
Video Upload Options
The demand for agricultural crops continues to escalate with the rapid growth of the population. Silica nanoparticles (SNPs) are beneficial for plant growth and production and can be used as nanopesticides, nanoherbicides, and nanofertilizers in agriculture. SNPs can be classified as porous or non-porous in structure and can be synthesized by chemical, physical, and biological methods. In agriculture, SNP can be sprayed on foliage or irrigated into the soil. SNPs can promote plant growth and development by increasing photosynthesis and nutrient uptake rates and enhancing plant resistance to environmental stress. In the future, SNPs will provide various solutions for the healthy growth of agricultural crops.
1. Introduction
The Food and Agriculture Organization (FAO) estimates that in 2050, the global population will increase to 9 billion, and global food production should increase by 70% to meet the growing population’s demand for food [1][2]. At present, biotic and abiotic stressors such as drought, extreme climate, salt, diseases, and pests cause crop loss worldwide. The influence of abiotic stresses on crop production causes an annual loss of crop yield of 51–82% [3]. To increase crop yield, farmers frequently use pesticides and fertilizers, creating a huge threat to the ecological environment. Therefore, environmentally friendly technologies should be developed to help plants overcome biotic and abiotic stresses and ensure optimum crop yield and agricultural sustainability.
Nanotechnology application in agriculture is an emerging interdisciplinary field, which plays a positive role in promoting plant growth and conferring stresses tolerance in plants and has a great potential for applications in agriculture [4][5][6]. Nanoparticles (NPs) are small particles with a size range of 1–100 nm. In comparison with bulk compounds, NPs exhibit different physical and chemical properties in terms of their surface, optical, thermal, and electrical properties [7]. NPs have unique physiological characteristics, large surface-area-to-weight ratios, and small sizes, which can increase their solubility and transportation speeds within plants [8]. Several nanomaterials such as Fe3O4, MgO, SiO2, and CeO2 are beneficial for plant growth, playing an important role in promoting seed germination, increasing plant resistance, degrading pesticide residues, and enhancing soil quality [9][10][11][12].
The hydrolyzed product of silica nanoparticles (SNPs) is monosilicic acid (H4SiO4) [13]. Silicon is the second most abundant element in the earth’s crust and plays a pivotal role in many biogeochemical processes. Silicon strengthens the physical barrier of plants through its deposition onto plant cell walls, thus promoting the ability of lodging tolerance and disease resistance [14]. The applications of silicon and SNPs reduce the oxidative stress response by priming defense reactions under biotic and abiotic stresses [15][16][17][18]. Moreover, SNPs have a small particle size and easily penetrate the plant cell wall and the organelles [19]. In comparison with bulk materials, NPs have a high surface-area-to-volume ratio, thus improving their reactivity and biochemical activity [20][21]. The SNPs’ accumulation in corn is 9.14% higher than for bulk silica [22]. Therefore, SNPs may be more effective than bulk silica in alleviating different adversity stresses [18][23][24][25].
2. Synthesis and Characteristics of SNPs
SNPs are synthesized via top-down and bottom-up approaches (Figure 1) [26][27]. Top-down strategies of SNPs mainly include mechanical and mechanochemical synthesis methods. The mechanical methods pulverize large solids via ball milling technology, while the mechanochemical approaches assist chemical reactions by mechanical pulverization [28]. The mechanochemical method involves the use of simple equipment at a low cost, and it could produce NPs in batches, but the produced NPs have a wide size range and uneven shape distribution, as well as contain a large amount of impurities [29]. The bottom-up syntheses of SNPs mainly include gas- and liquid-phase synthesis. Gas=phase synthesis has some drawbacks, such as the need for special equipment to produce films and the reaction chamber, and the emission of toxic gaseous by-products [30]. Liquid-phase synthesis covers precipitation, microemulsion, and solution-gel syn-thesis [31][32][33]. Solution-gel synthesis is one of the most common SNP synthesis methods. Stöber et al. (1986) reported tetraethylorthosilicate as the silicon source, ammonia as the catalyst, and different alcohol solutions as the solvent to synthesis SNPs with a particle size distribution of 0.05–2 μm [31]. Most mesoporous SNPs are synthesized by a modified Stöber’s method [34]. In addition, the biosynthesis of NPs by microorganisms, algae, and plant compounds is a green and eco-friendly technology [6][35].
