetic synchrotron X-ray microbeams. J. Synchrotron Radiat. 2011, 18, 630–636.
- Hausermann, D.; Hall, C.; Maksimenko, A.; Campbell, C. The Imaging and Medical Beam Line at the Australian Synchrotron. AIP Conf. Proc. 2010, 1266, 3–9.
- Livingstone, J.; Adam, J.-F.; Crosbie, J.C.; Hall, C.J.; Lye, J.E.; McKinlay, J.; Pelliccia, D.; Pouzoulet, F.; Prezado, Y.; Stevenson, A.W. Preclinical radiotherapy at the Australian Synchrotron’s Imaging and Medical Beamline: Instrumentation, dosimetry and a small-animal feasibility study. J. Synchrotron Radiat. 2017, 24, 854–865.
- Trappetti, V.; Fernandez-Palomo, C.; Smyth, L.; Klein, M.; Haberthur, D.; Butler, D.; Barnes, M.; Shintani, N.; de Veer, M.; Laissue, J.A.; et al. Synchrotron Microbeam Radiotherapy for the treatment of lung carcinoma: A pre-clinical study. Int. J. Radiat. Oncol Biol. Phys. 2021.
- Crosbie, J.C.; Fournier, P.; Bartzsch, S.; Donzelli, M.; Cornelius, I.; Stevenson, A.W.; Requardt, H.; Brauer-Krisch, E. Energy spectra considerations for synchrotron radiotherapy trials on the ID17 bio-medical beamline at the European Synchrotron Radiation Facility. J. Synchrotron Radiat. 2015, 22, 1035–1041.
- Duncan, M.; Donzelli, M.; Pellicioli, P.; Brauer-Krisch, E.; Davis, J.A.; Lerch, M.L.F.; Rosenfeld, A.B.; Petasecca, M. First experimental measurement of the effect of cardio-synchronous brain motion on the dose distribution during microbeam radiation therapy. Med. Phys. 2020, 47, 213–222.
- Bourhis, J.; Montay-Gruel, P.; Goncalves Jorge, P.; Bailat, C.; Petit, B.; Ollivier, J.; Jeanneret-Sozzi, W.; Ozsahin, M.; Bochud, F.; Moeckli, R.; et al. Clinical translation of FLASH radiotherapy: Why and how? Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2019, 139, 11–17.
- Montay-Gruel, P.; Bouchet, A.; Jaccard, M.; Patin, D.; Serduc, R.; Aim, W.; Petersson, K.; Petit, B.; Bailat, C.; Bourhis, J.; et al. X-rays can trigger the FLASH effect: Ultra-high dose-rate synchrotron light source prevents normal brain injury after whole brain irradiation in mice. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2018, 129, 582–588.
- de Kruijff, R.M. FLASH radiotherapy: Ultra-high dose rates to spare healthy tissue. Int. J. Radiat. Biol. 2020, 96, 419–423.
- Favaudon, V.; Caplier, L.; Monceau, V.; Pouzoulet, F.; Sayarath, M.; Fouillade, C.; Poupon, M.F.; Brito, I.; Hupe, P.; Bourhis, J.; et al. Ultrahigh dose-rate FLASH irradiation increases the differential response between normal and tumor tissue in mice. Sci. Transl. Med. 2014, 6, 245ra293.
- Vozenin, M.C.; De Fornel, P.; Petersson, K.; Favaudon, V.; Jaccard, M.; Germond, J.F.; Petit, B.; Burki, M.; Ferrand, G.; Patin, D.; et al. The Advantage of FLASH Radiotherapy Confirmed in Mini-pig and Cat-cancer Patients. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019, 25, 35–42.
- Eling, L.; Bouchet, A.; Nemoz, C.; Djonov, V.; Balosso, J.; Laissue, J.; Bräuer-Krisch, E.; Adam, J.F.; Serduc, R. Ultra high dose rate Synchrotron Microbeam Radiation Therapy. Preclinical evidence in view of a clinical transfer. Radiother. Oncol. 2019, 139, 56–61.
- Schultke, E.; Balosso, J.; Breslin, T.; Cavaletti, G.; Djonov, V.; Esteve, F.; Grotzer, M.; Hildebrandt, G.; Valdman, A.; Laissue, J. Microbeam radiation therapy—Grid therapy and beyond: A clinical perspective. Br. J. Radiol. 2017, 90, 20170073.
- Smyth, L.M.; Senthi, S.; Crosbie, J.C.; Rogers, P.A. The normal tissue effects of microbeam radiotherapy: What do we know, and what do we need to know to plan a human clinical trial? Int. J. Radiat. Biol. 2016, 92, 302–311.
- Fernandez-Palomo, C.; Fazzari, J.; Trappetti, V.; Smyth, L.; Janka, H.; Laissue, J.; Djonov, V. Animal Models in Microbeam Radiation Therapy: A Scoping Review. Cancers 2020, 12, 527.
