
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Maša Knez Marevci | + 3513 word(s) | 3513 | 2021-12-01 04:35:49 |
Video Upload Options
Chia (Salvia hispanica L.) is a small seed that comes from an annual herbaceous plant, Salvia hispanica L. Chia seeds contain healthy ω-3 fatty acids, polyunsaturated fatty acids, dietary fiber, proteins, vitamins, and some minerals. Besides this, the seeds are an excellent source of polyphenols and antioxidants, such as caffeic acid, rosmarinic acid, myricetin, quercetin, and others.
1. Introduction
2. Chemical Composition and Phytochemicals in Chia Seeds
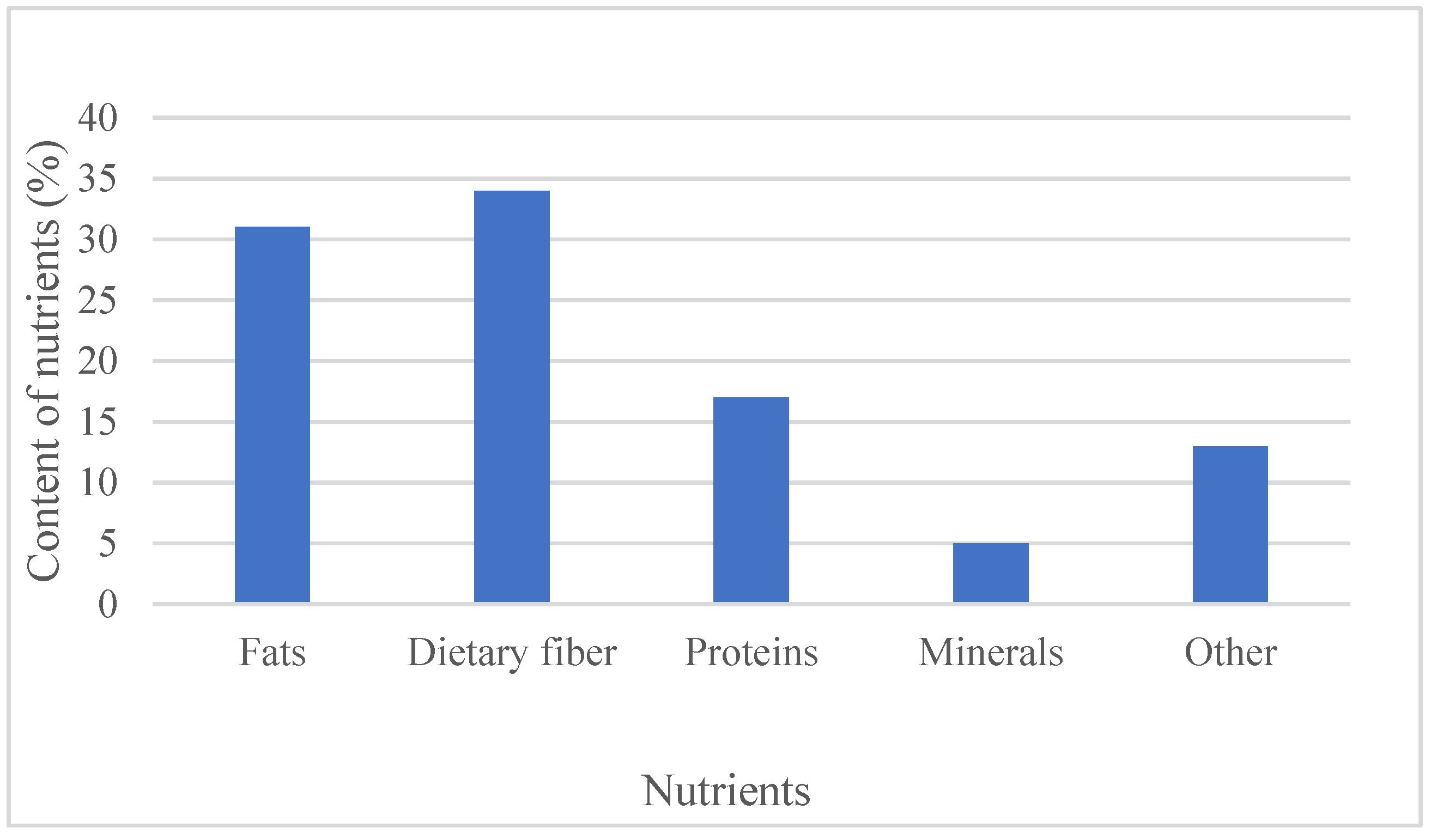
|
Chia Seeds |
Rice |
Corn |
Wheat |
Quinoa |
Amaranth |
|
|---|---|---|---|---|---|---|
|
Carbohydrates (g) |
42 |
80 |
74 |
71 |
64.2 |
71 |
|
Protein (g) |
17 |
6.5 |
9.4 |
12.6 |
14.1 |
12.6 |
|
Fat (g) |
31 |
1.5 |
1.92 |
1.5 |
||
|
Minerals (mg) |
||||||
|
Magnesium |
335 |
25 |
127 |
126 |
197 |
126 |
|
Phosphorus |
860 |
115 |
210 |
288 |
457 |
288 |
|
Calcium |
631 |
28 |
7 |
29 |
29 |
|
|
Potassium |
407 |
115 |
287 |
363 |
563 |
363 |
|
Natrium |
16 |
/ |
/ |
/ |
/ |
/ |
|
Other (g) |
13 |
/ |
/ |
/ |
/ |
/ |
|
Vitamins (mg) |
||||||
|
Vitamin A eq. |
54 μg |
0 |
214 |
9 |
0 |
n.d. |
|
Vitamin E |
0.5 |
0.11 |
0.49 |
1.01 |
0.63 |
1.19 |
|
Vitamin C |
1.6 |
0 |
0 |
0 |
0 |
4.2 |
|
Thiamine (B1) |
0.62 |
0.07 |
0.39 |
0.30 |
0.11 |
0.12 |
|
Riboflavin (B2) |
0.17 |
0.05 |
0.20 |
0.12 |
0.11 |
0.2 |
|
Niacin (B3) |
8.83 |
1.6 |
3.63 |
5.46 |
0.412 |
0.92 |
|
Fatty acid content (%) |
||||||
|
Linolenic acid (C18:3, ω-3) |
63.79 |
2.1 |
1 |
0.08 |
6.7 |
1.01 |
|
Linoleic acid (C18:2, ω-6) |
18.89 |
39.7 |
52 |
0.68 |
56.4 |
0.35 |
|
Olec acid (C18:1, ω-9) |
7.3 |
35.1 |
31 |
0.24 |
20.4 |
22.69 |
|
Palmitoleic acid (C16:1) |
0.03 |
/ |
/ |
/ |
n.d. |
0.08 |
|
Eicosenic acid (20:1) |
n.d. |
/ |
/ |
0.005 |
n.d. |
1.49 |
|
Palmitic acid (C16:0) |
7.04 |
20.8 |
13 |
3.02 |
9.7 |
18.59 |
|
Phenolic compunds (μg) |
||||||
|
Caffeic acid |
27 |
n.d. |
26 |
40 |
37 |
0.90 |
|
Quercetin |
0.17 |
/ |
/ |
30.1 |
43.3 |
/ |
|
Kaempferol |
0.013 |
/ |
/ |
/ |
36.7 |
/ |
|
Daidzin |
6.6 |
/ |
/ |
/ |
/ |
/ |
|
Glycitin |
1.4 |
/ |
/ |
/ |
/ |
/ |
|
Genistin |
3.4 |
/ |
/ |
/ |
/ |
/ |
3. Antioxidant and Antimicrobial Activity
4. Applications of Chia Seeds and Derived Products
4.1. Food Industry
4.2. Pharmaceutical Use
5. Therapeutic Value
|
Aim of the Study |