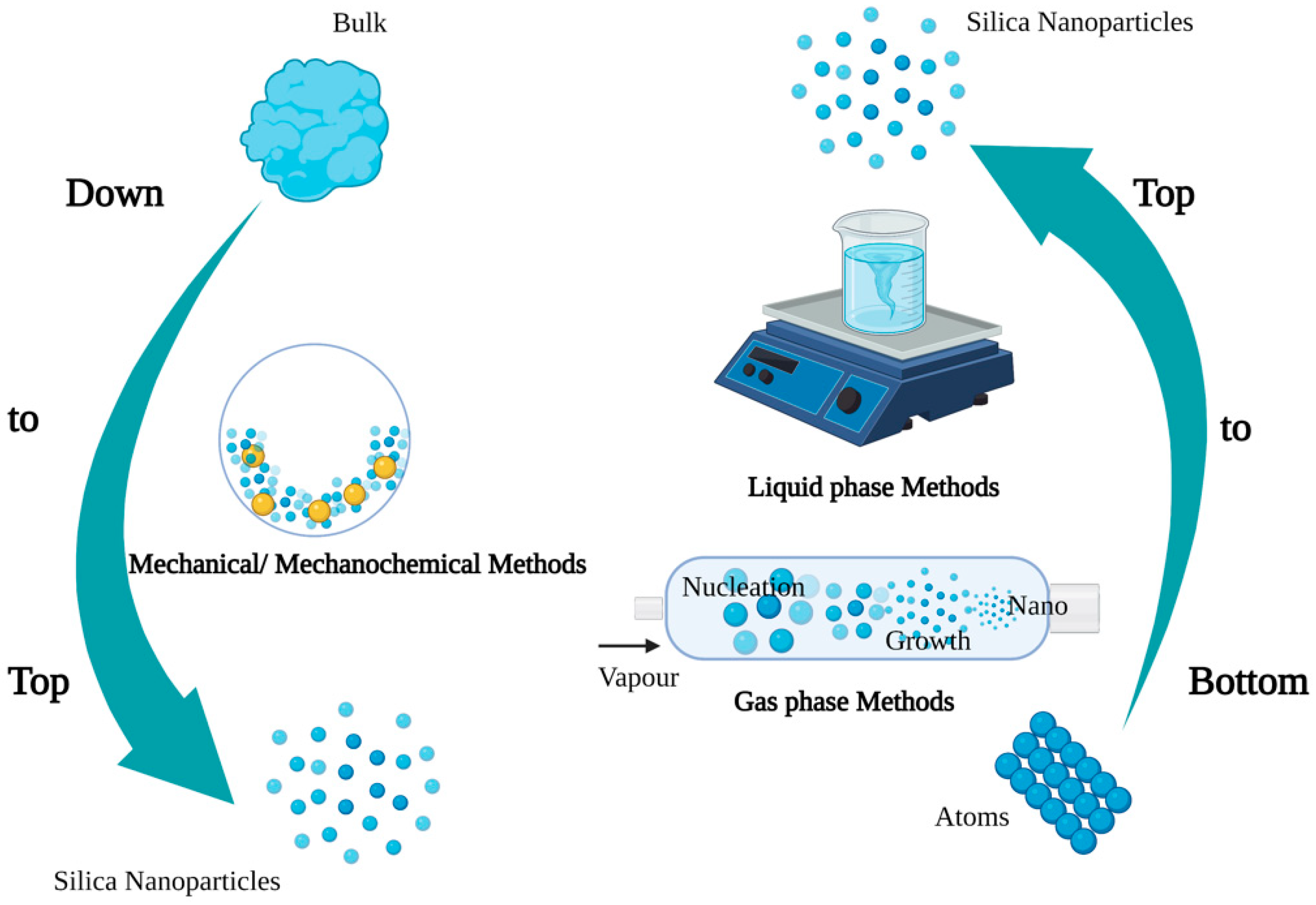
Figure 1. Synthesis methods of nanoparticles (Figure created using BioRender [https://biorender.com/] 6 February 2022).
SNPs could be classified as porous or nonporous in structure. The synthesis method of SNPs significantly affects their structure with variations in particle size, shape, pore size, and dissolution rate, which influence their application and manner in organisms [34][36]. The size of SNPs is usually governed via adjusting the pH of the reaction solution in the solution-gel method [31]. The particle size and shape of the SNPs greatly impact their cellular uptake and distribution in organisms [37][38]. However, previous studies on the influence of the shape and size of SNPs on organisms have mostly focused on the animal field. In contrast, there are relatively few studies in the plant field. The porosity of Stöber silica could be controlled by adjusting the ratio of water; in conditions of a high ratio of water to TEOS, SNPs with smooth particle surfaces were obtained. In contrast, rough particle surfaces with micropores were synthesized [39]. The type of surfactant added in the liquid phase synthesis process could change the pore size of SNPs [34]. The longer chain lengths of surfactants cause SNPs to have larger pores, and those surfactants with shorter chain lengths cause SNPs to have smaller pores [40]. Pore SNPs are usually used in the delivery of pesticides or herbicides in agriculture. The pores’ size and number of SNPs will affect their degradation rate, which is essential for effective drug delivery [41]. The dissolution rate of SNPs was accelerated as the increase of porosity increased [42]. The dissolution rate of SNPs in plants impacts their effectiveness. Fast-dissolving SNPs could increase plant biomass and fruit yield [11]. Furthermore, in the process of preparing SNPs, some compounds such as (3-aminopropyl) triethoxysilane, oligochitosan, and amino acids could also be added to the synthesis of functionalized SNPs [11][43][44]. For instance, after oligochitosan–nanosilica application, the resistance of chili plants to anthracnose disease rises significantly when compared to single nanosilica treatment [44]. At present, there is a lack of in-depth research about the influence of SNPs synthesis methods on the absorption and distribution of SNPs in plants, plant growth, and stress resistance. In addition, the release behavior of different SNPs (porous, non-porous, surface modification, etc.) in plants needs to be further studied.
