| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hassan Awada | + 10184 word(s) | 10184 | 2020-09-09 09:53:18 | | | |
| 2 | Bruce Ren | -3968 word(s) | 6216 | 2020-09-11 03:30:01 | | | | |
| 3 | Bruce Ren | -2235 word(s) | 3981 | 2020-10-20 11:08:45 | | |
Video Upload Options
Over the past decade, new insights have emerged on the pathophysiology of essential thrombocythemia (ET), its clinical management, and associated thrombohemostatic disturbances. Here, we review the latest diagnostic and risk stratification modalities of ET and its therapeutics. Moreover, we discuss the clinical evidence-based benefits, deriving from major clinical trials, of using cytoreductive therapy and antiplatelet agents to lower the risk of fatal vascular events. Also, we focus on the condition of extreme thrombocytosis (>1000 × 109/L) and bleeding risk, the development and pathogenesis of acquired von Willebrand syndrome, and the clinical approach to this paradoxical scenario in ET.
1. Introduction
Essential thrombocythemia (ET) is a clonal hematopoietic stem cell disorder that belongs to Philadelphia chromosome-negative myeloproliferative neoplasm (MPN) category [1][2]. ET is phenotypically expressed by persistent nonreactive thrombocytosis and increased risk of vascular events, resulting from the clonal proliferation of atypical megakaryocytes in the bone marrow. The prevalence of ET is estimated to be 38–57 per 100,000 population in the United States with a median age of 58 years on presentation and a female sex preponderance [3]. ET shares with other MPNs the classical driver mutations affecting genes like Janus kinase (JAK2 V617F; 50–60%), calreticulin (CALR; 25–35%), or myeloproliferative leukemia (MPL; 5–10%) [4][5][6][7]. Given the great deal of genotypic and phenotypic overlap with other MPN subtypes, the diagnosis of ET can be challenging in some situations. For instance, JAK2 V617F mutations and isolated thrombocytosis can be also seen in myelofibrosis (MF) and polycythemia vera (PV). [8][9] Additionally, it is of paramount importance to distinguish ET from prefibrotic myelofibrosis (PFMF), as the clinical picture of PFMF may resemble ET but its prognosis and management is more related to that of MF [10]. Hence, a stringent standard for ET diagnoses has been implemented and sequentially updated by the World Health Organization (WHO) with the latest 2016 revised version requiring the satisfaction of four major criteria, or three major criteria and one minor criterion to confirm the diagnosis [2] (Table 1). In fact, the presence of driver mutations influences disease evolution and has diagnostic and prognostic significance in ET [11][12][13]. Nevertheless, 10–20% of the patients are wild type for the aforementioned somatic hits (denoted hereafter as “triple-negative”) and express no driver mutations [14]. Interestingly, two studies have shown that about 8–10% of triple-negative patients carry activating mutations of JAK2 or MPL outside of the classical loci and these non-canonical mutations may be either acquired (somatic) or inherited (germline) suggesting that they are more likely benign disorders of platelet production rather than MPNs [15][16].
Table 1. The evolution of essential thrombocythemia diagnostic criteria according to World Health Organization (WHO).
|
WHO 2008 |
WHO 2016 (Revised) |
|
Major criteria |
Major criteria |
|
1. Platelet count ≥ 450 × 109/L |
1. Platelet count ≥ 450 × 109/L |
|
2. BM biopsy showing proliferation of the megakaryocyte lineage with increased numbers of enlarged, mature megakaryocytes |
2. BM biopsy showing proliferation of the megakaryocyte lineage with increased numbers of enlarged, mature megakaryocytes with hyperlobulated nuclei. No significant left-shift of neutrophil myelopoiesis or erythropoiesis and very rarely minor (grade 1) increase in reticulin fibers |
|
3. Not meeting WHO criteria for CML, PV, PMF, MDS, or other myeloid neoplasms |
3. Not meeting WHO criteria for BCR-ABL1 + CML, PV, PMF, MDS, or other myeloid neoplasms |
|
4. Presence of JAK2 V617F mutation or other clonal marker or lack of evidence of a secondary cause of thrombocytosis |
4. Presence of JAK2, CALR or MPL mutation |
|
No minor criteria |
Minor criteria |
|
All four major criteria required |
All four major criteria or three major and one minor required |
Adapted from Arber et al. [2]. Abbreviations: BM, bone marrow; CML, chronic myeloid leukemia; PV, polycythemia vera, PMF, primary myelofibrosis; MDS, myelodysplastic syndromes.
Indeed, ET is a chronic disorder with a 15-year survival rate of 80% and a natural history that is characterized by the potential for progression to MF as well as secondary acute myeloid leukemia (sAML) [17][18]. Furthermore, morbidity and mortality are more associated with vascular risks (thrombosis and hemorrhage), hence, the primary goal of current prognostication models and available therapeutics is mainly to identify and appropriately manage patients at higher risk of such complications. Finally, recent insights deriving from next-generation sequencing (NGS) revealed that 53% of an ET cohort (n = 183) had sequence variants/mutations other than the classical driver mutations, with TET2 and ASXL1 being the most frequently mutated genes [19]. Hits affecting other myeloid genes like SH2B3, IDH2, SF3B1, U2AF1, EZH2, and TP53 were found to have an adverse impact on the overall, leukemia-free, MF-free survival as well as an increased vascular risk in the studied ET population [19]. These observations raise the question of whether we need to implement the use of targeted NGS in routine management, surveillance, and design of therapeutic trajectory of patients with ET.
2. Clinical and Therapeutic Pitfalls
While the heterogeneous molecular profile of ET tend to drive the disease evolution, the phenotype, including the thrombo-hemorrhagic tendency and systemic symptom burden (e.g., fatigue, pruritus, microvascular symptoms, splenomegaly) [20], remains the major target of cytoreductive and antiplatelet therapies [21]. Hence, the goal is to relieve symptoms and decrease fatal vascular complications in a preventive fashion via lowering the persistent platelet elevation, ideally to less than 400 × 109/L.
ET comprises a wide spectrum of clinical complications, including thrombosis of major vessels, deep venous thrombosis or pulmonary embolism as well as other unusual sites [22][23][24]. The latter clinical scenario is more frequent among JAK2 V617F carriers and may represent the first sign of disease onset; e.g., development of thrombosis in the splanchnic vessels (Budd-Chiari syndrome) or cerebral venous sinus [25]. ET patients also suffer from microvascular occlusions involving small vessels, which can cause ocular migraine (amaurosis fugax), transient ischemic attack, or erythromelalgia [26][27]. On the other hand, minor bleeding or major hemorrhagic complications can paradoxically happen, especially with extreme thrombocytosis [28][29][30]. While this may be true, vascular complications tend to controversially correlate with the extent and degree of thrombocytosis. For instance, a platelet count (PC) > 1000 × 109/L can induce an acquired von Willebrand syndrome (AVWS) [31], caused by the proteolytic reduction of von Willebrand factor (VWF) multimers due to the passive adsorption to the platelet membrane. In contrast, lower PC (<1000 × 109/L) has been associated with arterial and venous thrombosis (ischemic stroke, deep venous thrombosis, pulmonary embolism, etc.), with an increased risk observed when JAK2 mutation is present [32]. All these observations illustrate the complexity of the disease and its intricate nature, as well as the controversy related to the therapeutic interventions in the clinical setting. In addition, as previously mentioned, patients with ET are at higher risk of fatal vascular events and the main therapeutic treatment, which is cytoreduction, only prevents vascular complications without reducing the risk of leukemic transformation or fibrotic progression [33].