Clinical Setting |
Study Description |
Result |
Reference |
|---|---|---|---|---|
|
Assessment of the effect of Salba-chia on body weight, visceral obesity and obesity-related risk factors in overweight and obese adults with type 2 diabetes. |
- Changes in body weight and in waist circumference, - body composition, - glycemic control, - level of C-reactive protein and obesity-related satiety hormones. |
- Two parallel groups with 77 over-weight or obese patients with type 2 diabetes were evaluated. |
- Significant weight loss, - reduction in waist circumference and C-reactive protein - increase of plasma adiponectin. |
[44] |
|
Comparison of the effect of two seeds (flax (Linum usitatissimum) and Salba-chia (Salvia hispanica L.)) on postprandial glycemia and satiety scores. |
Blood glucose samples and satiety ratings were collected at fasting and over 2 h postprandially. |
- Fifteen healthy participants - randomized to receive a 50 g glucose challenge, alone or supplemented with either 25 g ground Salba-chia or 31.5 g flax. |
- Salba-chia appears to have the ability to convert glucose into a slow-release carbohydrate - and affect satiety to a greater extent than flax (due to the higher fiber viscosity). |
[45] |
|
Influence of Ingesting Chia Seed Oil on Human Running Performance |
- A randomized (1:1 allocation, random number generator), - crossover approach, and - subjects engaged in two run-to-exhaustion trials after acute ingestion of flavored water with chia seed oil or flavored water alone (no blinding), with at least a two-week washout period. |
- After providing a blood sample at 8:00 am, subjects ingested 0.5 L flavored water alone or 0.5 L water with 7 kcal kg−1 chia seed oil (random order), provided another blood sample at 8:30 am, and then started running to exhaustion. - Additional blood samples were collected immediately post- and 1 h post-exercise. |
- Ingestion ofchia seed oil 30 min before running caused an increase in plasma ALA levels, - no discernable benefits for the athletes in this study. |
[46] |
|
Effect of chia supplementation (Salvia hispanica L.) on blood pressure (BP) and its associated cardiometabolic factors. |
- Hypertensive individuals of both sexes, –randomized, double-blind, experimental and placebo-controlled study. |
- Nutritional assessment, -clinical BP measurement, - ambulatory blood pressure monitoring (ABPM), - collection of blood samples. |