3. Absorption and Transmission of SNPs in Plants
In agriculture, SNPs can be applied by spraying on the leaves or directly into the root. The SNPs applied by the foliar application can enter the leaves and be transported to different parts through the cuticular or stomata (Figure 2). The transport path of solutes via the cuticle has two routes: one is the lipophilic pathway for non-polar solutes via diffusion and penetration, and the other is the hydrophilic pathway for polar solutes via water pores (the effective diameter of these pores is 0.6–0.8 nm) [45][46][47]. In addition, the hydrophilic substances are transported via stomatal pores in the stomatal pathway. However, the unique geometric structure and physiological function of the stomata is complex, and the actual size exclusion limit of the pores penetrated by nanomaterials is still unclear. Hu et al. (2020) investigated SNPs in the guard cells of leaves after spraying SNPs (18 nm) for 3 h [48]. Similarly, SNPs (54 ± 7 nm) could enter the air spaces of the leaf via the stomata and the outer edge of the cell walls [18]. After nanomaterials enter leaves, they could trans-locate from leaves back to the roots via the phloem [49][50]. In the roots, nanomaterials could form complexes with carrier proteins or root exudates [51]. These complexes can pass through the root cell wall, enter plant cells through intracellular phagocytosis, or directly penetrate the cytoplasmic membrane and then disperse in the cytoplasm [52][53][54][55]. Moreover, nanomaterials can also be transported from the roots to shoots through the xylem [54][56]. Furthermore, the active mode of silicon uptake from roots to shoots via specific transporter proteins includes transport protein (Lsi1), transport protein (Lsi2), and transport protein (Lsi6) [14][57]. SNPs enter the root through the transporter Lsi1 [54][58]. However, the concentration of SNPs application does not affect the expression of the Lsi1 gene in plants. Therefore, SNPs enter the plant body mainly through extra mass infiltration [59]. In addition, the expression of silicon transported genes Lsi1 and Lsi2 in rice treated with SNPs increases under salt stress [25]. Moreover, OsLsi1 is a Si-transporting aquaporin (AQP), which was upregulated by Si supplementation, and the AQP could establish the link between Si and molecular signaling [60].
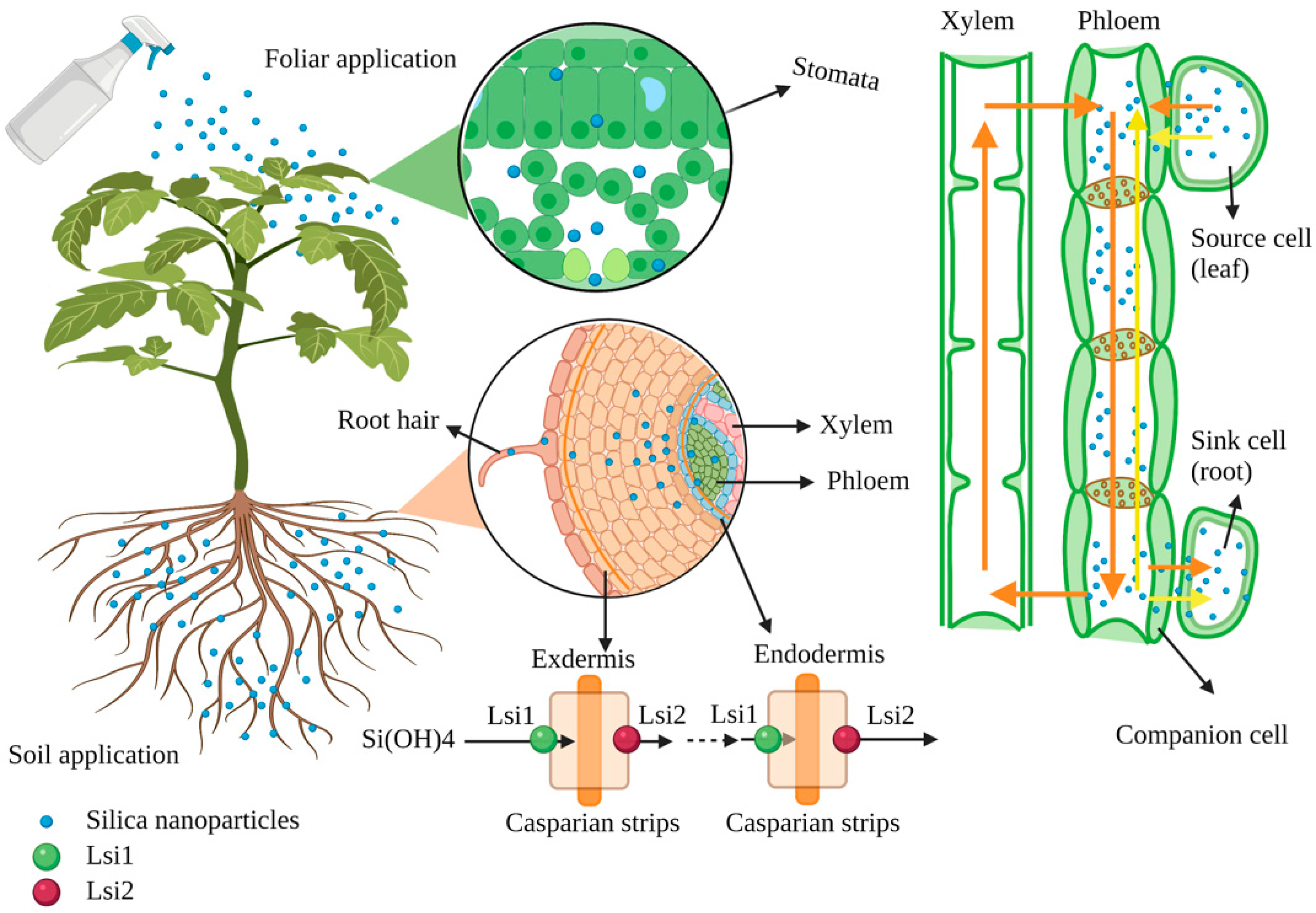