3. Risk Factors and Stratification Models
3.1. The Evolution of Prognostication Systems
The higher thrombotic or bleeding risk in ET is not always well-defined because of commonalities and shadowlands in the clinical presentation of such situations, taking into account the possible simultaneous evolution of a case of extreme thrombocytosis. So far, patients with ET have been stratified by various models for vascular complications’ risk (mainly thrombotic) using factors like age (<40, 40–59 and 60 years), history of thrombosis, and JAK2/MPL/CALR mutation status and assessed for cardiovascular risk factors including hypertension, diabetes, and others.
Historically, the European LeukemiaNet (ELN) categorized ET patients into high and low-risk groups according to a two-tiered model that takes into consideration the absence or presence of either age >60 years or history of thrombosis. Patients not meeting any of these two factors, fall in the low-risk category. In 2012, Barbui et al. proposed a 3-tiered score (with the addition of an intermediate-risk group) called the “International Prognostic Score of Thrombosis for Essential Thrombocythemia” (IPSET-thrombosis model), which accounted for the presence of cardiovascular risk factors and JAK2 V617F mutation [34]. A more recent risk stratification modality is the new version of IPSET model that revised the individualized and combined contribution of cardiovascular risk factors and JAK2/MPL mutations and divided ET patients into 4-tiered risk categories, a) very-low-risk patients, who have no adverse features (age <61 years, no prior history of thrombosis or major hemorrhage, and absent JAK2 V617F/MPL mutations), b) low-risk patients, who harbor JAK2 V617F/MPL mutations but no adverse features, c) intermediate-risk patients, who are older (age >60 or older), but have no prior history of thrombosis or major hemorrhage and lack JAK2 V617F/MPL mutations, and d) high-risk patients, who have a thrombosis history, or are older and harbor JAK2 V617F/MPL mutations [35][36][37](Table 2). As concluded from the serial revisions of the prognostication models, thrombosis risk may vary according to the mutational status. For instance, the integration of MPL mutations in the revised IPSET model relied on the association of this mutation with older age (a main risk factor for ET prognosis) [38]. Although MPL-mutated patients share a similar clinical picture with CALR-mutants, the latter group has a lower incidence of thrombosis, especially when compared to JAK2 V617F mutated counterpart (10.5 vs. 25.1%, respectively; p = 0.01) [39]. As a matter of fact, also JAK2 V617F mutant allele burden has been related to both arterial (values >25%; p = 0.055) and venous (values > 90%; p = 0.036) thrombosis [40]. In the meantime, Passamonti et al. proposed a prognostic model (IPSET-survival) based on age (≥60 years), leukocyte count (WBC; ≥11 × 109/L), and history of thrombosis which enabled survival prediction at the time of diagnosis [41]. The IPSET-survival model consented to stratify ET patients into categories of significantly different survivals with median values ranging from 18.6 years in the low-risk category, to 7.1 years in the high-risk category.
Table 2. Evolution of prognostication systems in essential thrombocythemia.
|
Traditional ELN Guidelines |
|
(a) High-risk: age ≥ 60 years or previous thrombosis |
|
(b) Low-risk: none of the above |
|
IPSET-thrombosis |
|
Risk factors: Age > 60, = 1 point; Cardiovascular risk factors (tobacco use, diabetes, hypercholesterolaemia, hypertension), = 1 point; Previous thrombosis, = 2 point; JAK2 V617F, = 2 points |
|
(a) Low risk: 0–1 |
|
(b) Intermediate risk: 2 |
|
(c) High-risk: ≥3 |
|
IPSET- thrombosis (Revised) |
|
(a) Very low risk: no thrombosis history, age ≤ 60 years and JAK2/MPL-unmutated |
|
(b) Low risk: no thrombosis history, age ≤ 60 years and JAK2/MPL-mutated |
|
(c) Intermediate risk: no thrombosis history, age > 60 years and JAK2/MPL-unmutated |
|
(d) High risk: thrombosis history or age > 60 years with JAK2/MPL mutation |
|
MIPSS-ET |
|
Risk factors: Adverse mutations (SRSF2, SF3B1, U2AF1 and TP53) = 2 points; age > 60 years = 4 points, male sex = 1 point and leukocyte count ≥ 11 × 109/L = 1 point |
|
(a) Low risk: 0–1 |
|
(b) Intermediate risk: 2–3 |
|
(c) High-risk: ≥4. |
Little is known about the other non-driver mutations’ impact on the vascular risk assessment; however, the enrichment in TET2 mutations in ET has been identified to be associated with a higher incidence of thrombotic risk . After the new insights on the molecular characterization of MF, which led to the development of newer risk models that included genetic biomarkers such as MIPSS70 (Mutation-enhanced international prognostic systems) [42], Tefferi and colleagues developed a similar approach for ET and PV called the “MIPSS-ET/PV” [43]. After analyzing data from two different cohorts in this recent study, multivariable analysis confirmed an independent survival effect associated with the presence of adverse mutations (SRSF2, SF3B1, U2AF1, and TP53), age >60 years, male sex and a leukocyte count ≥11 × 109/L in ET patients. This led to a 3-tiered model that has been shown to be superior to the conventional IPSET-survival score in predicting survival rates of patients with ET. The main difference from the previous models is that thrombosis history is no longer considered a risk factor (Table 2).
3.2. Mutational Profile
As discussed, due to the chronic manifestation of this disease, the main goal of ET management remains the prevention of fatal vascular complications which have been reported to be the leading cause of death . Up to 24% of ET patients develop a vascular event before (13%) or after (11%) diagnosis with a lower rate in CALR-mutants as compared to JAK2 V617F/MPL mutants and triple-negative cases [44]. Apart from having different molecular lesions and survival outcomes, JAK2 V617F-mutated ET patients had a higher hemoglobin level and WBC (the so-called “PV-like phenotype”), and a lower PC while a substantial fraction of CALR-mutants had a PC >1000 × 109/L . Despite this association, CALR-mutated patients had a lower risk of thrombosis, pointing out that the PC per se is not the only determinant of vascular complications . Platelets from patients with CALR mutations were significantly less activated following adenosine diphosphate (ADP) stimulation compared to that of a control group or JAK2 mutants (p < 0.001) [45]. Moreover, among the two predominant variants of CALR mutations (type 1 and 2), patients harboring type 2 CALR mutations seemed to have a more indolent disease course [46].
3.3. Leukocytosis
As observed in PV, many studies demonstrated a correlation between leukocytosis and vascular events in ET . For instance, it has been observed that a near to linear correlation between thrombosis and WBC existed in ET patients . A recent meta-analysis highlighted the dual effect of leukocytosis on both thrombosis and bleeding events [47]. Moreover, this negative impact seemed to be independent of the PC per se as observed in patients treated with anagrelide with optimized PC < 575 × 109/L but WBC > 9.66 × 109/L [48]. This observation emphasizes the role of WBC beyond PC per se on triggering vascular complications, as highlighted and taken into consideration by risk assessment models. Finally, we might assume that the use of anagrelide, not providing a normalization of WBC, should be reserved for lower-risk patients without evidence of leukocytosis.