- The consumption of the chia or the placebo caused no gastrointestinal, hepatic or renal disorders, - decrease of the BP in hypertensive individuals. |
[47] |
|
Effectiveness of milled and whole chia seed in altering disease risk factors in overweight, postmenopausal women. |
- Metabolomics approach using gas chromatography–mass spectrometry with multivariate statistical methods, - including principal component analysis and partial least-square discriminant analysis (PLS-DA). |
- Subjects ingested 25 g chia seed or placebo supplements each day for 10 weeks, - body mass and composition, blood pressure and augmentation index, serum lipid profile, inflammation markers from fasting blood samples, plasma fatty acids, and metabolic profiling. |
Ingestion of 25 g/day milled chia seed compared to whole chia seed or placebo for 10 weeks by overweight women increased plasma ALA and EPA, but had no influence on inflammation or disease risk factors using both traditional and metabolomics-based measures. |
[48] |
|
Evaluation of the effects of a dietary pattern (DP; soy protein, nopal, chia seed, and oat) on the biochemical variables of MetS, the AUC for glucose and insulin, glucose intolerance (GI), the relationship of the presence of certain polymorphisms related to MetS, and the response to the DP. |
A single-center, randomized, placebo-controlled, double-blind, parallel-arm study. |
- In the first stage, participants were instructed to consume a reduced energy diet according to (23) and a low-saturated fat and low-cholesterol diet for 2 wk (5). - During the second stage of the study, participants were randomly assigned to consume either the dietary pattern (DP) or placebo (P) in addition to the reduced energy diet for 2 mo. |
- BW, BMI, and WC decreased, - no changes in the percentages of the lean or fat mass in either group after the dietary treatment. |
[49] |
|
Assessment of Omega 3 chia seed loading as a means of Carbohydrate loading. |
-CHO-loading treatments were based on the subject’s body weight and were thus isocaloric. |
Comparison of the performance testing results between 2 different CHO-loading treatments |
- No statistical difference between Omega 3 Chia loading and CHO loading. |
[50] |
References
- Ciau-Solís, N.; Rosado-Rubio, G.; Segura-Campos, M.R.; Betancur-Ancona, D.; Chel-Guerrero, L. Chemical and Functional Properties of Chia Seed (Salvia hispanica L.) Gum. Int. J. Food Sci. 2014, 2014, 1–5.