Figure 2. Schematic diagram of the transportation of nanomaterials in the plant (figure created using BioRender [https://biorender.com/] 6 February 2022).
The accumulation and absorption of nanomaterials in plants differ, and the process mainly depends on the type of plant, chemical composition, and the size of nanomaterials [51][61]. Slomberg and Schoenfisch (2012) reported different sizes of SNPs (14, 50, and 200 nm) that could enter Arabidopsis roots, but the accumulation of SNPs in root cells decreases with the increase of SNP size [62]. The efficient transport of nanomaterials into guard cells, extracellular space, and chloroplasts is related to the size and charge of the nanomaterials [48]. Cui et al. (2017) applied different particle sizes (19, 48, and 202 nm) of SNPs to treat rice cell suspension cultures under chromium stress and found that SNPs with small particle sizes had viable rice cells in the suspension [24]. Similarly, the bactericidal effect of SNPs on Pseudomonas aeruginosa also increases with the decrease of its particle size [63].
References
- Agata Tyczewska; Ewa Woźniak; Joanna Gracz; Jakub Kuczyński; Tomasz Twardowski; Towards Food Security: Current State and Future Prospects of Agrobiotechnology. Trends in Biotechnology 2018, 36, 1219-1229, 10.1016/j.tibtech.2018.07.008.
- Edwin Gitobu Mwobobia; Arthur Sichangi; Kuria B. Thiong’O; Characterization of wheat production using earth-based observations: a case study of Meru County, Kenya. Modeling Earth Systems and Environment 2019, 6, 13-25, 10.1007/s40808-019-00699-4.
- Suarau O. Oshunsanya, Nkem J. Nwosu, Yong Li. Abiotic Stress in Agricultural Crops under Climatic Conditions//Sustainable Agriculture, Forest and Environmental Management; Manoj Kumar Jhariya, Eds.; Springer Nature Singapore Pte Ltd.: Singapore, 2019; pp. 71-100.
- Alia Servin; Wade Elmer; Arnab Mukherjee; Roberto De la Torre-Roche; Helmi Hamdi; Jason C. White; Prem Bindraban; Christian Dimkpa; A review of the use of engineered nanomaterials to suppress plant disease and enhance crop yield. Journal of Nanoparticle Research 2015, 17, 1-21, 10.1007/s11051-015-2907-7.
- Hemraj Chhipa; Nanofertilizers and nanopesticides for agriculture. Environmental Chemistry Letters 2016, 15, 15-22, 10.1007/s10311-016-0600-4.
- Peerzada Gh Jeelani; Prajakta Mulay; Rajesh Venkat; C. Ramalingam; Multifaceted Application of Silica Nanoparticles. A Review. Silicon 2019, 12, 1337-1354, 10.1007/s12633-019-00229-y.