3.4. Inherited Thrombophilia
As aforementioned, about 25% of patients with ET experience an episode of thrombosis during the disease course. In this context, it is also important to identify possible carriers of other inherited thrombophilic risk-factors, including antithrombin and protein C functional activities, free protein S antigen, fasting homocysteine, Factor V Leiden, PT G20210A, and antiphospholipid antibodies (lupus anticoagulant, anticardiolipin antibodies, and anti-β2glycoprotein I). For example, De Stefano et al [49] reported that younger patients with ET are at higher risk of thrombotic events in the case of the concomitant presence of both JAK2 V617F mutation and inherited thrombophilia (5-fold increase as compared to non-carriers of either alteration). Particularly, Factor V Leiden seemed to be associated with an increased thrombotic risk in patients with ET [50]. Nevertheless, literature reports have been controversial and failed to demonstrate a higher prevalence of thrombophilic conditions in patients with ET.
4. Cytoreductive Therapies
Risk stratification is an early step that follows diagnosis and is critically important to guide clinicians towards appropriate therapeutic interventions. Cytoreductive therapies (e.g., hydroxyurea (HU), anagrelide, pegylated interferon α (P-IFNα), pipobroman, busulphan, and radioactive phosphorus) remain the backbone of treatments available for ET patients of the high-risk group. The specific drug of choice is basically selected according to the individual risk, defined as already discussed, by age, past medical history, type of driver mutation and, last but not least, the patient’s preferences in the context of a patient-physician therapeutic alliance. A detailed description of all the cytoreductive agents and their main conducted clinical trials in ET are reviewed below.
4.1. Hydroxyurea
The PT-1 randomized controlled trial (RCT) led to the wide use of HU as frontline therapy [51]. The study enrolled 809 high-risk patients with ET and showed that HU is superior to anagrelide in reducing arterial thrombosis, serious hemorrhage, and myelofibrotic progression. When combined with aspirin (ASA), HU decreased the rate of thrombotic events by 20.4% compared with single-agent ASA in a study of 114 patients [52]. However, the phase III ANAHYDRET trial concluded that anagrelide, as a selective platelet-lowering agent, was not inferior to HU in preventing thrombotic complications when ET patients were diagnosed according to the 2008 WHO criteria [53].
4.2. Interferons
An alternative frontline therapy is the recombinant interferon α [e.g., interferon α-2b, pegylated interferon α-2a (P-IFNα-2a), and pegylated interferon α-2b (P-IFNα-2b)] which has been traditionally used in ET and had successful hematologic and molecular responses [54][55]. Although the hematologic response was not different, JAK2 V617F-positive patients were more likely to achieve higher molecular response with P-IFNα, as compared to CALR-mutant individuals[55]and according to this evidence, a recent study suggested that higher doses are needed in the latter population [56]. Long-term efficacy and safety data of P-IFNα-2a treatment were reported in a single-center, open-label, phase 2 trial after a median follow up of 83 months [57]. The study showed that P-IFNα-2a can produce durable hematological and molecular responses in ET patients. Moreover, the Myeloproliferative Neoplasms Research Consortium (MPN-RC) 112 randomized trial that compared P-IFNα-2a vs. HU for the treatment of high-risk PV and ET, revealed that complete response rates were similar at 12 and 24 months, however, P-IFNα-2a was associated with a higher rate of grade 3/4 toxicity [58]. Both arms appeared to induce comparable effects on the spleen size, karyotypic abnormalities, histopathological parameters, incidence of thrombotic complications and disease evolution, making them equally capable of modifying the natural history of high-risk ET/PV.
Resistance or intolerance to HU occurs in 25 to 35% of ET patients who then become at a higher risk of overall mortality and leukemic transformation [59]. A prospective, open-label, phase II clinical trial for treatment with P-IFNα was conducted by the MPN-RC across several sites in North America and Europe and included 65 ET patients who were either resistant or intolerant to HU [60]. Treatment with P-IFNα showed an overall response in approximately two-thirds of patients with high-risk ET and PV. Moreover, the presence of CALR mutations was associated with higher complete response rates to P-IFNα-2a suggesting a stronger indication for it when the mutational status is known [60].
A known frontline therapy for PV that has been recently introduced and studied in ET is the very long-acting monopegylated interferon, ropeginterferon alfa-2b (R-INFα-2b). A prospective, open-label, multicenter phase 1/2 dose-escalation study (PEGINVERA) showed that R-INFα-2b induced high response rates at a low dose and with minimal toxicity in patients with PV [61]. The drug also showed non-inferiority to HU in PV when given every 2 weeks followed by once monthly as per the 3-year follow-up data [62]. The non-inferiority to HU in terms of hematological response and normal spleen size was not evident at 12 months; however, improved responses happened at 36 months [62]. It has been also reported that R-INFα-2b can induce significant partial and complete molecular response rates, as reflected by the reduction of the JAK2 allelic burden and more recently also of TET2 [63]. R-INFα-2b is a promising medication for ET patients and is currently being investigated as a potential second-line therapy in the setting of HU resistance or intolerance. Presumably, future trials will be conducted to study it as a promising frontline treatment for ET as compared to HU or best available therapy (BAT).
4.3. Ruxolitinib
Besides, a long-term phase 2 study of ruxolitinib, a JAK1/2 inhibitor, demonstrated that patients with ET who are refractory to or intolerant of HU can achieve clinically meaningful and durable reductions in PC and WBC, and improvements in ET-related symptoms with this treatment [64]. When compared with BAT in a randomized phase II trial in patients resistant or intolerant to HU (MAJIC-ET), no significant difference in the rate of complete or partial hematologic remission was found [65]. In addition, the incidence rate of thrombosis, hemorrhage, and leukemic transformation at 2 years was not significantly different. However, symptomatic relief with improved itching and weight loss was reported with ruxolitinib [65]. Hence, the use of this drug remains controversial. Of note, the phase IIb RUXBETA trial that compared ruxolitinib vs. BAT in patients with high-risk ET refractory after first-line treatment (NCT02962388) has been abandoned. Ongoing clinical trials assessing ruxolitinib in adults with ET include: (i) phase 3 Ruxo-BEAT trial that compares ruxolitinib vs. BAT in patients with high-risk PV or high-risk ET(NCT02577926) and (ii) phase 2 RESET-272 trial with ruxolitinib vs. anagrelide in subjects with ET who are resistant to or intolerant of HU (NCT03123588). Perhaps, future trials will aim to understand the potential benefit and efficacy of combination therapies with ruxolitinib as compared to monotherapy.
4.4. Therapeutic-Choice Considerations
In summary, cytoreductive agents showed comparable capabilities of achieving the primary endpoints of complete or partial hematologic remission, molecular response (a new therapy achievement in the NGS era), tolerability, and prevention of thrombosis. Perhaps the main influence on the physician’s therapeutic decision-making remains the patient’s circumstances, life events, and history. For example, P-IFNα is quite safer than HU for young female patients wishing to conceive [66][67][68][69][70]. The long-term follow-up of patients treated with these therapies in the clinical practice has helped describe the main adverse effects related to their use. For instance, HU can cause oral ulcers, skin hyperpigmentation, rashes, and teratogenicity [71]; anagrelide has been associated with fluid retention, headaches, heart palpitations, and cardiomyopathy [72][73]; P-IFNα can cause depression, flu-like symptoms, headache, malaise, fevers, arthralgia, pruritus, injection-site reactions, gonadal toxicity, and thyroid dysfunction. Other cytoreductive agents like pipobroman, busulfan, and radioactive phosphorus have been historically used but are reported to accelerate leukemic transformation [74].
