Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Tiago Carvalho | + 2086 word(s) | 2086 | 2021-12-14 05:29:22 | | | |
| 2 | Lindsay Dong | + 178 word(s) | 2264 | 2022-02-24 08:15:46 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Carvalho, T. Tumor Microenvironment Features and in Pancreatic Ductal Adenocarcinoma. Encyclopedia. Available online: https://encyclopedia.pub/entry/19816 (accessed on 07 February 2026).
Carvalho T. Tumor Microenvironment Features and in Pancreatic Ductal Adenocarcinoma. Encyclopedia. Available at: https://encyclopedia.pub/entry/19816. Accessed February 07, 2026.
Carvalho, Tiago. "Tumor Microenvironment Features and in Pancreatic Ductal Adenocarcinoma" Encyclopedia, https://encyclopedia.pub/entry/19816 (accessed February 07, 2026).
Carvalho, T. (2022, February 23). Tumor Microenvironment Features and in Pancreatic Ductal Adenocarcinoma. In Encyclopedia. https://encyclopedia.pub/entry/19816
Carvalho, Tiago. "Tumor Microenvironment Features and in Pancreatic Ductal Adenocarcinoma." Encyclopedia. Web. 23 February, 2022.
Copy Citation
Pancreatic ductal adenocarcinoma (PDAC) has a prominent desmoplastic stromal microenvironment that includes a dense extracellular matrix together with a series of activated cell types, hypoxia, and an acidic extracellular pH.
pancreatic ductal adenocarcinoma
tumor microenvironment
1. Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the fourth leading cause of cancer-related mortality in western countries and is projected to be the second-leading cause of cancer-related death in the United States by 2030 [1]. A prominent feature of the PDAC microenvironment is an extensive desmoplasia that consists of a highly fibrotic and stiff extracellular matrix (ECM) principally composed of collagen I, fibronectin and hyaluronan, which are secreted by alpha-muscle actin-positive fibroblasts (also known as myofibroblasts or activated pancreatic stellate cells (PSCs)) [2]. Modifications that the ECM architecture suffers during cancer progression have been deeply explored, since it has been recognized that atypical ECM architecture influences therapeutic outcomes specifically by modulating (i) tumor biomechanics [3]; (ii) cancer cell migration/invasion [4][5][6]; and (iii) drug penetration into the tumor [7]. The existence of this dense tumor microenvironment (TME) may be the main reason that therapies targeting specifically only cancer-associated molecular pathways have not given satisfactory results [8].
The TME was first proposed by Ioannides in 1993, and referred especially to the local environment where tumors occurred and developed [9]. Commonly, the TME of PDAC is characterized by abundant stroma, hypoxia, a deficient blood supply and elevated immunosuppression [10]. Studies have shown that the TME, including cancer-related fibroblasts (CAFs), stellate cells, diverse immune cells and cytokines released by them, are involved in the control of the proliferation, metastasis, chemoresistance and immunotherapy of pancreatic cancer cells [11]. Factors associated with TME such as cell plasticity, heterogeneity of the tumor, composition of the tumor stroma, epithelial-to-mesenchymal transition (EMT), reprogrammed metabolism, acidic extracellular pH (pHe) and hypoxia can heavily impact treatment outcomes. Therefore, finding new therapeutic targets within the TME of PDAC is an encouraging and potential research direction in order to understand the lack of efficacy of current treatments for pancreatic cancer.
2. Desmoplastic Reaction in the Pancreatic Tumor Microenvironment
2.1. Desmoplasia: The Impact on Tumor Development and Progression
Despite the sizeable improvement in recent years, chemotherapy remains markedly incompetent in improving PDAC patient survival [12]. There are many factors that contribute to the failure of chemotherapy in this pathology, including the occurrence of desmoplasia in the PDAC TME [12].
Desmoplasia, also known as the desmoplastic reaction, consists of a dense ECM together with myofibroblast-like cells, including CAFs and is a fundamental feature of the pancreatic cancer TME [13]. Initially, the role of this phenomenon was overlooked; however, many studies have since demonstrated that during PDAC development, the cancer cells expend a large amount of energy to promote the recruitment, proliferation, and activation of fibroblasts. Consequent to their activation, CAFs are able to deposit ECM components and secrete several types of factors that strongly affect the behavior of cancer cells [14][15][16]. Indeed, pharmacologic inhibition of the desmoplastic reaction in combination with chemotherapy showed better results in inhibiting PDAC progression than chemotherapy alone, thus highlighting desmoplasia as a likely therapeutic target in pancreatic cancer [17][18][19][20]. Desmoplasia can be divided histopathologically into two groups: (i) overproduction of ECM proteins, and (ii) extensive proliferation of the PSCs [21][22].