- Anshu Rastogi; Durgesh Kumar Tripathi; Saurabh Yadav; Devendra Kumar Chauhan; Marek Živčák; Mansour Ghorbanpour; Nabil Ibrahim El-Sheery; Marian Brestic; Application of silicon nanoparticles in agriculture. 3 Biotech 2019, 9, 1-11, 10.1007/s13205-019-1626-7.
- Mehdi Salajegheh; Mohammadreza Yavarzadeh; Arezo Payandeh; Mohammad Mehdi Akbarian; Effects of titanium and silicon nanoparticles on antioxidant enzymes activity and some biochemical properties of Cuminum cyminum L. under drought stress. Banat's Journal of Biotechnology 2020, XI, 19-25, 10.7904/2068-4738-xi(21)-19.
- Stephen Joseph; Hossain M. Anawar; Paul Storer; Paul Blackwell; Chee Chia; Yun Lin; Paul Munroe; Scott Donne; Joseph Horvat; Jianli Wang; et al.Zakaria M. Solaiman Effects of Enriched Biochars Containing Magnetic Iron Nanoparticles on Mycorrhizal Colonisation, Plant Growth, Nutrient Uptake and Soil Quality Improvement. Pedosphere 2015, 25, 749-760, 10.1016/s1002-0160(15)30056-4.
- Lin Cai; Juanni Chen; Zhongwei Liu; Hancheng Wang; Huikuan Yang; Wei Ding; Magnesium Oxide Nanoparticles: Effective Agricultural Antibacterial Agent Against Ralstonia solanacearum. Frontiers in Microbiology 2018, 9, 790, 10.3389/fmicb.2018.00790.
- Hyunho Kang; Wade Elmer; Yu Shen; Nubia Zuverza-Mena; Chuanxin Ma; Pablo Botella; Jason C. White; Christy L. Haynes; Silica Nanoparticle Dissolution Rate Controls the Suppression of Fusarium Wilt of Watermelon (Citrullus lanatus). Environmental Science & Technology 2021, 55, 13513-13522, 10.1021/acs.est.0c07126.
- Hao Wu; Qian Sun; Jingyi Chen; Guan-Yu Wang; Dan Wang; Xiao-Fei Zeng; Jie-Xin Wang; Citric acid-assisted ultrasmall CeO2 nanoparticles for efficient photocatalytic degradation of glyphosate. Chemical Engineering Journal 2021, 425, 130640, 10.1016/j.cej.2021.130640.
- Gregory R. Choppin; Priyanath Pathak; Punam Thakur; Polymerization and Complexation Behavior of Silicic Acid: A Review. Main Group Metal Chemistry 2008, 31, 53-72, 10.1515/mgmc.2008.31.1-2.53.
- J. F. Ma; N. Yamaji; Functions and transport of silicon in plants. Cellular and Molecular Life Sciences 2008, 65, 3049-3057, 10.1007/s00018-008-7580-x.
- Yongxing Zhu; Haijun Gong; Beneficial effects of silicon on salt and drought tolerance in plants. Agronomy for Sustainable Development 2013, 34, 455-472, 10.1007/s13593-013-0194-1.
- Daniel Debona; Fabrício A. Rodrigues; Lawrence E. Datnoff; Silicon's Role in Abiotic and Biotic Plant Stresses. Annual Review of Phytopathology 2017, 55, 85-107, 10.1146/annurev-phyto-080516-035312.
- Alexandra de Sousa; Ahmed M. Saleh; Talaat H. Habeeb; Yasser Mahmoud Hassan; Rafat Zrieq; Mohammed A.M. Wadaan; Wael N. Hozzein; Samy Selim; Manuela Matos; Hamada AbdElgawad; et al. Silicon dioxide nanoparticles ameliorate the phytotoxic hazards of aluminum in maize grown on acidic soil. Science of The Total Environment 2019, 693, 133636, 10.1016/j.scitotenv.2019.133636.
- Mohamed El-Shetehy; Aboubakr Moradi; Mattia Maceroni; Didier Reinhardt; Alke Petri-Fink; Barbara Rothen-Rutishauser; Felix Mauch; Fabienne Schwab; Silica nanoparticles enhance disease resistance in Arabidopsis plants. Nature Nanotechnology 2020, 16, 344-353, 10.1038/s41565-020-00812-0.
- Hashmath I. Hussain; Zhifeng Yi; James E. Rookes; Lingxue X. Kong; David M. Cahill; Mesoporous silica nanoparticles as a biomolecule delivery vehicle in plants. Journal of Nanoparticle Research 2013, 15, 1-15, 10.1007/s11051-013-1676-4.