5. Extreme Thrombocytosis and Acquired von Willebrand Syndrome: The Paradox of Hemorrhagic Thrombocythemia
Since the first report in 1934, it has been recognized that extreme thrombocytosis (PC > 1000 × 109/L) in ET may be associated with bleeding complications [75]. However, the paradox of a bleeding diathesis in a patient with high PC is a clinical conundrum and the correct identification and management of AVWS is still a matter of an individual clinical acumen among physicians.
AVWS is a rare bleeding disorder that is similar to its inherited counterpart in terms of clinical and laboratory manifestations. VWF is a large multimeric glycoprotein that is mostly produced and stored in Wiebel-Palade bodies in endothelial cells. While inactive in circulation, VWF is bound to factor VIII, which in turn protects it from bloodstream degradation. The facilitated interaction between platelets factor receptor GPIb and VWF due to high platelet numbers, as seen in ET, permits the degradation of VWF via ADAMTS13. More in general, AVWS is the result of low levels of VWF due to its accelerated removal from plasma by: (i) antibodies, as in the case of lymphoproliferative or immunologic conditions; (ii) in vivo absorption onto malignant cells, as in ET or solid tumors; (iii) conditions of high shear stress, as in cardiovascular disorders [76]. When the last two mechanisms are at play, a preferential removal of high molecular weight (HMW) VWF multimers occurs and results in a phenotype resembling type 2A or 2B VWF disease [77]. Thus, AVWS in ET is more characterized by a qualitative deficiency rather than a quantitative defect of VWF. This translates into normal levels of VWF:Ag (VWF antigen) with decreased ristocetin cofactor activity (VWF:RCoA usually below 20% in AVWS) and ultimately a VWF: RCoA/VWF:Ag ratio < 0.7 (normal value around 1) [78]. The pattern of VWF multimers is almost identical to type 2 VWD as depicted by multimeric analysis of patients with ET and extreme thrombocytosis before treatment with desmopressin (DDAVP) which corrects the VWF multimeric distribution [77] Therefore, patients with AVWS are prone to experience bleeding complications.
The scenario is even more complicated when considering patients with ET presenting with extreme thrombocytosis who are often under ASA or anticoagulant treatment (Figure 1). This subset of patients shows a significantly prolonged Ivy bleeding time when compared to normal control and patients with reactive thrombocytosis (RT) [79]. The difference in bleeding time in RT and normal controls is explained by the thrombocytopathy of patients with ET. Malignant platelets are hypersensitive, activated by the high shear stress in the microcirculation, and show a secondary storage pool disease with pseudopodia and centralized granules . Based on these insights, the use of antiplatelet agents, a common clinical practice in patients with ET as aforementioned, requires caution. Indeed, situations of extreme thrombocytosis may render clinicians’ choices a cause of anxiety due to an unpredictable thrombo-hemorrhagic balance.
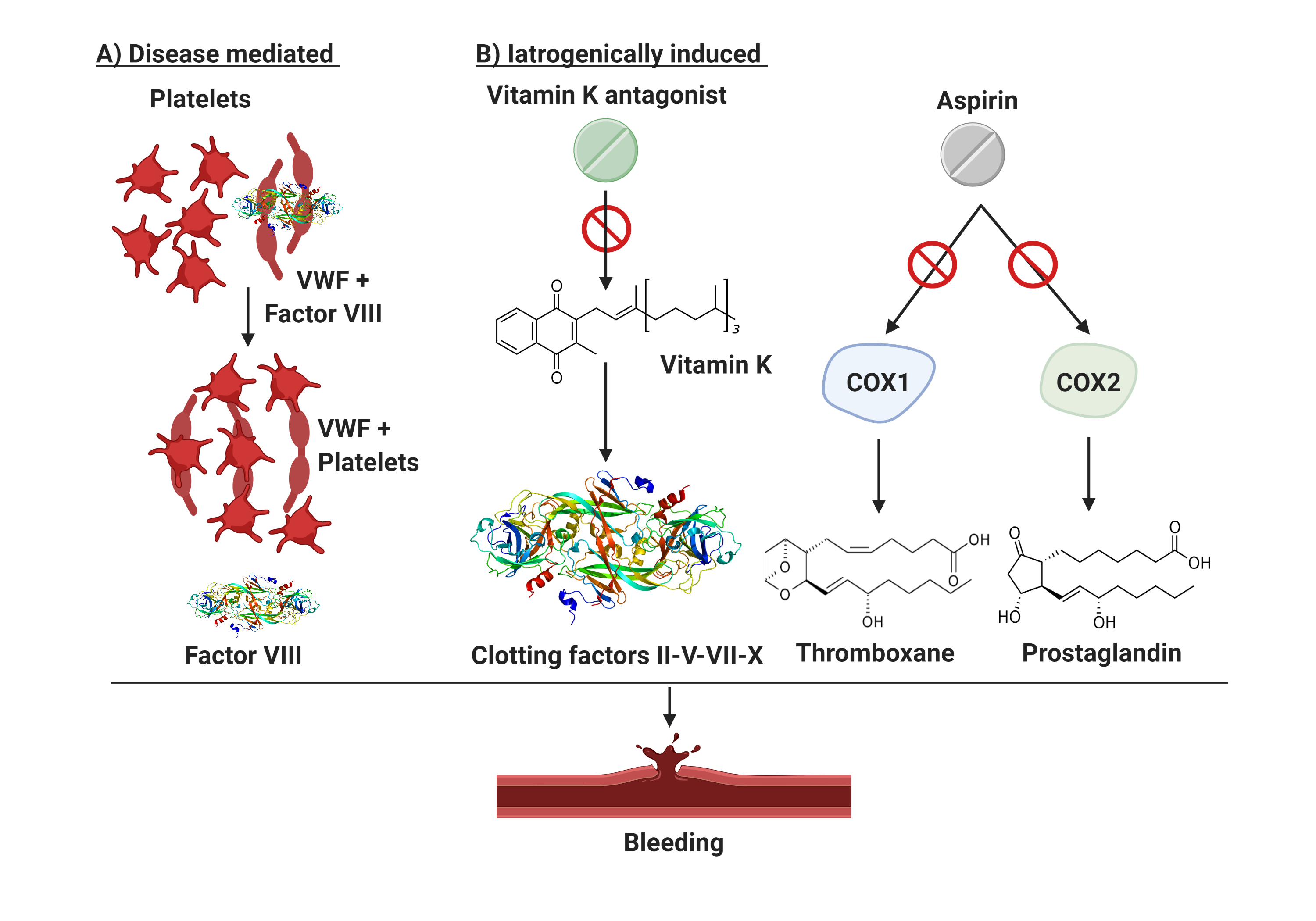
Figure 1. Bleeding mechanisms in patients with essential thrombocythemia. Patients with ET are prone to bleeding from AVWS or due to the use of antiplatelet/anticoagulant drugs. (A) illustrates the disease mediated mechanism in setting of excessive thrombocytosis which may cause AVWS due to in vivo absorption of VWF multimers. (B) describes iatrogenically induced bleeding risk in ET. Aspirin suppresses the production of thromboxane and prostaglandins by inactivating cyclooxygenase enzymes 1 and 2 (COX1/2) and thus impairing platelets aggregation. Vitamin K antagonist (used in selected patients with ET with active or prior thrombosis) have an intrinsic bleeding risk because of their narrow therapeutic window. Finally, excessive thrombocytosis may cause AVWS due to in vivo absorption of VWF multimers. COX1/2, cyclooxygenase enzymes type 1 and 2; VWF, von Willebrand factor.