Among all non-cellular components of desmoplasia, the importance of many ECM proteins, namely collagen types I, III and IV, fibronectin, laminin, hyaluronan, as well as the glycoprotein osteonectin [23][24] should be emphasized. Desmoplastic progression derives from the abnormal activation of several intercellular and intracellular signaling processes, such as the transforming growth factor beta (TGFβ), the basic fibroblast growth factor (bFGF), the connective tissue growth factor (CTGF) and interleukin-1β, which by stimulating ECM production drive desmoplastic progression [25][26][27][28][29]. The ECM components can also be divided into two categories: the fibrous proteins, such as collagens, and the polysaccharide chain glycosaminoglycans (GAGs), such as hyaluronan [30][31][32][33]. In the normal pancreas, GAGs structurally function to sustain compressive forces on the tissue, whereas the fibrous proteins act to support the tensile forces on the tissue [34]. On other hand, the marked overproduction of ECM constituents in PDAC has been suggested to be a failed wound healing, leading to fibrosis [34]. The increased deposition of collagen type I, III, and IV in PDAC tissues [35][36][37] is directly linked to the TGFβ/Smad signaling and is a product of the activity of the fibroblasts [38]. Remarkably, in PDAC, the elevated levels of collagen I reduce tissue elasticity and raise interstitial fluid pressure, causing a reduction in drug perfusion [39].
The protein-free GAG, hyaluronan, is also an important component of the ECM, contributing to tissue rigidity and thereby decreasing elasticity [40] and its accumulation within damaged tissue is a product of increased secretion by CAFs in pancreatic cancer [41]. Moreover, hyaluronan maintains its interaction with water molecules, to preserve tissue hydration in normal pancreas [42]. Nevertheless, in the “unhealthy” pancreas the increased deposition of hyaluronan might result in interstitial edema and, consequently, augmented interstitial fluid pressure that, in turn, leads to decreased fluid conveyance [43]. Accordingly, this enhanced interstitial edema together with the absence of an efficient lymphatic system in tumor tissue, results in a remarkable reduction in the exchange of several substances with the bloodstream, including chemotherapeutics [44]. Therefore, this significant deposition of hyaluronan in pancreatic cancer stroma is one of the main features of pancreatic TME responsible for decreased chemotherapeutic penetration (Figure 1) [45][46].
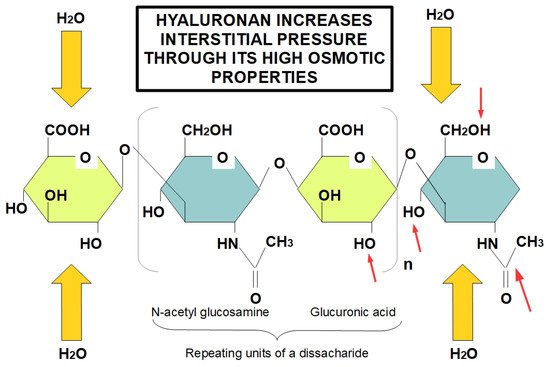
Figure 1. Role of hyaluronan in increasing tissue interstitial pressure. Hyaluronan forms long chains creating a highly osmotic environment that produces edema and increased interstitial pressure. Despite the fact that the diagram only shows a tetrasacharide, hyaluronan is a very lengthy unbranched chain of repeating disaccharides. Red arrows indicate the hydrophilic parts of glucuronic acid and N-acetyl glucosamine, proving the highly hydrophilic ability of hyaluronan. Increased hyaluronan in tumors is an early event occurring in TME, which leads to increased interstitial pressure due to its hygroscopic properties, causing an obstacle to the adequate delivery of chemotherapeutic drugs.
Lastly, fibronectin is also a crucial ECM component which binds to adhesion receptors in several cell types [47]. Additionally, it supports cell–ECM interactions and is a key factor for wound healing and development and the maintenance of tissue homeostasis [48][49][50][51][52]. While several cell types including tumor cells and endothelial cells are able to produce fibronectin, fibroblasts are the main producers [50]. The elevated expression of fibronectin is displayed by several solid tumors, particularly in pancreatic cancer [53]. Therefore, the interaction of multiple ECM proteins to produce the desmoplastic reaction in PDAC is a feature which clearly contributes to the pathogenesis and ultimately to chemoresistance.
2.2. The Contribution of Desmoplastic Components towards Chemoresistance
Desmoplasia can promote chemoresistance through several mechanisms which can be divided in two main groups: biological and physiological chemoresistance [22]. Biological chemoresistance can arise from different mechanisms. Target cells can (a) acquire resistance to drug uptake, (b) reduce their sensitivity to drugs by increasing the expression of anti-apoptotic proteins and activating protective mechanisms such as autophagy [54], (c) use DNA repair mechanisms to counteract the drug-dependent destruction of the tumor cell DNA [55][56] and (d) recruit more transporters/proteins responsible for drug efflux, thus preventing their action in the cancer cell. In PDAC, both physiological and biological chemoresistance are present and constitute a considerable problem for effective chemotherapy [22]. Moreover, the non-cellular components of the desmoplastic reaction can contribute to biological chemoresistance. Indeed, the binding of hyaluronan to its receptor, CD44, caused a Stat-3-mediated increase in the expression of the multi-drug resistance protein 1 (MDR1) in pancreatic cancer cell lines [57]. Moreover, the interaction of hyaluronan with CD44 is able to activate the phosphatidylinositol-3-kinase (PI3K/AkT) signaling pathway, which is upregulated in several cancers, resulting in the phosphorylation of Bad, and the consequent downregulation of apoptosis (Figure 2) [58][59].