- Grancieri, M.; Martino, H.S.D.; Gonzalez de Mejia, E. Chia Seed (Salvia hispanica L.) as a Source of Proteins and Bioactive Peptides with Health Benefits: A Review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 480–499.
- Ullah, R.; Nadeem, M.; Khalique, A.; Imran, M.; Mehmood, S.; Javid, A.; Hussain, J. Nutritional and therapeutic perspectives of Chia (Salvia hispanica L.): A review. J. Food Sci. Technol. 2016, 53, 1750–1758.
- Campos, B.E.; Dias Ruivo, T.; da Silva Scapim, M.R.; Madrona, G.S.; Bergamasco, R.d.C. Optimization of the mucilage extraction process from chia seeds and application in ice cream as a stabilizer and emulsifier. LWT-Food Sci. Technol. 2016, 65, 874–883.
- Das, A. Advances in Chia Seed Research. Adv. Biotechnol. Microbiol. 2018, 5, 5–7.
- Mohd Ali, N.; Yeap, S.K.; Ho, W.Y.; Beh, B.K.; Tan, S.W.; Tan, S.G. The promising future of chia, Salvia hispanica L. J. Biomed. Biotechnol. 2012, 2012, 1–9.
- de Falco, B.; Amato, M.; Lanzotti, V. Chia seeds products: An overview. Phytochem. Rev. 2017, 16, 745–760.
- Gérard, F. Chia seed CO2 extract: A revolutionary ingredient for food and cosmetics. Wellness Foods Eur. 2006, 5, 1–4.
- Knez Hrnčič, M.; Cör, D.; Knez, Ž. Subcritical extraction of oil from black and white chia seeds with n-propane and comparison with conventional techniques. J. Supercrit. Fluids 2018, 140, 182–187.
- Silva, C.; Garcia, V.A.S.; Zanette, C.M. Chia (Salvia hispanica L.) oil extraction using different organic solvents: Oil yield, fatty acids profile and technological analysis of defatted meal. Int. Food Res. J. 2016, 23, 998–1004.
- Coorey, R.; Grant, A.; Jayasena, V. Effects of Chia Flour Incorporation on the Nutritive Quality and Consumer Acceptance of Chips. J. Food Res. 2012, 1, 85.
- Ixtaina, V.Y.; Nolasco, S.M.; Tomás, M.C. Physical properties of chia (Salvia hispanica L.) seeds. Ind. Crops Prod. 2008, 28, 286–293.
- Reyes-Caudillo, E.; Tecante, A.; Valdivia-López, M.A. Dietary fibre content and antioxidant activity of phenolic compounds present in Mexican chia (Salvia hispanica L.) seeds. Food Chem. 2008, 107, 656–663.
- Ayerza, R. Crop year effects on seed yields, growing cycle length, and chemical composition of chia (Salvia hispanica L) growing in Ecuador and Bolivia. Emir. J. Food Agric. 2016, 28, 196–200.
- Muñoz, L.A.; Cobos, A.; Diaz, O.; Aguilera, J.M. Chia seeds: Microstructure, mucilage extraction and hydration. J. Food Eng. 2012, 108, 216–224.