References
- Campo, E.; Swerdlow, S.H.; Harris, N.L.; Pileri, S.; Stein, H.; Jaffe, E.S. The 2008 WHO classification of lymphoid neoplasms and beyond: Evolving concepts and practical applications. Blood 2011, 117, 5019–5032, doi:10.1182/blood-2011-01-293050.
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405, doi:10.1182/blood-2016-03-643544.
- Mehta, J.; Wang, H.; Iqbal, S.U.; Mesa, R. Epidemiology of myeloproliferative neoplasms in the United States. Leuk. Lymphoma 2014, 55, 595–600, doi:10.3109/10428194.2013.813500.
- Boyd, E.M.; Bench, A.J.; Goday-Fernandez, A.; Anand, S.; Vaghela, K.J.; Beer, P.; Scott, M.A.; Bareford, D.; Green, A.R.; Huntly, B.; et al. Clinical utility of routine MPL exon 10 analysis in the diagnosis of essential thrombocythaemia and primary myelofibrosis. Br. J. Haematol. 2010, 149, 250–257, doi:10.1111/j.1365-2141.2010.08083.x.
- Tefferi, A. Myeloproliferative neoplasms: A decade of discoveries and treatment advances. Am. J. Hematol. 2016, 91, 50–58, doi:10.1002/ajh.24221.
- Vannucchi, A.M.; Antonioli, E.; Guglielmelli, P.; Rambaldi, A.; Barosi, G.; Marchioli, R.; Marfisi, R.M.; Finazzi, G.; Guerini, V.; Fabris, F.; et al. Clinical profile of homozygous JAK2 617V>F mutation in patients with polycythemia vera or essential thrombocythemia. Blood 2007, 110, 840–846, doi:10.1182/blood-2006-12-064287.
- Klampfl, T.; Gisslinger, H.; Harutyunyan, A.S.; Nivarthi, H.; Rumi, E.; Milosevic, J.D.; Them, N.C.; Berg, T.; Gisslinger, B.; Pietra, D.; et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N. Engl. J. Med. 2013, 369, 2379–2390, doi:10.1056/NEJMoa1311347.
- Nangalia, J.; Green, T.R. The evolving genomic landscape of myeloproliferative neoplasms. Hematol. Am Soc. Hematol. Educ. Program 2014, 2014, 287–296, doi:10.1182/asheducation-2014.1.287.
- Cervantes, F.; Alvarez-Larran, A.; Talarn, C.; Gomez, M.; Montserrat, E. Myelofibrosis with myeloid metaplasia following essential thrombocythaemia: Actuarial probability, presenting characteristics and evolution in a series of 195 patients. Br. J. Haematol. 2002, 118, 786–790, doi:10.1046/j.1365-2141.2002.03688.x.
- Tefferi, A.; Pardanani, A. Essential Thrombocythemia. N. Engl. J. Med. 2019, 381, 2135–2144, doi:10.1056/NEJMcp1816082.
- Tefferi, A.; Lasho, T.L.; Tischer, A.; Wassie, E.A.; Finke, C.M.; Belachew, A.A.; Ketterling, R.P.; Hanson, C.A.; Pardanani, A.D. The prognostic advantage of calreticulin mutations in myelofibrosis might be confined to type 1 or type 1-like CALR variants. Blood 2014, 124, 2465–2466, doi:10.1182/blood-2014-07-588426.
- Tefferi, A.; Lasho, T.L.; Finke, C.M.; Knudson, R.A.; Ketterling, R.; Hanson, C.H.; Maffioli, M.; Caramazza, D.; Passamonti, F.; Pardanani, A. CALR vs JAK2 vs MPL-mutated or triple-negative myelofibrosis: Clinical, cytogenetic and molecular comparisons. Leukemia 2014, 28, 1472–1477, doi:10.1038/leu.2014.3.
- Shammo, J.M.; Stein, B.L. Mutations in MPNs: Prognostic implications, window to biology, and impact on treatment decisions. Hematol. Am. Soc. Hematol. Educ. Program 2016, 2016, 552–560, doi:10.1182/asheducation-2016.1.552.
- Tefferi, A.; Guglielmelli, P.; Larson, D.R.; Finke, C.; Wassie, E.A.; Pieri, L.; Gangat, N.; Fjerza, R.; Belachew, A.A.; Lasho, T.L.; et al. Long-term survival and blast transformation in molecularly annotated essential thrombocythemia, polycythemia vera, and myelofibrosis. Blood 2014, 124, 2507–2513, quiz 2615, doi:10.1182/blood-2014-05-579136.
- Milosevic Feenstra, J.D.; Nivarthi, H.; Gisslinger, H.; Leroy, E.; Rumi, E.; Chachoua, I.; Bagienski, K.; Kubesova, B.; Pietra, D.; Gisslinger, B.; et al. Whole-exome sequencing identifies novel MPL and JAK2 mutations in triple-negative myeloproliferative neoplasms. Blood 2016, 127, 325–332, doi:10.1182/blood-2015-07-661835.
- Cabagnols, X.; Favale, F.; Pasquier, F.; Messaoudi, K.; Defour, J.P.; Ianotto, J.C.; Marzac, C.; Le Couédic, J.P.; Droin, N.; Chachoua, I.; et al. Presence of atypical thrombopoietin receptor (MPL) mutations in triple-negative essential thrombocythemia patients. Blood 2016, 127, 333–342, doi:10.1182/blood-2015-07-661983.
- Abdulkarim, K.; Girodon, F.; Johansson, P.; Maynadie, M.; Kutti, J.; Carli, P.M.; Bovet, E.; Andreasson, B. AML transformation in 56 patients with Ph- MPD in two well defined populations. Eur. J. Haematol. 2009, 82, 106–111, doi:10.1111/j.1600-0609.2008.01163.x.
- Spivak, J.L. Myeloproliferative Neoplasms. N. Engl. J. Med. 2017, 376, 2168–2181, doi:10.1056/NEJMra1406186.
- Tefferi, A.; Lasho, T.L.; Guglielmelli, P.; Finke, C.M.; Rotunno, G.; Elala, Y.; Pacilli, A.; Hanson, C.A.; Pancrazzi, A.; Ketterling, R.P.; et al. Targeted deep sequencing in polycythemia vera and essential thrombocythemia. Blood Adv. 2016, 1, 21–30, doi:10.1182/bloodadvances.2016000216.
- Radaelli, F.; Colombi, M.; Calori, R.; Zilioli, V.R.; Bramanti, S.; Iurlo, A.; Zanella, A. Analysis of risk factors predicting thrombotic and/or haemorrhagic complications in 306 patients with essential thrombocythemia. Hematol. Oncol. 2007, 25, 115–120, doi:10.1002/hon.816.
- Cervantes, F. Management of essential thrombocythemia. Hematol. Am Soc. Hematol. Educ. Program 2011, 2011, 215–221, doi:10.1182/asheducation-2011.1.215.
- Cortelazzo, S.; Viero, P.; Finazzi, G.; D’Emilio, A.; Rodeghiero, F.; Barbui, T. Incidence and risk factors for thrombotic complications in a historical cohort of 100 patients with essential thrombocythemia. J. Clin. Oncol. 1990, 8, 556–562, doi:10.1200/jco.1990.8.3.556.