- Dilmanian, F.A.; Qu, Y.; Feinendegen, L.E.; Pena, L.A.; Bacarian, T.; Henn, F.A.; Kalef-Ezra, J.; Liu, S.; Zhong, Z.; McDonald, J.W. Tissue-sparing effect of x-ray microplanar beams particularly in the CNS: Is a bystander effect involved? Exp. Hematol. 2007, 35, 69–77.
- Potez, M.; Fernandez-Palomo, C.; Bouchet, A.; Trappetti, V.; Donzelli, M.; Krisch, M.; Laissue, J.; Volarevic, V.; Djonov, V. Synchrotron Microbeam Radiation Therapy as a New Approach for the Treatment of Radioresistant Melanoma: Potential Underlying Mechanisms. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 1126–1136.
- Fernandez-Palomo, C.; Trappetti, V.; Potez, M.; Pellicioli, P.; Krisch, M.; Laissue, J.; Djonov, V. Complete Remission of Mouse Melanoma after Temporally Fractionated Microbeam Radiotherapy. Cancers 2020, 12, 2656.
- Hall, E.J. The bystander effect. Health Phys. 2003, 85, 31–35.
- Prise, K.M.; O’Sullivan, J.M. Radiation-induced bystander signalling in cancer therapy. Nat. Rev. Cancer 2009, 9, 351–360.
- Mothersill, C.; Seymour, C. Medium from irradiated human epithelial cells but not human fibroblasts reduces the clonogenic survival of unirradiated cells. Int. J. Radiat. Biol. 1997, 71, 421–427.
- Azzam, E.I.; de Toledo, S.M.; Gooding, T.; Little, J.B. Intercellular communication is involved in the bystander regulation of gene expression in human cells exposed to very low fluences of alpha particles. Radiat. Res. 1998, 150, 497–504.
- Nagasawa, H.; Little, J.B. Induction of sister chromatid exchanges by extremely low doses of alpha-particles. Cancer Res. 1992, 52, 6394–6396.
- Azzam, E.I.; de Toledo, S.M.; Raaphorst, G.P.; Mitchel, R.E. Low-dose ionizing radiation decreases the frequency of neoplastic transformation to a level below the spontaneous rate in C3H 10T1/2 cells. Radiat. Res. 1996, 146, 369–373.
- Mothersill, C.; Seymour, C. Radiation-induced bystander effects: Evidence for an adaptive response to low dose exposures? Dose Response 2006, 4, 283–290.
- Matsumoto, H.; Takahashi, A.; Ohnishi, T. Radiation-induced adaptive responses and bystander effects. Biol. Sci. Space 2004, 18, 247–254.
- Mole, R.H. Whole body irradiation; radiobiology or medicine? Br. J. Radiol. 1953, 26, 234–241.
- Koturbash, I.; Rugo, R.E.; Hendricks, C.A.; Loree, J.; Thibault, B.; Kutanzi, K.; Pogribny, I.; Yanch, J.C.; Engelward, B.P.; Kovalchuk, O. Irradiation induces DNA damage and modulates epigenetic effectors in distant bystander tissue in vivo. Oncogene 2006, 25, 4267–4275.
- Koturbash, I.; Loree, J.; Kutanzi, K.; Koganow, C.; Pogribny, I.; Kovalchuk, O. In vivo bystander effect: Cranial X-irradiation leads to elevated DNA damage, altered cellular proliferation and apoptosis, and increased p53 levels in shielded spleen. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 554–562.
- Mancuso, M.; Pasquali, E.; Leonardi, S.; Tanori, M.; Rebessi, S.; Di Majo, V.; Pazzaglia, S.; Toni, M.P.; Pimpinella, M.; Covelli, V.; et al. Oncogenic bystander radiation effects in Patched heterozygous mouse cerebellum. Proc. Natl. Acad. Sci. USA 2008, 105, 12445–12450.
- Dubrova, Y.E. Radiation-induced transgenerational instability. Oncogene 2003, 22, 7087–7093.
- Tamminga, J.; Koturbash, I.; Baker, M.; Kutanzi, K.; Kathiria, P.; Pogribny, I.P.; Sutherland, R.J.; Kovalchuk, O. Paternal cranial irradiation induces distant bystander DNA damage in the germline and leads to epigenetic alterations in the offspring. Cell Cycle 2008, 7, 1238–1245.
- Hall, E.J. Intensity-modulated radiation therapy, protons, and the risk of second cancers. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 1–7.
- Zhong, N.; Morris, G.M.; Bacarian, T.; Rosen, E.M.; Avraham Dilmanian, F. Response of rat skin to high-dose unidirectional X-ray microbeams: A histological study. Radiat. Res. 2003, 160, 133–142.
- Smilowitz, H.; Blattmann, H.; Bräuer-Krisch, E.; Bravin, A.; Di Michiel, M.; Gebbers, J.-O.; Hanson, A.; Lyubimova, N.; Slatkin, D.; Stepanek, J. Synergy of gene-mediated immunoprophylaxis and microbeam radiation therapy for advanced intracerebral rat 9L gliosarcomas. J. Neuro-Oncol. 2006, 78, 135–143.