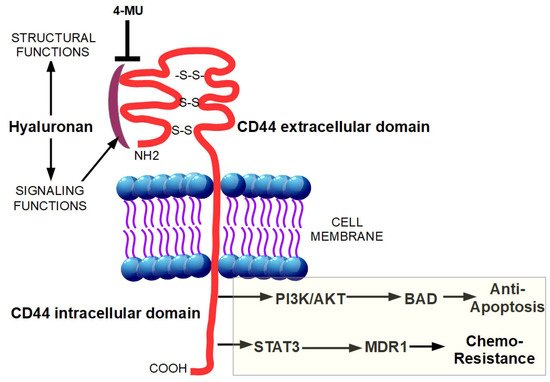
Figure 2. Binding of hyaluronan to CD44 unleashes a pro-tumoral intracellular signaling. The intracellular signaling functions of hyaluronan are triggered after its binding with CD44. This interaction results in the increased expression of the multi-drug resistance protein 1 (MDR1) through STAT3 activation and in the activation of phosphatidylinositol-3-kinase (PI3K/AkT) signaling pathway, causing phosphorylation of Bad, and the subsequent downregulation of apoptosis. The hyaluronan synthesis inhibitor, 4-methylumbelliferone (4-MU), inhibits cell migration, proliferation, and invasion by blocking the interaction between hyaluronan and CD44.
The ECM components are also involved in physiological chemoresistance and probably to a larger extent than in biological chemoresistance [60]. Physiological chemoresistance is due to poor tissue vascularization in the tumor tissue caused by the overproduction of ECM proteins and the consequent increased interstitial fluid pressure, which together produce a barrier to drug absorption into the target tissue [22]. As mentioned before, collagen type I, III, and IV are highly secreted into the PDAC TME and it was demonstrated, in a PDAC orthotopic xenograft mouse model, that the increased collagen I content decreased the penetration of nanoparticles, resulting in a significant reduction in the response to doxorubicin treatment [61]. Moreover, the collagen-reducing effects of the anti-hypertensive agent, losartan, resulted in a significant augmentation of nanoparticle penetration towards the target cells [61]. However, the decreased collagen I content induced a switch in cancer stem cells (CSCs) from slow growing, avascular-type cells to fast-growing, highly autophagic endothelial-like cells, creating a favorable mechanism for tumor progression and chemoresistance to gemcitabine [54][62]. Altogether, these data suggest that the correct manipulation of ECM collagen composition may be able to enhance the accessibility of drugs to tumor tissues and decrease chemoresistance.
2.3. Targeting the Desmoplasia Improves Chemotherapy Outcomes
Several stroma-targeting drugs are currently being tested as new treatment strategies to reduce chemoresistance in PDAC. One of the more appealing targets of the tumor stroma is the potent cytokine TGFβ which regulates developments, differentiation, and homeostasis in mammalian [63]. TGFβ binds to TGFβ receptor 1 or 2 to inhibits cell proliferation, motility, invasion, EMT, and metastasis [64] and its tumor-inhibitory effect is controlled by Smad-dependent TGFβ signaling [65]. Indeed, in human prostate cancer, overexpression of TGFβ1 correlates with collagen I levels, suggesting that TGFβ can be directly linked to the desmoplastic process [26]. Furthermore, knocking down Smad 3 abolishes collagen fibrosis induced by the EMT-regulator Snail, hence corroborating the role of TGFβ as a crucial signaling pathway in the propagation of the desmoplastic reaction [66]. Moreover, Smad4 is frequently mutated in PDAC and this could be one of the reasons for the resistance to the growth inhibitory effects of TGFβ [26]. In this line, some TGFβ receptor 1 inhibitors, such as SB431542 and SB525334, have been developed and tested in combination with gemcitabine, resulting in a higher cytotoxic effect compared to gemcitabine alone due to an increased delivery and penetration of the drugs into the tumor (desmoplastic) tissues [67].
Moreover, the hyaluronan cell surface receptor CD44 has a critical role in pancreatic carcinogenesis [68] such that disruption of the hyaluronan-CD44 complex is a crucial therapeutic target to prevent PDAC drug resistance [68]. Indeed, the compound, 4-methylumbelliferone (4-MU), by inhibiting hyaluronan synthesis and accumulation on cancer cells and their surrounding stroma, blocked cell proliferation, migration, and spreading in several tumor cell types [69][70] and reduced bone metastases in breast cancer (Figure 2) [71]. In both PDAC cell lines and in vivo, 4-MU slowed the development and progression of the disease and also increased tumor response to gemcitabine [72][73][74]. In a similar way, PEGylated human recombinant PH20 hyaluronidase (PEGPH20) acted as a hyaluronan “consumer” enhancing the delivery of chemotherapeutic agents such as doxorubicin and gemcitabine [46][75]. Injection of PEGPH20 into KPC mice tumors rapidly degraded hyaluronan, restored the patency of intra-tumoral vessels, increased vessel diameter and highly reduced the high interstitial fluid pressure within the tumors to normal levels [7]. Importantly, these alterations increased the macromolecules permeability and the combined treatment of gemcitabine with PEGPH20 remarkably improved treatment efficacy by reducing metastases and doubling the median survival time in mice [76].