- S. Dubchak; A. Ogar; J. W. Mietelski; K. Turnau; Influence of silver and titanium nanoparticles on arbuscular mycorrhiza colonization and accumulation of radiocaesium in Helianthus annuus. Spanish Journal of Agricultural Research 2010, 8, 103, 10.5424/sjar/201008s1-1228.
- Rangaraj Suriyaprabha; Gopalu Karunakaran; R. Yuvakkumar; P. Prabu; V. Rajendran; N. Kannan; Growth and physiological responses of maize (Zea mays L.) to porous silica nanoparticles in soil. Journal of Nanoparticle Research 2012, 14, 1-14, 10.1007/s11051-012-1294-6.
- Rangaraj Suriyaprabha; Gopalu Karunakaran; Kandiah Kavitha; Rathinam Yuvakkumar; Venkatachalam Rajendran; Narayanasamy Kannan; Application of silica nanoparticles in maize to enhance fungal resistance. IET Nanobiotechnology 2014, 8, 133-137, 10.1049/iet-nbt.2013.0004.
- Durgesh KumarTripathi,Vijay PratapSingh,Sheo MohanPrasad,Devendra KumarChauhan, Nawal KishoreDubey; Silicon nanoparticles (SiNp) alleviate chromium (VI) phytotoxicity in Pisumsativum (L.) seedlings. Plant Physiology and Biochemistry 2015, 96, 189-198.
- Jianghu Cui; Tongxu Liu; Fangbai Li; Jicai Yi; Chuanping Liu; Huan-Yun Yu; Silica nanoparticles alleviate cadmium toxicity in rice cells: Mechanisms and size effects. Environmental Pollution 2017, 228, 363-369, 10.1016/j.envpol.2017.05.014.
- Mahmoud Abdel-Haliem; Hegazy S. Hegazy; Noaman S. Hassan; Deyala Naguib; Effect of silica ions and nano silica on rice plants under salinity stress. Ecological Engineering 2017, 99, 282-289, 10.1016/j.ecoleng.2016.11.060.
- Namita Rajput; Methods of preparation of nanoparticles-a review. International Journal of Advances in Engineering & Technology 2015, 7, 1806.
- Ayuk, E.L. and Ugwu, M.O. and Aronimo, Samuel B.; A review on synthetic methods of nanostructured materials. Chemistry Research Journal 2017, 2, 97-123.
- C Lam; Y.F Zhang; Y.H Tang; Chun-Sing Lee; I Bello; S.T Lee; Large-scale synthesis of ultrafine Si nanoparticles by ball milling. Journal of Crystal Growth 2000, 220, 466-470, 10.1016/s0022-0248(00)00882-4.
- Thakur Prasad Yadav; Ram Manohar Yadav; Dinesh Singh; Mechanical Milling: a Top Down Approach for the Synthesis of Nanomaterials and Nanocomposites. Nanoscience and Nanotechnology 2012, 2, 22-48, 10.5923/j.nn.20120203.01.
- Giyaullah Habibullah; Jitka Viktorova; Tomas Ruml; Current Strategies for Noble Metal Nanoparticle Synthesis. Nanoscale Research Letters 2021, 16, 1-12, 10.1186/s11671-021-03480-8.
- Werner Stöber; Arthur Fink; Ernst Bohn; Controlled growth of monodisperse silica spheres in the micron size range. Journal of Colloid and Interface Science 1968, 26, 62-69, 10.1016/0021-9797(68)90272-5.
- Aixi Hu,Zhigang Yao,Xi Yu; Phase behavior of a sodium dodec anolallyl sulfosuccinicdiester/n-pentanol/methyl acrylate/butyl acrylate/water microemulsion system and preparation of acrylate latexes by microemulsion polymerization. Journal of applied polymer science 2009, 113, 2202-2208.
- Ping Lu; You-Lo Hsieh; Highly pure amorphous silica nano-disks from rice straw. Powder Technology 2012, 225, 149-155, 10.1016/j.powtec.2012.04.002.
- Reema Narayan; Usha Y. Nayak; Ashok M. Raichur; Sanjay Garg; Mesoporous Silica Nanoparticles: A Comprehensive Review on Synthesis and Recent Advances. Pharmaceutics 2018, 10, 118, 10.3390/pharmaceutics10030118.
- Hamed Zamani; Arezou Jafari; Seyyed Mohammad Mousavi; Esmaeel Darezereshki; Biosynthesis of silica nanoparticle using Saccharomyces cervisiae and its application on enhanced oil recovery. Journal of Petroleum Science and Engineering 2020, 190, 107002, 10.1016/j.petrol.2020.107002.
- Jonas G. Croissant; Kimberly S. Butler; Jeffrey I. Zink; C. Jeffrey Brinker; Synthetic amorphous silica nanoparticles: toxicity, biomedical and environmental implications. Nature Reviews Materials 2020, 5, 886-909, 10.1038/s41578-020-0230-0.