- de Falco, B.; Fiore, A.; Rossi, R.; Amato, M.; Lanzotti, V. Metabolomics driven analysis by UAEGC-MS and antioxidant activity of chia (Salvia hispanica L.) commercial and mutant seeds. Food Chem. 2018, 254, 137–143.
- Kulczyński, B.; Kobus-Cisowska, J.; Taczanowski, M.; Kmiecik, D.; Gramza-Michałowska, A. The Chemical Composition and Nutritional Value of Chia Seeds-Current State of Knowledge. Nutrients 2019, 11, 1242.
- Ho, H.; Lee, A.S.; Jovanovski, E.; Jenkins, A.L.; DeSouza, R.; Vuksan, V. Effect of whole and ground Salba seeds (Salvia Hispanica L.) on postprandial glycemia in healthy volunteers: A randomized controlled, dose-response trial. Eur. J. Clin. Nutr. 2013, 67, 786–788.
- da Luz, J.M.R.; Nunes, M.D.; Paes, S.A.; Torres, D.P.; Silva, M.D.C.S.D.; Kasuya, M.C.M. Lignocellulolytic enzyme production of Pleurotus ostreatus growth in agroindustrial wastes. Braz. J. Microbiol. 2012, 43, 1508–1515.
- Coates, W.; Ayerza, R. Pontencial De Produção Da Chia No Noroeste Argentino. Ind. Crops. Prod. 1996, 6690.
- Villanueva-Bermejo, D.; Calvo, M.V.; Castro-Gómez, P.; Fornari, T.; Fontecha, J. Production of omega 3-rich oils from underutilized chia seeds. Comparison between supercritical fluid and pressurized liquid extraction methods. Food Res. Int. 2019, 115, 400–407.
- Musa Özcan, M.; Al-Juhaimi, F.Y.; Mohamed Ahmed, I.A.; Osman, M.A.; Gassem, M.A. Effect of different microwave power setting on quality of chia seed oil obtained in a cold press. Food Chem. 2019, 278, 190–196.
- Noshe, A.S.; Al-bayyar, A.H. Effect of extraction method of Chia seeds Oil on its content of fatty acids and antioxidants. Int. Res. J. Eng. Technol. 2017, 234, 1–9.
- Singh, D. Quinoa (Chenopodium Quinoa Willd); Scientific Publishers: Rajasthan, India, 2019; ISBN 978-93-87991-67-5.
- Siger, A.; Nogala-Kalucka, M.; Lampart-Szczapa, E. The Content and Antioxidant Activity of Phenolic Compounds in Cold-Pressed Plant Oils. J. Food Lipids 2008, 15, 137–149.
- Gunstone, F.D. Fatty Acid and Lipid Chemistry; Springer: New York, NY, USA, 2012; ISBN 978-1-4615-4131-8.
- Paśko, P.; Sajewicz, M.; Gorinstein, S.; Zachwieja, Z. Analysis of selected phenolic acids and flavonoids in Amaranthus cruentus and Chenopodium quinoa seeds and sprouts by HPLC. Acta Chromatogr. 2008, 20, 661–672.
- Călinoiu, L.F.; Vodnar, D.C. Whole Grains and Phenolic Acids: A Review on Bioactivity, Functionality, Health Benefits and Bioavailability. Nutrients 2018, 10, 1615.
- Rosero, O.; Marounek, M.; Břeňová, N.; Lukeova, D. Phytase activity and comparison of chemical composition, phytic acid P content of four varieties of quinoa grain (Chenopodium quinoa Willd.). Acta Agronómica 2013, 62, 13–20.
- Martirosyan, D.M.; Miroshnichenko, L.A.; Kulakova, S.N.; Pogojeva, A.V.; Zoloedov, V.I. Amaranth oil application for coronary heart disease and hypertension. Lipids Health Dis. 2007, 6, 1.