Further, the enzymatic degradation of ECM components has also been proposed as a possible complementary therapeutic approach against PDAC. Several studies using hyaluronidase showed improved cancer sensitivity to chemotherapeutics and enhanced permeability to drugs in cultured multicellular spheroids [77]. Similarly, collagenases have also displayed beneficial characteristics by increasing the penetration of macromolecules. However, their sensitivity to and stability at physiological pH might be a therapeutic hindrance that has not yet allowed this enzyme to be clinically available [78]. It has proposed a double edged targeting of the hyaluronan-CD44 pathway by combining 4-MU as hyaluronan production inhibitor and bromelain as CD44 inhibitor [79], since this combination has not been experimentally tested yet. Lastly, Hedgehog (Hh) is a signaling pathway that is abnormally activated in most pancreatic cancers leading to cancer initiation, progression and metastatic development [80][81]. More recently, it has been related to the beginning and maintenance of the desmoplastic reaction. Indeed, Hh stimulates the differentiation of myofibroblasts and induces stroma-derived growth promoting molecules [82][83]. There is also some evidence that supports the existence of an interplay between Hh signaling and TGFβ, both being tightly connected with the desmoplastic reaction and involved in fibrosis [83]. Further, it was demonstrated that blocking Hh signaling, both in vitro and in vivo, with the small molecule compound, cyclopamine, markedly improved drug delivery and abrogated pancreatic metastasis [84][85]. Multiple studies have been conducted to verify whether the combined treatment of Hh signaling inhibitors with chemotherapeutic agents can have synergistic anticancer effects [86]. Indeed, the inhibition of the Hh signal with the semisynthetic analogue of cyclopamine, IP-926, reduced the desmoplastic reaction and improved tumor vascularity [87]. A phase Ib trial demonstrated that IPI-926 reduced tumor desmoplasia and increased gemcitabine delivery, such that 31% of patients displayed a partial response and 63% of them showed a reduced expression of the marker Carbohydrate Antigen 19-9 (CA 19-9) in their tumor tissues [88].
In summary, the desmoplastic reaction creates a unique microenvironment, which stimulates tumor growth/progression and forms a “physical barrier” to chemotherapy permeability. Hence, several strategies have arisen to improve chemotherapeutic efficacy by blocking or interfering with the desmoplastic process and enhance tumor penetration, accumulation, and drug distribution. Thus, targeting stroma components of the desmoplastic reaction might be a promising new area of investigation in pancreatic cancer treatment.
References
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014, 74, 2913–2921.
- Schober, M.; Jesenofsky, R.; Faissner, R.; Weidenauer, C.; Hagmann, W.; Michl, P.; Heuchel, R.L.; Haas, S.L.; Löhr, J.M. Desmoplasia and chemoresistance in pancreatic cancer. Cancers 2014, 6, 2137–2154.
- Stylianopoulos, T. The Solid Mechanics of Cancer and Strategies for Improved Therapy. J. Biomech. Eng. 2017, 139, 021004.
- Goetz, J.G.; Minguet, S.; Navarro-Lérida, I.; Lazcano, J.J.; Samaniego, R.; Calvo, E.; Tello, M.; Osteso-Ibáñez, T.; Pellinen, T.; Echarri, A.; et al. Biomechanical remodeling of the microenvironment by stromal caveolin-1 favors tumor invasion and metastasis. Cell 2011, 146, 148–163.
- Erdogan, B.; Ao, M.; White, L.M.; Means, A.L.; Brewer, B.M.; Yang, L.; Washington, M.K.; Shi, C.; Franco, O.E.; Weaver, A.M.; et al. Cancer-associated fibroblasts promote directional cancer cell migration by aligning fibronectin. J. Cell Biol. 2017, 216, 3799–3816.
- Stanisavljevic, J.; Loubat-Casanovas, J.; Herrera, M.; Luque, T.; Peña, R.; Lluch, A.; Albanell, J.; Bonilla, F.; Rovira, A.; Peña, C.; et al. Snail1-expressing fibroblasts in the tumor microenvironment display mechanical properties that support metastasis. Cancer Res. 2015, 75, 284–295.
- Provenzano, P.P.; Cuevas, C.; Chang, A.E.; Goel, V.K.; Von Hoff, D.D.; Hingorani, S.R. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell 2012, 21, 418–429.
- Adamska, A.; Domenichini, A.; Falasca, M. Pancreatic Ductal Adenocarcinoma: Current and Evolving Therapies. Int. J. Mol. Sci. 2017, 18, 1338.
- Ioannides, C.G.; Whiteside, T.L. T cell recognition of human tumors: Implications for molecular immunotherapy of cancer. Clin. Immunol. Immunopathol. 1993, 66, 91–106.
- Apte, M.V.; Xu, Z.; Pothula, S.; Goldstein, D.; Pirola, R.C.; Wilson, J.S. Pancreatic cancer: The microenvironment needs attention too! Pancreatology 2015, 15 (Suppl. S4), S32–S38.
- Chang, J.H.; Jiang, Y.; Pillarisetty, V.G. Role of immune cells in pancreatic cancer from bench to clinical application: An updated review. Medicine 2016, 95, e5541.
- Hall, B.R.; Cannon, A.; Atri, P.; Wichman, C.S.; Smith, L.M.; Ganti, A.K.; Are, C.; Sasson, A.R.; Kumar, S.; Batra, S.K. Advanced pancreatic cancer: A meta-analysis of clinical trials over thirty years. Oncotarget 2018, 9, 19396.