- Besses, C.; Cervantes, F.; Pereira, A.; Florensa, L.; Sole, F.; Hernandez-Boluda, J.C.; Woessner, S.; Sans-Sabrafen, J.; Rozman, C.; Montserrat, E. Major vascular complications in essential thrombocythemia: A study of the predictive factors in a series of 148 patients. Leukemia 1999, 13, 150–154, doi:10.1038/sj.leu.2401270.
- Alvarez-Larran, A.; Cervantes, F.; Bellosillo, B.; Giralt, M.; Julia, A.; Hernandez-Boluda, J.C.; Bosch, A.; Hernandez-Nieto, L.; Clapes, V.; Burgaleta, C.; et al. Essential thrombocythemia in young individuals: Frequency and risk factors for vascular events and evolution to myelofibrosis in 126 patients. Leukemia 2007, 21, 1218–1223, doi:10.1038/sj.leu.2404693.
- Stein, B.L.; Martin, K. From Budd-Chiari syndrome to acquired von Willebrand syndrome: Thrombosis and bleeding complications in the myeloproliferative neoplasms. Hematol. Am. Soc. Hematol. Educ. Program 2019, 2019, 397–406, doi:10.1182/hematology.2019001318.
- Moulard, O.; Mehta, J.; Fryzek, J.; Olivares, R.; Iqbal, U.; Mesa, R.A. Epidemiology of myelofibrosis, essential thrombocythemia, and polycythemia vera in the European Union. Eur. J. Haematol. 2014, 92, 289–297, doi:10.1111/ejh.12256.
- Passamonti, F.; Rumi, E.; Pungolino, E.; Malabarba, L.; Bertazzoni, P.; Valentini, M.; Orlandi, E.; Arcaini, L.; Brusamolino, E.; Pascutto, C.; et al. Life expectancy and prognostic factors for survival in patients with polycythemia vera and essential thrombocythemia. Am. J. Med. 2004, 117, 755–761, doi:10.1016/j.amjmed.2004.06.032.
- Campbell, P.J.; MacLean, C.; Beer, P.A.; Buck, G.; Wheatley, K.; Kiladjian, J.J.; Forsyth, C.; Harrison, C.N.; Green, A.R. Correlation of blood counts with vascular complications in essential thrombocythemia: Analysis of the prospective PT1 cohort. Blood 2012, 120, 1409–1411, doi:10.1182/blood-2012-04-424911.
- Tefferi, A.; Fonseca, R.; Pereira, D.L.; Hoagland, H.C. A long-term retrospective study of young women with essential thrombocythemia. Mayo Clin. Proc. 2001, 76, 22–28, doi:10.4065/76.1.22.
- Falchi, L.; Bose, P.; Newberry, K.J.; Verstovsek, S. Approach to patients with essential thrombocythaemia and very high platelet counts: What is the evidence for treatment? Br. J. Haematol. 2017, 176, 352–364, doi:10.1111/bjh.14443.
- Michiels, J.J. Acquired von Willebrand disease due to increasing platelet count can readily explain the paradox of thrombosis and bleeding in thrombocythemia. Clin. Appl. Thromb. Hemost. 1999, 5, 147–151, doi:10.1177/107602969900500301.
- Lussana, F.; Carobbio, A.; Salmoiraghi, S.; Guglielmelli, P.; Vannucchi, A.M.; Bottazzi, B.; Leone, R.; Mantovani, A.; Barbui, T.; Rambaldi, A. Driver mutations (JAK2V617F, MPLW515L/K or CALR), pentraxin-3 and C-reactive protein in essential thrombocythemia and polycythemia vera. J. Hematol. Oncol. 2017, 10, 54, doi:10.1186/s13045-017-0425-z.
- Cervantes, F.; Pereira, A. Does ruxolitinib prolong the survival of patients with myelofibrosis? Blood 2017, 129, 832–837, doi:10.1182/blood-2016-11-731604.
- Barbui, T.; Barosi, G.; Birgegard, G.; Cervantes, F.; Finazzi, G.; Griesshammer, M.; Harrison, C.; Hasselbalch, H.C.; Hehlmann, R.; Hoffman, R.; et al. Philadelphia-negative classical myeloproliferative neoplasms: Critical concepts and management recommendations from European LeukemiaNet. J. Clin. Oncol. 2011, 29, 761–770, doi:10.1200/jco.2010.31.8436.
- Barbui, T.; Finazzi, G.; Carobbio, A.; Thiele, J.; Passamonti, F.; Rumi, E.; Ruggeri, M.; Rodeghiero, F.; Randi, M.L.; Bertozzi, I.; et al. Development and validation of an International Prognostic Score of thrombosis in World Health Organization-essential thrombocythemia (IPSET-thrombosis). Blood 2012, 120, 5128–5133, quiz 5252, doi:10.1182/blood-2012-07-444067.
- Barbui, T.; Vannucchi, A.M.; Buxhofer-Ausch, V.; De Stefano, V.; Betti, S.; Rambaldi, A.; Rumi, E.; Ruggeri, M.; Rodeghiero, F.; Randi, M.L.; et al. Practice-relevant revision of IPSET-thrombosis based on 1019 patients with WHO-defined essential thrombocythemia. Blood Cancer J. 2015, 5, e369, doi:10.1038/bcj.2015.94.
- Haider, M.; Gangat, N.; Lasho, T.; Abou Hussein, A.K.; Elala, Y.C.; Hanson, C.; Tefferi, A. Validation of the revised International Prognostic Score of Thrombosis for Essential Thrombocythemia (IPSET-thrombosis) in 585 Mayo Clinic patients. Am. J. Hematol. 2016, 91, 390–394, doi:10.1002/ajh.24293.
- Alvarez-Larran, A.; Martinez, D.; Arenillas, L.; Rubio, A.; Arellano-Rodrigo, E.; Hernandez Boluda, J.C.; Papaleo, N.; Caballero, G.; Martinez, C.; Ferrer-Marin, F.; et al. Essential thrombocythaemia with mutation in MPL: Clinicopathological correlation and comparison with JAK2V617F-mutated and CALR-mutated genotypes. J. Clin. Pathol. 2018, 71, 975–980, doi:10.1136/jclinpath-2018-205227.
- Rumi, E.; Pietra, D.; Ferretti, V.; Klampfl, T.; Harutyunyan, A.S.; Milosevic, J.D.; Them, N.C.; Berg, T.; Elena, C.; Casetti, I.C.; et al. JAK2 or CALR mutation status defines subtypes of essential thrombocythemia with substantially different clinical course and outcomes. Blood 2014, 123, 1544–1551, doi:10.1182/blood-2013-11-539098.
- Horvat, I.; Boban, A.; Zadro, R.; Antolic, M.R.; Serventi-Seiwerth, R.; Roncevic, P.; Radman, I.; Sertic, D.; Vodanovic, M.; Pulanic, D.; et al. Influence of Blood Count, Cardiovascular Risks, Inherited Thrombophilia, and JAK2 V617F Burden Allele on Type of Thrombosis in Patients With Philadelphia Chromosome Negative Myeloproliferative Neoplasms. Clin. Lymphoma Myeloma Leuk. 2019, 19, 53–63, doi:10.1016/j.clml.2018.08.020.
- Passamonti, F.; Thiele, J.; Girodon, F.; Rumi, E.; Carobbio, A.; Gisslinger, H.; Kvasnicka, H.M.; Ruggeri, M.; Randi, M.L.; Gangat, N.; et al. A prognostic model to predict survival in 867 World Health Organization-defined essential thrombocythemia at diagnosis: A study by the International Working Group on Myelofibrosis Research and Treatment. Blood 2012, 120, 1197–1201, doi:10.1182/blood-2012-01-403279.