- Repo-Carrasco-Valencia, R.; Hellström, J.K.; Pihlava, J.-M.; Mattila, P.H. Flavonoids and other phenolic compounds in Andean indigenous grains: Quinoa (Chenopodium quinoa), kañiwa (Chenopodium pallidicaule) and kiwicha (Amaranthus caudatus). Food Chem. 2010, 120, 128–133.
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules 2016, 21, 901.
- Sargi, S.C.; Silva, B.C.; Santos, H.M.C.; Montanher, P.F.; Boeing, J.S.; Santos Júnior, O.O.; Souza, N.E.; Visentainer, J.V. Antioxidant capacity and chemical composition in seeds rich in omega-3: Chia, flax, and perilla. Food Sci. Technol. 2013, 33, 541–548.
- Guindani, C.; Podestá, R.; Block, J.M.; Rossi, M.J.; Mezzomo, N.; Ferreira, S.R.S. Valorization of chia (Salvia hispanica) seed cake by means of supercritical fluid extraction. J. Supercrit. Fluids 2016, 112, 67–75.
- Alcântara, M.A.; de Lima Brito Polari, I.; de Albuquerque Meireles, B.R.L.; de Lima, A.E.A.; da Silva Junior, J.C.; de Andrade Vieira, É.; dos Santos, N.A.; de Magalhães Cordeiro, A.M.T. Effect of the solvent composition on the profile of phenolic compounds extracted from chia seeds. Food Chem. 2019, 275, 489–496.
- Kondo, S.; Teongtip, R.; Srichana, D.; Itharat, A. Antimicrobial activity of rice bran extracts for diarrheal disease. J. Med. Assoc. Thail. Chotmaihet Thangphaet 2011, 94, S117–S121.
- Morshed, S.; Islam, S.S. Antimicrobial activity and phytochemical properties of corn (Zea mays L.) silk. SKUAST J. Res. 2015, 17, 8–14.
- Saha, S.; Islam, Z.; Islam, S.; Hossain, M.S.; Islam, S.M. Evaluation of antimicrobial activity of wheat (Triticum aestivum L.) against four bacterial strains. SKUAST J. Res. 2018, 20, 58–62.
- Park, J.H.; Lee, Y.J.; Kim, Y.H.; Yoon, K.S. Antioxidant and Antimicrobial Activities of Quinoa (Chenopodium quinoa Willd.) Seeds Cultivated in Korea. Prev. Nutr. Food Sci. 2017, 22, 195–202.
- Cherian, P.; Sheela, D. Antimicrobial activity of Amaranth Alkaloid against pathogenic microbes. Int. J. Herb. Med. 2016, 4, 70–72.
- Julio, L.M.; Ixtaina, V.Y.; Fernández, M.A.; Sánchez, R.M.T.; Wagner, J.R.; Nolasco, S.M.; Tomás, M.C. Chia seed oil-in-water emulsions as potential delivery systems of ω-3 fatty acids. J. Food Eng. 2015, 162, 48–55.
- Julio, L.M.; Copado, C.N.; Diehl, B.W.K.; Ixtaina, V.Y.; Tomás, M.C. Chia bilayer emulsions with modified sunflower lecithins and chitosan as delivery systems of omega-3 fatty acids. LWT 2018, 89, 581–590.
- Timilsena, Y.P.; Adhikari, R.; Barrow, C.J.; Adhikari, B. Digestion behaviour of chia seed oil encapsulated in chia seed protein-gum complex coacervates. Food Hydrocoll. 2017, 66, 71–81.
- Vuksan, V.; Jenkins, A.L.; Brissette, C.; Choleva, L.; Jovanovski, E.; Gibbs, A.L.; Bazinet, R.P.; Au-Yeung, F.; Zurbau, A.; Ho, H.V.T.; et al. Salba-chia (Salvia hispanica L.) in the treatment of overweight and obese patients with type 2 diabetes: A double-blind randomized controlled trial. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 138–146.