- Apte, M.; Park, S.; Phillips, P.; Santucci, N.; Goldstein, D.; Kumar, R.; Ramm, G.; Buchler, M.; Friess, H.; McCarroll, J. Desmoplastic reaction in pancreatic cancer: Role of pancreatic stellate cells. Pancreas 2004, 29, 179–187.
- Lu, J.; Zhou, S.; Siech, M.; Habisch, H.; Seufferlein, T.; Bachem, M. Pancreatic stellate cells promote hapto-migration of cancer cells through collagen I-mediated signalling pathway. Br. J. Cancer 2014, 110, 409–420.
- Hwang, R.F.; Moore, T.; Arumugam, T.; Ramachandran, V.; Amos, K.D.; Rivera, A.; Ji, B.; Evans, D.B.; Logsdon, C.D. Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer Res. 2008, 68, 918–926.
- Dunér, S.; Lindman, J.L.; Ansari, D.; Gundewar, C.; Andersson, R. Pancreatic cancer: The role of pancreatic stellate cells in tumor progression. Pancreatology 2010, 10, 673–681.
- Sherman, M.H.; Ruth, T.Y.; Engle, D.D.; Ding, N.; Atkins, A.R.; Tiriac, H.; Collisson, E.A.; Connor, F.; Van Dyke, T.; Kozlov, S. Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell 2014, 159, 80–93.
- Carapuça, E.F.; Gemenetzidis, E.; Feig, C.; Bapiro, T.E.; Williams, M.D.; Wilson, A.S.; Delvecchio, F.R.; Arumugam, P.; Grose, R.P.; Lemoine, N.R. Anti-stromal treatment together with chemotherapy targets multiple signalling pathways in pancreatic adenocarcinoma. J. Pathol. 2016, 239, 286–296.
- Ene-Obong, A.; Clear, A.J.; Watt, J.; Wang, J.; Fatah, R.; Riches, J.C.; Marshall, J.F.; Chin-Aleong, J.; Chelala, C.; Gribben, J.G. Activated pancreatic stellate cells sequester CD8+ T cells to reduce their infiltration of the juxtatumoral compartment of pancreatic ductal adenocarcinoma. Gastroenterology 2013, 145, 1121–1132.
- Masamune, A.; Hamada, S.; Kikuta, K.; Takikawa, T.; Miura, S.; Nakano, E.; Shimosegawa, T. The angiotensin II type I receptor blocker olmesartan inhibits the growth of pancreatic cancer by targeting stellate cell activities in mice. Scand. J. Gastroenterol. 2013, 48, 602–609.
- Whatcott, C.J.; Posner, R.G.; Von Hoff, D.D.; Han, H. Desmoplasia and chemoresistance in pancreatic cancer. In Pancreatic Cancer and Tumor Microenvironment; Transworld Research Network: Trivandrum, India, 2012.
- Yen, T.W.; Aardal, N.P.; Bronner, M.P.; Thorning, D.R.; Savard, C.E.; Lee, S.P.; Bell, R.H., Jr. Myofibroblasts are responsible for the desmoplastic reaction surrounding human pancreatic carcinomas. Surgery 2002, 131, 129–134.
- Bachem, M.G.; Schneider, E.; Groß, H.; Weidenbach, H.; Schmid, R.M.; Menke, A.; Siech, M.; Beger, H.; Grünert, A.; Adler, G. Identification, culture, and characterization of pancreatic stellate cells in rats and humans. Gastroenterology 1998, 115, 421–432.
- Apte, M.; Haber, P.; Darby, S.; Rodgers, S.; McCaughan, G.; Korsten, M.; Pirola, R.; Wilson, J. Pancreatic stellate cells are activated by proinflammatory cytokines: Implications for pancreatic fibrogenesis. Gut 1999, 44, 534–541.
- Aoyagi, Y.; Oda, T.; Kinoshita, T.; Nakahashi, C.; Hasebe, T.; Ohkohchi, N.; Ochiai, A. Overexpression of TGF-β by infiltrated granulocytes correlates with the expression of collagen mRNA in pancreatic cancer. Br. J. Cancer 2004, 91, 1316–1326.
- Löhr, M.; Schmidt, C.; Ringel, J.; Kluth, M.; Müller, P.; Nizze, H.; Jesnowski, R. Transforming growth factor-β1 induces desmoplasia in an experimental model of human pancreatic carcinoma. Cancer Res. 2001, 61, 550–555.
- Awaji, M.; Futakuchi, M.; Heavican, T.; Iqbal, J.; Singh, R.K. Cancer-associated fibroblasts enhance survival and progression of the aggressive pancreatic tumor Via FGF-2 and CXCL8. Cancer Microenviron. 2019, 12, 37–46.
- Hartel, M.; di Mola, F.F.; Gardini, A.; Zimmermann, A.; Di Sebastiano, P.; Guweidhi, A.; Innocenti, P.; Giese, T.; Giese, N.; Büchler, M.W. Desmoplastic reaction influences pancreatic cancer growth behavior. World J. Surg. 2004, 28, 818–825.