- Quan Zhang; Xiaoling Wang; Pei-Zhou Li; Kim Truc Nguyen; Xiao-Jun Wang; Zhong Luo; Huacheng Zhang; Nguan Soon Tan; Yanli Zhao; Biocompatible, Uniform, and Redispersible Mesoporous Silica Nanoparticles for Cancer-Targeted Drug Delivery In Vivo. Advanced Functional Materials 2013, 24, 2450-2461, 10.1002/adfm.201302988.
- Li Linlin,Liu Tianlong,Fu Changhui,Tan Longfei, Meng Xianwei,Liu Huiyu; Nanomedicine: Nanotechnology, Biology and Medicine et al. Biodistribution, excretion, and toxicity of mesoporous silica nanoparticles after oral administration depend on their shape. Nanomedicine: Nanotechnology, Biology and Medicine 2015, 11, 1915-1924.
- A. van Blaaderen; A.P.M. Kentgens; Particle morphology and chemical microstructure of colloidal silica spheres made from alkoxysilanes. Journal of Non-Crystalline Solids 1992, 149, 161-178, 10.1016/0022-3093(92)90064-q.
- Aparna Ganguly; Tokeer Ahmad; Ashok K. Ganguli; Silica Mesostructures: Control of Pore Size and Surface Area Using a Surfactant-Templated Hydrothermal Process. Langmuir 2010, 26, 14901-14908, 10.1021/la102510c.
- Jonas Croissant; Yevhen Fatieiev; Niveen Khashab; Degradability and Clearance of Silicon, Organosilica, Silsesquioxane, Silica Mixed Oxide, and Mesoporous Silica Nanoparticles. Advanced Materials 2017, 29, 1604634, 10.1002/adma.201604634.
- Biana Godin; Jianhua Gu; Rita E. Serda; Rohan Bhavane; Ennio Tasciotti; Ciro Chiappini; Xuewu Liu; Takemi Tanaka; Paolo Decuzzi; Mauro Ferrari; et al. Tailoring the degradation kinetics of mesoporous silicon structures through PEGylation. Journal of Biomedical Materials Research Part A 2010, 9999A, 1236-1243, 10.1002/jbm.a.32807.
- Showkat AM, Zhang YP, Kim MS, Gopalan AI, Reddy KR, Lee K; Analysis of Heavy Metal Toxic Ions by Adsorption onto Amino-functionalized Ordered Mesoporous Silica. Bulletin of the Korean Chemical Society 2007, 28, 1985-1992, 10.5012/bkcs.2007.28.11.1985.
- Ngoc Thuy Nguyen; Dai Hai Nguyen; Dinh Dzung Pham; Van Phu Dang; Quoc Hien Nguyen; Dong Quy Hoang; New oligochitosan-nanosilica hybrid materials: preparation and application on chili plants for resistance to anthracnose disease and growth enhancement. Polymer Journal 2017, 49, 861-869, 10.1038/pj.2017.58.
- Christian Popp; Markus Burghardt; Adrian Friedmann; Markus Riederer; Characterization of hydrophilic and lipophilic pathways of Hedera helix L. cuticular membranes: permeation of water and uncharged organic compounds. Journal of Experimental Botany 2005, 56, 2797-2806, 10.1093/jxb/eri272.
- Lukas Schreiber; Polar Paths of Diffusion across Plant Cuticles: New Evidence for an Old Hypothesis. Annals of Botany 2005, 95, 1069-1073, 10.1093/aob/mci122.
- Thomas Eichert; Andreas Kurtz; Ulrike Steiner; Heiner E. Goldbach; Size exclusion limits and lateral heterogeneity of the stomatal foliar uptake pathway for aqueous solutes and water-suspended nanoparticles. Physiologia Plantarum 2008, 134, 151-160, 10.1111/j.1399-3054.2008.01135.x.
- Peiguang Hu; Jing An; Maquela M. Faulkner; Honghong Wu; Zhaohu Li; Xiaoli Tian; Juan Pablo Giraldo; Nanoparticle Charge and Size Control Foliar Delivery Efficiency to Plant Cells and Organelles. ACS Nano 2020, 14, 7970-7986, 10.1021/acsnano.9b09178.
- Zhenyu Wang; Xiaoyan Xie; Jian Zhao; Xiaoyun Liu; Wenqiang Feng; Jason C. White; Baoshan Xing; Xylem- and Phloem-Based Transport of CuO Nanoparticles in Maize (Zea mays L.). Environmental Science & Technology 2012, 46, 4434-4441, 10.1021/es204212z.
- Jitao Lv; Peter Christie; Shuzhen Zhang; Uptake, translocation, and transformation of metal-based nanoparticles in plants: recent advances and methodological challenges. Environmental Science: Nano 2018, 6, 41-59, 10.1039/c8en00645h.