- Vuksan, V.; Choleva, L.; Jovanovski, E.; Jenkins, A.L.; Au-Yeung, F.; Dias, A.G.; Ho, H.V.T.; Zurbau, A.; Duvnjak, L. Comparison of flax (Linum usitatissimum) and Salba-chia (Salvia hispanica L.) seeds on postprandial glycemia and satiety in healthy individuals: a randomized, controlled, crossover study. Eur. J. Clin. Nutr. 2017, 71, 234.
- Nieman, D.; Gillitt, N.; Meaney, M.; Dew, D. No positive influence of ingesting chia seed oil on human running performance. Nutrients 2015, 7, 3666–3676.
- Toscano, L.T.; da Silva, C.S.O.; Toscano, L.T.; de Almeida, A.E.M.; da Cruz Santos, A.; Silva, A.S. Chia flour supplementation reduces blood pressure in hypertensive subjects. Plant Foods Hum. Nutr. 2014, 69, 392–398.
- Nieman, D.C.; Gillitt, N.; Jin, F.; Henson, D.A.; Kennerly, K.; Shanely, R.A.; Ore, B.; Su, M.; Schwartz, S. Chia seed supplementation and disease risk factors in overweight women: A metabolomics investigation. J. Altern. Complement. Med. 2012, 18, 700–708.
- Guevara-Cruz, M.; Tovar, A.R.; Aguilar-Salinas, C.A.; Medina-Vera, I.; Gil-Zenteno, L.; Hernández-Viveros, I.; López-Romero, P.; Ordaz-Nava, G.; Canizales-Quinteros, S.; Guillen Pineda, L.E. A dietary pattern including nopal, chia seed, soy protein, and oat reduces serum triglycerides and glucose intolerance in patients with metabolic syndrome. J. Nutr. 2011, 142, 64–69.
- Illian, T.G.; Casey, J.C.; Bishop, P.A. Omega 3 chia seed loading as a means of carbohydrate loading. J. Strength Cond. Res. 2011, 25, 61–65.
- Ayerza, R.; Coates, W. Seed yield, oil content and fatty acid composition of three botanical sources of ω-3 fatty acid planted in the Yungas ecosystem of tropical Argentina. Trop. Sci. 2007, 47, 183–187.
- Segura Campos, M.R.; Peralta González, F.; Chel Guerrero, L.; Betancur Ancona, D. Angiotensin I-Converting Enzyme Inhibitory Peptides of Chia (Salvia hispanica) Produced by Enzymatic Hydrolysis. Int. J. Food Sci. 2013, 2013, 158482.
- Rodea-González, D.A.; Cruz-Olivares, J.; Román-Guerrero, A.; Rodríguez-Huezo, M.E.; Vernon-Carter, E.J.; Pérez-Alonso, C. Spray-dried encapsulation of chia essential oil (Salvia hispanica L.) in whey protein concentrate-polysaccharide matrices. J. Food Eng. 2012, 111, 102–109.
- Ayerza, R.; Coates, W. Chia: Rediscovering a Forgotten Crop of the Aztecs; University of Arizona Press: Tuscon, AZ, USA, 2005; ISBN 978-0-8165-2488-4.
- Nieman, D.C.; Cayea, E.J.; Austin, M.D.; Henson, D.A.; McAnulty, S.R.; Jin, F. Chia seed does not promote weight loss or alter disease risk factors in overweight adults. Nutr. Res. 2009, 29, 414–418.
- Adams, J.D.; Wang, R.; Yang, J.; Lien, E.J. Preclinical and clinical examinations of Salvia miltiorrhiza and its tanshinones in ischemic conditions. Chin. Med. 2006, 1, 3.
- Adams, J.D.; Wall, M.; Garcia, C. Salvia columbariae contains tanshinones. Evid. Based Complement. Alternat. Med. 2005, 2, 107–110.