- Guglielmelli, P.; Lasho, T.L.; Rotunno, G.; Mudireddy, M.; Mannarelli, C.; Nicolosi, M.; Pacilli, A.; Pardanani, A.; Rumi, E.; Rosti, V.; et al. MIPSS70: Mutation-Enhanced International Prognostic Score System for Transplantation-Age Patients With Primary Myelofibrosis. J. Clin. Oncol. 2018, 36, 310–318, doi:10.1200/jco.2017.76.4886.
- Tefferi, A.; Guglielmelli, P.; Lasho, T.L.; Coltro, G.; Finke, C.M.; Loscocco, G.G.; Sordi, B.; Szuber, N.; Rotunno, G.; Pacilli, A.; et al. Mutation-enhanced international prognostic systems for essential thrombocythaemia and polycythaemia vera. Br. J. Haematol. 2020, 189, 291–302, doi:10.1111/bjh.16380.
- Santoro, M.; Accurso, V.; Mancuso, S.; Carlisi, M.; Raso, S.; Tarantino, G.; Di Piazza, F.; Perez, A.; Russo, A.; Siragusa, S. Comparison between thrombotic risk scores in essential thrombocythemia and survival implications. Hematol. Oncol. 2019, 37, 434–437, doi:10.1002/hon.2670.
- Hauschner, H.; Bokstad Horev, M.; Misgav, M.; Nagar, M.; Seligsohn, U.; Rosenberg, N.; Koren-Michowitz, M. Platelets from Calreticulin mutated essential thrombocythemia patients are less reactive than JAK2 V617F mutated platelets. Am. J. Hematol. 2019, doi:10.1002/ajh.25713.
- Cottin, L.; Riou, J.; Orvain, C.; Ianotto, J.C.; Boyer, F.; Renard, M.; Truchan-Graczyk, M.; Murati, A.; Jouanneau-Courville, R.; Allangba, O.; et al. Sequential mutational evaluation of CALR -mutated myeloproliferative neoplasms with thrombocytosis reveals an association between CALR allele burden evolution and disease progression. Br. J. Haematol. 2020, 188, 935–944, doi:10.1111/bjh.16276.
- Carobbio, A.; Ferrari, A.; Masciulli, A.; Ghirardi, A.; Barosi, G.; Barbui, T. Leukocytosis and thrombosis in essential thrombocythemia and polycythemia vera: A systematic review and meta-analysis. Blood Adv. 2019, 3, 1729–1737, doi:10.1182/bloodadvances.2019000211.
- Buxhofer-Ausch, V.; Steurer, M.; Sormann, S.; Schloegl, E.; Schimetta, W.; Gisslinger, B.; Schalling, M.; Krauth, M.T.; Thiele, J.; Ruckser, R.; et al. Impact of white blood cells on thrombotic risk in patients with optimized platelet count in essential thrombocythemia. Eur. J. Haematol. 2018, doi:10.1111/ejh.13070.
- De Stefano, V.; Za, T.; Rossi, E.; Fiorini, A.; Ciminello, A.; Luzzi, C.; Chiusolo, P.; Sica, S.; Leone, G. Influence of the JAK2 V617F mutation and inherited thrombophilia on the thrombotic risk among patients with essential thrombocythemia. Haematologica 2009, 94, 733–737, doi:10.3324/haematol.13869.
- Trifa, A.P.; Cucuianu, A.; Popp, R.A.; Coada, C.A.; Costache, R.M.; Militaru, M.S.; Vesa, S.C.; Pop, I.V. The relationship between factor V Leiden, prothrombin G20210A, and MTHFR mutations and the first major thrombotic episode in polycythemia vera and essential thrombocythemia. Ann. Hematol. 2014, 93, 203–209, doi:10.1007/s00277-013-1838-6.
- Harrison, C.N.; Campbell, P.J.; Buck, G.; Wheatley, K.; East, C.L.; Bareford, D.; Wilkins, B.S.; van der Walt, J.D.; Reilly, J.T.; Grigg, A.P.; et al. Hydroxyurea compared with anagrelide in high-risk essential thrombocythemia. N. Engl. J. Med. 2005, 353, 33–45, doi:10.1056/NEJMoa043800.
- Cortelazzo, S.; Finazzi, G.; Ruggeri, M.; Vestri, O.; Galli, M.; Rodeghiero, F.; Barbui, T. Hydroxyurea for patients with essential thrombocythemia and a high risk of thrombosis. N. Engl. J. Med. 1995, 332, 1132–1136, doi:10.1056/nejm199504273321704.
- Gisslinger, H.; Gotic, M.; Holowiecki, J.; Penka, M.; Thiele, J.; Kvasnicka, H.M.; Kralovics, R.; Petrides, P.E. Anagrelide compared with hydroxyurea in WHO-classified essential thrombocythemia: The ANAHYDRET Study, a randomized controlled trial. Blood 2013, 121, 1720–1728, doi:10.1182/blood-2012-07-443770.
- Kiladjian, J.J.; Chomienne, C.; Fenaux, P. Interferon-alpha therapy in bcr-abl-negative myeloproliferative neoplasms. Leukemia 2008, 22, 1990–1998, doi:10.1038/leu.2008.280.
- Quintas-Cardama, A.; Kantarjian, H.; Manshouri, T.; Luthra, R.; Estrov, Z.; Pierce, S.; Richie, M.A.; Borthakur, G.; Konopleva, M.; Cortes, J.; et al. Pegylated interferon alfa-2a yields high rates of hematologic and molecular response in patients with advanced essential thrombocythemia and polycythemia vera. J. Clin. Oncol. 2009, 27, 5418–5424, doi:10.1200/jco.2009.23.6075.
- Czech, J.; Cordua, S.; Weinbergerova, B.; Baumeister, J.; Crepcia, A.; Han, L.; Maie, T.; Costa, I.G.; Denecke, B.; Maurer, A.; et al. JAK2V617F but not CALR mutations confer increased molecular responses to interferon-alpha via JAK1/STAT1 activation. Leukemia 2019, 33, 995–1010, doi:10.1038/s41375-018-0295-6.
- Masarova, L.; Patel, K.P.; Newberry, K.J.; Cortes, J.; Borthakur, G.; Konopleva, M.; Estrov, Z.; Kantarjian, H.; Verstovsek, S. Pegylated interferon alfa-2a in patients with essential thrombocythaemia or polycythaemia vera: A post-hoc, median 83 month follow-up of an open-label, phase 2 trial. Lancet Haematol. 2017, 4, e165–e175, doi:10.1016/s2352-3026(17)30030-3.
- Mascarenhas, J.; Kosiorek, H.E.; Prchal, J.T.; Rambaldi, A.; Berenzon, D.; Yacoub, A.; Harrison, C.N.; McMullin, M.F.; Vannucchi, A.M.; Ewing, J.; et al. Results of the Myeloproliferative Neoplasms - Research Consortium (MPN-RC) 112 Randomized Trial of Pegylated Interferon Alfa-2a (PEG) Versus Hydroxyurea (HU) Therapy for the Treatment of High Risk Polycythemia Vera (PV) and High Risk Essential Thrombocythemia (ET). Blood 2018, 132, 577–577, doi:10.1182/blood-2018-99-111946.