- Cyren M. Rico; Sanghamitra Majumdar; Maria Duarte-Gardea; Jose R. Peralta-Videa; Jorge L. Gardea-Torresdey; Interaction of Nanoparticles with Edible Plants and Their Possible Implications in the Food Chain. Journal of Agricultural and Food Chemistry 2011, 59, 3485-3498, 10.1021/jf104517j.
- Jasmina Kurepa; Tatjana Paunesku; Stefan Vogt; Hans Arora; Bryan M. Rabatic; Jinju Lu; M. Beau Wanzer; Gayle E. Woloschak; Jan A. Smalle; Uptake and Distribution of Ultrasmall Anatase TiO2 Alizarin Red S Nanoconjugates in Arabidopsis thaliana. Nano Letters 2010, 10, 2296-2302, 10.1021/nl903518f.
- Feng-Peng Chang; Lin-Yun Kuang; Chia-An Huang; Wann-Neng Jane; Yann Hung; Yue-Ie C. Hsing; Chung-Yuan Mou; A simple plant gene delivery system using mesoporous silica nanoparticles as carriers. Journal of Materials Chemistry B 2013, 1, 5279-5287, 10.1039/c3tb20529k.
- Dequan Sun; Hashmath I. Hussain; Zhifeng Yi; Rainer Siegele; Tom Cresswell; Lingxue Kong; David Cahill; Uptake and cellular distribution, in four plant species, of fluorescently labeled mesoporous silica nanoparticles. Plant Cell Reports 2014, 33, 1389-1402, 10.1007/s00299-014-1624-5.
- Durgesh Kumar Tripathi; Shweta; Shweta Singh; Swati Singh; Rishikesh Pandey; Vijay Pratap Singh; Nilesh C. Sharma; Sheo Mohan Prasad; Nawal Dubey; Devendra Kumar Chauhan; et al. An overview on manufactured nanoparticles in plants: Uptake, translocation, accumulation and phytotoxicity. Plant Physiology and Biochemistry 2016, 110, 2-12, 10.1016/j.plaphy.2016.07.030.
- Dequan Sun; Hashmath I. Hussain; Zhifeng Yi; James E. Rookes; Lingxue Kong; David M. Cahill; Mesoporous silica nanoparticles enhance seedling growth and photosynthesis in wheat and lupin. Chemosphere 2016, 152, 81-91, 10.1016/j.chemosphere.2016.02.096.
- Jian Feng Ma; Naoki Yamaji; Silicon uptake and accumulation in higher plants. Trends in Plant Science 2006, 11, 392-397, 10.1016/j.tplants.2006.06.007.
- Sanam Nazaralian; Ahmad Majd; Saeed Irian; Farzaneh Najafi; Farrokh Ghahremaninejad; Tommy Landberg; Maria Greger; Comparison of silicon nanoparticles and silicate treatments in fenugreek. Plant Physiology and Biochemistry 2017, 115, 25-33, 10.1016/j.plaphy.2017.03.009.
- Faride Asgari; Ahmad Majd; Parissa Jonoubi; Farzaneh Najafi; Effects of silicon nanoparticles on molecular, chemical, structural and ultrastructural characteristics of oat (Avena sativa L.). Plant Physiology and Biochemistry 2018, 127, 152-160, 10.1016/j.plaphy.2018.03.021.
- Durgesh Kumar Tripathi; Kanchan Vishwakarma; Vijay Pratap Singh; Ved Prakash; Shivesh Sharma; Sowbiya Muneer; Miroslav Nikolic; Rupesh Deshmukh; Marek Vaculík; Francisco J. Corpas; et al. Silicon crosstalk with reactive oxygen species, phytohormones and other signaling molecules. Journal of Hazardous Materials 2020, 408, 124820, 10.1016/j.jhazmat.2020.124820.
- Farzad Aslani; Samira Bagheri; Nurhidayatullaili Muhd Julkapli; Abdul Shukor Juraimi; Farahnaz Sadat Golestan Hashemi; Ali Baghdadi; Effects of Engineered Nanomaterials on Plants Growth: An Overview. The Scientific World Journal 2014, 2014, 1-28, 10.1155/2014/641759.
- Danielle L. Slomberg; Mark H. Schoenfisch; Silica Nanoparticle Phytotoxicity to Arabidopsis thaliana. Environmental Science & Technology 2012, 46, 10247-10254, 10.1021/es300949f.
- Alexis W. Carpenter; Danielle L. Slomberg; Kavitha S. Rao; Mark H. Schoenfisch; Influence of Scaffold Size on Bactericidal Activity of Nitric Oxide-Releasing Silica Nanoparticles. ACS Nano 2011, 5, 7235-7244, 10.1021/nn202054f.