- Das, S.; Shapiro, B.; Vucic, E.A.; Vogt, S.; Bar-Sagi, D. Tumor Cell-Derived IL-1β Promotes Desmoplasia and Immune Suppression in Pancreatic Cancer. Cancer Res. 2020, 80, 1088–1101.
- Stern, R. Hyaluronidases in cancer biology. Hyaluronan Cancer Biol. 2008, 207–220.
- Marastoni, S.; Ligresti, G.; Lorenzon, E.; Colombatti, A.; Mongiat, M. Extracellular matrix: A matter of life and death. Connect. Tissue Res. 2008, 49, 203–206.
- Kaspar, M.; Zardi, L.; Neri, D. Fibronectin as target for tumor therapy. Int. J. Cancer 2006, 118, 1331–1339.
- Mahadevan, D.; Von Hoff, D.D. Tumor-stroma interactions in pancreatic ductal adenocarcinoma. Mol. Cancer Ther. 2007, 6, 1186–1197.
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. The extracellular matrix of animals. In Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002.
- Imamura, T.; Iguchi, H.; Manabe, T.; Ohshio, G.; Yoshimura, T.; Wang, Z.; Suwa, H.; Ishigami, S.; Imamura, M. Quantitative analysis of collagen and collagen subtypes I, III, and V in human pancreatic cancer, tumor-associated chronic pancreatitis, and alcoholic chronic pancreatitis. Pancreas 1995, 11, 357–364.
- Mollenhauer, J.; Roether, I.; Kern, H. Distribution of extracellular matrix proteins in pancreatic ductal adenocarcinoma and its influence on tumor cell proliferation in vitro. Pancreas 1987, 2, 14–24.
- Linder, S.; Castaños-Velez, E.; Biberfeld, P. Immunohistochemical expression of extracellular matrix proteins and adhesion molecules in pancreatic carcinoma. Hepatogastroenterology 2001, 48, 1321–1327.
- Verrecchia, F.; Mauviel, A. TGF-β and TNF-α: Antagonistic cytokines controlling type I collagen gene expression. Cell. Signal. 2004, 16, 873–880.
- Nieskoski, M.D.; Marra, K.; Gunn, J.R.; Hoopes, P.J.; Doyley, M.M.; Hasan, T.; Trembly, B.S.; Pogue, B.W. Collagen complexity spatially defines microregions of total tissue pressure in pancreatic cancer. Sci. Rep. 2017, 7, 1–12.
- Laurent, T.C.; Fraser, J. Hyaluronan. FASEB J. 1992, 6, 2397–2404.
- Yu, M.; Tannock, I.F. Targeting tumor architecture to favor drug penetration: A new weapon to combat chemoresistance in pancreatic cancer? Cancer Cell 2012, 21, 327–329.
- Provenzano, P.; Hingorani, S. Hyaluronan, fluid pressure, and stromal resistance in pancreas cancer. Br. J. Cancer 2013, 108, 1–8.
- Johnsson, C.; Hällgren, R.; Tufveson, G. Role of hyaluronan in acute pancreatitis. Surgery 2000, 127, 650–658.
- Voutouri, C.; Polydorou, C.; Papageorgis, P.; Gkretsi, V.; Stylianopoulos, T. Hyaluronan-derived swelling of solid tumors, the contribution of collagen and cancer cells, and implications for cancer therapy. Neoplasia 2016, 18, 732–741.
- Kultti, A.; Zhao, C.; Singha, N.C.; Zimmerman, S.; Osgood, R.J.; Symons, R.; Jiang, P.; Li, X.; Thompson, C.B.; Infante, J.R. Accumulation of extracellular hyaluronan by hyaluronan synthase 3 promotes tumor growth and modulates the pancreatic cancer microenvironment. BioMed Res. Int. 2014, 2014, 817613.
- Jacobetz, M.A.; Chan, D.S.; Neesse, A.; Bapiro, T.E.; Cook, N.; Frese, K.K.; Feig, C.; Nakagawa, T.; Caldwell, M.E.; Zecchini, H.I. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut 2013, 62, 112–120.
- Akiyama, S.K.; Olden, K.; Yamada, K.M. Fibronectin and integrins in invasion and metastasis. Cancer Metastasis Rev. 1995, 14, 173–189.
- Lenselink, E.A. Role of fibronectin in normal wound healing. Int. Wound J. 2015, 12, 313–316.
- George, E.L.; Georges-Labouesse, E.N.; Patel-King, R.S.; Rayburn, H.; Hynes, R.O. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development 1993, 119, 1079–1091.
- Pankov, R.; Yamada, K.M. Fibronectin at a glance. J. Cell Sci. 2002, 115, 3861–3863.
- To, W.S.; Midwood, K.S. Plasma and cellular fibronectin: Distinct and independent functions during tissue repair. Fibrogenes. Tissue Repair 2011, 4, 21.
- Midwood, K.S.; Williams, L.V.; Schwarzbauer, J.E. Tissue repair and the dynamics of the extracellular matrix. Int. J. Biochem. Cell Biol. 2004, 36, 1031–1037.
- Ramaswamy, S.; Ross, K.N.; Lander, E.S.; Golub, T.R. A molecular signature of metastasis in primary solid tumors. Nat. Genet. 2003, 33, 49–54.