- Alvarez-Larran, A.; Pereira, A.; Cervantes, F.; Arellano-Rodrigo, E.; Hernandez-Boluda, J.C.; Ferrer-Marin, F.; Angona, A.; Gomez, M.; Muina, B.; Guillen, H.; et al. Assessment and prognostic value of the European LeukemiaNet criteria for clinicohematologic response, resistance, and intolerance to hydroxyurea in polycythemia vera. Blood 2012, 119, 1363–1369, doi:10.1182/blood-2011-10-387787.
- Yacoub, A.; Mascarenhas, J.; Kosiorek, H.; Prchal, J.T.; Berenzon, D.; Baer, M.R.; Ritchie, E.; Silver, R.T.; Kessler, C.; Winton, E.; et al. Pegylated interferon alfa-2a for polycythemia vera or essential thrombocythemia resistant or intolerant to hydroxyurea. Blood 2019, 134, 1498–1509, doi:10.1182/blood.2019000428.
- Gisslinger, H.; Zagrijtschuk, O.; Buxhofer-Ausch, V.; Thaler, J.; Schloegl, E.; Gastl, G.A.; Wolf, D.; Kralovics, R.; Gisslinger, B.; Strecker, K.; et al. Ropeginterferon alfa-2b, a novel IFNalpha-2b, induces high response rates with low toxicity in patients with polycythemia vera. Blood 2015, 126, 1762–1769, doi:10.1182/blood-2015-04-637280.
- Gisslinger, H.; Klade, C.; Georgiev, P.; Krochmalczyk, D.; Gercheva-Kyuchukova, L.; Egyed, M.; Rossiev, V.; Dulicek, P.; Illes, A.; Pylypenko, H.; et al. Ropeginterferon alfa-2b versus standard therapy for polycythaemia vera (PROUD-PV and CONTINUATION-PV): A randomised, non-inferiority, phase 3 trial and its extension study. Lancet Haematol. 2020, 7, e196–e208, doi:10.1016/s2352-3026(19)30236-4.
- Kralovics, R.; Klade, C.; Georgiev, P.; Krochmalczyk, D.; Gercheva-Kyuchukova, L.; Egyed, M.; Rossiev, V.; Dulicek, P.; Illes, A.; Pylypenko, H.; et al. Ropeginterferon Alpha-2b is efficacious and reduces variant Tet2 allele burden in patients with polycythaemia vera and Tet2 mutation: genetic analysis of Phase III PROUD-PV/CONTINUATION-PV studies. Hemasphere 2020, 4, 1–1168.
- Verstovsek, S.; Passamonti, F.; Rambaldi, A.; Barosi, G.; Rumi, E.; Gattoni, E.; Pieri, L.; Zhen, H.; Granier, M.; Assad, A.; et al. Ruxolitinib for essential thrombocythemia refractory to or intolerant of hydroxyurea: Long-term phase 2 study results. Blood 2017, 130, 1768–1771, doi:10.1182/blood-2017-02-765032.
- Harrison, C.N.; Mead, A.J.; Panchal, A.; Fox, S.; Yap, C.; Gbandi, E.; Houlton, A.; Alimam, S.; Ewing, J.; Wood, M.; et al. Ruxolitinib vs best available therapy for ET intolerant or resistant to hydroxycarbamide. Blood 2017, 130, 1889–1897, doi:10.1182/blood-2017-05-785790.
- Harrison, C. Pregnancy and its management in the Philadelphia negative myeloproliferative diseases. Br. J. Haematol. 2005, 129, 293–306, doi:10.1111/j.1365-2141.2005.05400.x.
- Harrison, C.N.; Bareford, D.; Butt, N.; Campbell, P.; Conneally, E.; Drummond, M.; Erber, W.; Everington, T.; Green, A.R.; Hall, G.W.; et al. Guideline for investigation and management of adults and children presenting with a thrombocytosis. Br. J. Haematol. 2010, 149, 352–375, doi:10.1111/j.1365-2141.2010.08122.x.
- Beauverd, Y.; Radia, D.; Cargo, C.; Knapper, S.; Drummond, M.; Pillai, A.; Harrison, C.; Robinson, S. Pegylated interferon alpha-2a for essential thrombocythemia during pregnancy: Outcome and safety. A case series. Haematologica 2016, 101, e182–e184, doi:10.3324/haematol.2015.139691.
- Singh, N.; Kumar, S.; Roy, K.K.; Sharma, V.; Jalak, A. Successful maternal and fetal outcome in a rare case of essential Thrombocythemia with pregnancy using Interferon alpha. Platelets 2012, 23, 319–321, doi:10.3109/09537104.2011.611919.
- Martinelli, P.; Martinelli, V.; Agangi, A.; Maruotti, G.M.; Paladini, D.; Ciancia, R.; Rotoli, B. Interferon alfa treatment for pregnant women affected by essential thrombocythemia: Case reports and a review. Am. J. Obs. Gynecol. 2004, 191, 2016–2020, doi:10.1016/j.ajog.2004.05.001.
- Antonioli, E.; Guglielmelli, P.; Pieri, L.; Finazzi, M.; Rumi, E.; Martinelli, V.; Vianelli, N.; Luigia Randi, M.; Bertozzi, I.; De Stefano, V.; et al. Hydroxyurea-related toxicity in 3,411 patients with Ph’-negative MPN. Am. J. Hematol. 2012, 87, 552–554, doi:10.1002/ajh.23160.
- Anagrelide, a therapy for thrombocythemic states: Experience in 577 patients. Anagrelide Study Group. Am. J. Med. 1992, 92, 69–76, doi:10.1016/0002-9343(92)90017-6.
- Jurgens, D.J.; Moreno-Aspitia, A.; Tefferi, A. Anagrelide-associated cardiomyopathy in polycythemia vera and essential thrombocythemia. Haematologica 2004, 89, 1394–1395.
- Harrison, C.N. Essential thrombocythaemia: Challenges and evidence-based management. Br. J. Haematol. 2005, 130, 153–165, doi:10.1111/j.1365-2141.2005.05543.x.
- Epstein, E.; Goedel, A. Hämorrhagische Thrombocythämie bei vasculärer Schrumpfmilz. Virchows Archiv Pathologische Anatomie Physiologie Klinische Medizin 1934, 292, 233–248, doi:10.1007/BF01891529.
- Federici, A.B. Acquired von Willebrand syndrome: An underdiagnosed and misdiagnosed bleeding complication in patients with lymphoproliferative and myeloproliferative disorders. Semin. Hematol. 2006, 43, S48–S58, doi:10.1053/j.seminhematol.2005.11.003.
- Michiels, J.J.; Berneman, Z.; Schroyens, W.; Finazzi, G.; Budde, U.; van Vliet, H.H. The paradox of platelet activation and impaired function: Platelet-von Willebrand factor interactions, and the etiology of thrombotic and hemorrhagic manifestations in Essential thrombocythemia and polycythemia vera. Semin. Thromb. Hemost. 2006, 32, 589–604, doi:10.1055/s-2006-949664.
- Tefferi, A.; Smock, K.J.; Divgi, A.B. Polycythemia vera-associated acquired von Willebrand syndrome despite near-normal platelet count. Am. J. Hematol. 2010, 85, 545, doi:10.1002/ajh.21730.
- Troost, M.M.; van Genderen, P.J. Excessive prolongation of the Ivy bleeding time after aspirin in essential thrombocythemia is also demonstrable in vitro in the high shear stress system PFA-100. Ann. Hematol. 2002, 81, 353–354.