- Forciniti, S.; Dalla Pozza, E.; Greco, M.R.; Amaral Carvalho, T.M.; Rolando, B.; Ambrosini, G.; Carmona-Carmona, C.A.; Pacchiana, R.; Di Molfetta, D.; Donadelli, M. Extracellular Matrix Composition Modulates the Responsiveness of Differentiated and Stem Pancreatic Cancer Cells to Lipophilic Derivate of Gemcitabine. Int. J. Mol. Sci. 2021, 22, 29.
- Chand, S.N.; Zarei, M.; Schiewer, M.J.; Kamath, A.R.; Romeo, C.; Lal, S.; Cozzitorto, J.A.; Nevler, A.; Scolaro, L.; Londin, E. Posttranscriptional regulation of PARG mRNA by HuR facilitates DNA repair and resistance to PARP inhibitors. Cancer Res. 2017, 77, 5011–5025.
- Perkhofer, L.; Gout, J.; Roger, E.; de Almeida, F.K.; Simões, C.B.; Wiesmüller, L.; Seufferlein, T.; Kleger, A. DNA damage repair as a target in pancreatic cancer: State-of-the-art and future perspectives. Gut 2021, 70, 606–617.
- Hong, S.P.; Wen, J.; Bang, S.; Park, S.; Song, S.Y. CD44-positive cells are responsible for gemcitabine resistance in pancreatic cancer cells. Int. J. Cancer 2009, 125, 2323–2331.
- Fujita, Y.; Kitagawa, M.; Nakamura, S.; Azuma, K.; Ishii, G.; Higashi, M.; Kishi, H.; Hiwasa, T.; Koda, K.; Nakajima, N. CD44 signaling through focal adhesion kinase and its anti-apoptotic effect. FEBS Lett. 2002, 528, 101–108.
- Mitsiades, C.S.; Mitsiades, N.; Koutsilieris, M. The Akt pathway: Molecular targets for anti-cancer drug development. Curr. Cancer Drug Targets 2004, 4, 235–256.
- Henke, E.; Nandigama, R.; Ergün, S. Extracellular matrix in the tumor microenvironment and its impact on cancer therapy. Front. Mol. Biosci. 2020, 6, 160.
- Diop-Frimpong, B.; Chauhan, V.P.; Krane, S.; Boucher, Y.; Jain, R.K. Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumors. Proc. Natl. Acad. Sci. USA 2011, 108, 2909–2914.
- Biondani, G.; Zeeberg, K.; Greco, M.R.; Cannone, S.; Dando, I.; Dalla Pozza, E.; Mastrodonato, M.; Forciniti, S.; Casavola, V.; Palmieri, M. Extracellular matrix composition modulates PDAC parenchymal and stem cell plasticity and behavior through the secretome. FEBS J. 2018, 285, 2104–2124.
- Clark, D.A.; Coker, R. Transforming growth factor-beta (TGF-beta). Int. J. Biochem. Cell Biol. 1998, 30, 293–298.
- Hilbig, A.; Oettle, H. Transforming growth factor beta in pancreatic cancer. Curr. Pharm. Biotechnol. 2011, 12, 2158–2164.
- García-Montero, A.C.; Vasseur, S.; Giono, L.E.; Canepa, E.; Moreno, S.; Dagorn, J.C.; Iovanna, J.L. Transforming growth factor β-1 enhances Smad transcriptional activity through activation of p8 gene expression. Biochem. J. 2001, 357, 249–253.
- Shields, M.A.; Dangi-Garimella, S.; Krantz, S.B.; Bentrem, D.J.; Munshi, H.G. Pancreatic cancer cells respond to type I collagen by inducing snail expression to promote membrane type 1 matrix metalloproteinase-dependent collagen invasion. J. Biol. Chem. 2011, 286, 10495–10504.
- Kim, Y.J.; Hwang, J.S.; Hong, Y.B.; Bae, I.; Seong, Y.-S. Transforming growth factor beta receptor I inhibitor sensitizes drug-resistant pancreatic cancer cells to gemcitabine. Anticancer Res. 2012, 32, 799–806.
- Toole, B.P.; Slomiany, M.G. Hyaluronan, CD44 and Emmprin: Partners in cancer cell chemoresistance. Drug Resist. Updates 2008, 11, 110–121.
- Edward, M.; Quinn, J.; Pasonen-Seppänen, S.; McCann, B.; Tammi, R. 4-Methylumbelliferone inhibits tumour cell growth and the activation of stromal hyaluronan synthesis by melanoma cell-derived factors. Br. J. Dermatol. 2010, 162, 1224–1232.
- Kultti, A.; Pasonen-Seppänen, S.; Jauhiainen, M.; Rilla, K.J.; Kärnä, R.; Pyöriä, E.; Tammi, R.H.; Tammi, M.I. 4-Methylumbelliferone inhibits hyaluronan synthesis by depletion of cellular UDP-glucuronic acid and downregulation of hyaluronan synthase 2 and 3. Exp. Cell Res. 2009, 315, 1914–1923.
- Urakawa, H.; Nishida, Y.; Wasa, J.; Arai, E.; Zhuo, L.; Kimata, K.; Kozawa, E.; Futamura, N.; Ishiguro, N. Inhibition of hyaluronan synthesis in breast cancer cells by 4-methylumbelliferone suppresses tumorigenicity in vitro and metastatic lesions of bone in vivo. Int. J. Cancer 2012, 130, 454–466.
- Hajime, M.; Shuichi, Y.; Makoto, N.; Masanori, Y.; Ikuko, K.; Atsushi, K.; Mutsuo, S.; Keiichi, T. Inhibitory effect of 4-methylesculetin on hyaluronan synthesis slows the development of human pancreatic cancer in vitro and in nude mice. Int. J. Cancer 2007, 120, 2704–2709.
- Morohashi, H.; Kon, A.; Nakai, M.; Yamaguchi, M.; Kakizaki, I.; Yoshihara, S.; Sasaki, M.; Takagaki, K. Study of hyaluronan synthase inhibitor, 4-methylumbelliferone derivatives on human pancreatic cancer cell (KP1-NL). Biochem. Biophys. Res. Commun. 2006, 345, 1454–1459.
- Nakazawa, H.; Yoshihara, S.; Kudo, D.; Morohashi, H.; Kakizaki, I.; Kon, A.; Takagaki, K.; Sasaki, M. 4-methylumbelliferone, a hyaluronan synthase suppressor, enhances the anticancer activity of gemcitabine in human pancreatic cancer cells. Cancer Chemother. Pharmacol. 2006, 57, 165–170.
- Thompson, C.B.; Shepard, H.M.; O’Connor, P.M.; Kadhim, S.; Jiang, P.; Osgood, R.J.; Bookbinder, L.H.; Li, X.; Sugarman, B.J.; Connor, R.J. Enzymatic depletion of tumor hyaluronan induces antitumor responses in preclinical animal models. Mol. Cancer Ther. 2010, 9, 3052–3064.
- Michl, P.; Gress, T.M. Improving drug delivery to pancreatic cancer: Breaching the stromal fortress by targeting hyaluronic acid. Gut 2012, 61, 1377–1379.
- Kohno, N.; Ohnuma, T.; Truog, P. Effects of hyaluronidase on doxorubicin penetration into squamous carcinoma multicellular tumor spheroids and its cell lethality. J. Cancer Res. Clin. Oncol. 1994, 120, 293–297.
- Magzoub, M.; Jin, S.; Verkman, A. Enhanced macromolecule diffusion deep in tumors after enzymatic digestion of extracellular matrix collagen and its associated proteoglycan decorin. FASEB J. 2008, 22, 276–284.
- Koltai, T.; Reshkin, S.J.; Carvalho, T.; Cardone, R.A. Targeting the Stromal Pro-Tumoral Hyaluronan-CD44 Pathway in Pancreatic Cancer. Int. J. Mol. Sci. 2021, 22, 3953.
- Kelleher, F.C. Hedgehog signaling and therapeutics in pancreatic cancer. Carcinogenesis 2011, 32, 445–451.
- Ji, Z.; Mei, F.C.; Xie, J.; Cheng, X. Oncogenic KRAS activates hedgehog signaling pathway in pancreatic cancer cells. J. Biol. Chem. 2007, 282, 14048–14055.
- Bailey, J.M.; Swanson, B.J.; Hamada, T.; Eggers, J.P.; Singh, P.K.; Caffery, T.; Ouellette, M.M.; Hollingsworth, M.A. Sonic hedgehog promotes desmoplasia in pancreatic cancer. Clin. Cancer Res. 2008, 14, 5995–6004.
- Bai, Y.; Bai, Y.; Dong, J.; Li, Q.; Jin, Y.; Chen, B.; Zhou, M. Hedgehog signaling in pancreatic fibrosis and cancer. Medicine 2016, 95, e2996.
- Feldmann, G.; Fendrich, V.; McGovern, K.; Bedja, D.; Bisht, S.; Alvarez, H.; Koorstra, J.-B.M.; Habbe, N.; Karikari, C.; Mullendore, M. An orally bioavailable small-molecule inhibitor of Hedgehog signaling inhibits tumor initiation and metastasis in pancreatic cancer. Mol. Cancer Ther. 2008, 7, 2725–2735.
- Kelleher, F.C.; McDermott, R. Aberrations and therapeutics involving the developmental pathway Hedgehog in pancreatic cancer. Vitam. Horm. 2012, 88, 355–378.
- Bisht, S.; Brossart, P.; Maitra, A.; Feldmann, G. Agents targeting the Hedgehog pathway for pancreatic cancer treatment. Curr. Opin. Investig. Drugs 2010, 11, 1387–1398.
- Olive, K.P.; Jacobetz, M.A.; Davidson, C.J.; Gopinathan, A.; McIntyre, D.; Honess, D.; Madhu, B.; Goldgraben, M.A.; Caldwell, M.E.; Allard, D. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 2009, 324, 1457–1461.
- Richards, D.A.; Stephenson, J.; Wolpin, B.M.; Becerra, C.; Hamm, J.T.; Messersmith, W.A.; Devens, S.; Cushing, J.; Schmalbach, T.; Fuchs, C.S. A phase Ib trial of IPI-926, a hedgehog pathway inhibitor, plus gemcitabine in patients with metastatic pancreatic cancer. J. Clin. Oncol. 2012, 30 (Suppl. S4), 213.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
554
Revisions:
2 times
(View History)
Update Date:
25 Feb 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




