Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Xin Jin | + 2253 word(s) | 2253 | 2022-02-07 09:40:21 | | | |
| 2 | Catherine Yang | Meta information modification | 2253 | 2022-02-21 02:16:48 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Jin, X. Lung Transplant Models in Mice and Rats. Encyclopedia. Available online: https://encyclopedia.pub/entry/19664 (accessed on 07 February 2026).
Jin X. Lung Transplant Models in Mice and Rats. Encyclopedia. Available at: https://encyclopedia.pub/entry/19664. Accessed February 07, 2026.
Jin, Xin. "Lung Transplant Models in Mice and Rats" Encyclopedia, https://encyclopedia.pub/entry/19664 (accessed February 07, 2026).
Jin, X. (2022, February 21). Lung Transplant Models in Mice and Rats. In Encyclopedia. https://encyclopedia.pub/entry/19664
Jin, Xin. "Lung Transplant Models in Mice and Rats." Encyclopedia. Web. 21 February, 2022.
Copy Citation
Lung transplantation (LTx) is the ultimate curative treatment for end-stage pulmonary disease. Lung transplantation improves the outcome and quality of life of patients with end-stage pulmonary disease. Over the past decades, translational experiments in animal models have led to a better understanding of physiology and immunopathology following the lung transplant procedure. Small animal models (e.g., rats and mice) are mostly used in experiments regarding immunology and pathobiology and are preferred over large animal models due to the ethical aspects, the cost–benefit balance, and the high throughput possibility.
lung transplantation
small animal model
1. The Advantage of Rodent Models
The ideal animal model should provide strong physiological and anatomical similarity to the human disease process, and the model should be balanced between resource and cost. Unfortunately, the ideal model does not exist. Depending on the research objectives, a choice should be made after surveying the advantages and disadvantages of a particular model. Elements that should be taken into consideration are outlined in Table 1.
Table 1. Differences between large and small animal LTx models and between rats and mice.
| Large Animal Models | Small Animal Models | ||
|---|---|---|---|
| Porcine | Rats | Mice | |
| Size | Large size: 40–50 kg | Medium size: 250–300 g | Smaller size: 20–30 g |
| Surgical complexity | Demanding surgical skills | Microsurgery training required | |
| Cost | High costs: purchase, housing |
Lower costs | |
| Facility | Large facility, equipment, and housing | Easier to house, although surgical microscope required for procedure | |
| Anatomy | Closest related to humans | Larger evolutionary gap between rodents and humans | |
| Lifespan | Long lifespan | Short lifespan, fast metabolism rate, short gestation time | |
| Application | Surgical training, ex vivo lung perfusion, artificial lung |
Complex applications: ex vivo lung perfusion, re-transplantation |
Genetic modification: knock-out, knock-in, transgenic strains, etc. |
Considering the physiological and anatomical similarities, pigs are more closely related to humans than rodents. For this purpose, research in pigs has a stronger translational value. However, experiments with pigs are demanding in required personnel (e.g., surgeons, veterinarians, animal caretakers) and facilities. It is challenging to study immunological processes in outbred transplant pigs due to the higher sensibility to infection and higher variability. The longer lifespan and slower metabolic rate result in difficulties in observing chronic processes after LTx. Accordingly, rodents should be the first step to study a hypothesis, including several experimental groups, which could be later confirmed with a smaller study design in pigs.
2. Surgical LTx Procedure
2.1. Rat Donor Organ Procurement
The orthotopic (transplantation of an organ into its normal position) single-lung transplantation is more frequently conducted using the left lung because it is one complete entity, whereas the right lung has four lobes. The donor and recipient should be matched in body shape, but choosing a smaller donor may provide convenience in anastomosis to a certain extent. The donor rat can be anesthetized with an intraperitoneal injection of ketamine (100 mg/mL) and xylazine (20 mg/mL) or with a mixture of oxygen and isoflurane depending on facilities and local regulations. The donor is positioned in supine position. A tracheotomy is performed, and an endotracheal tube is inserted and connected to the ventilator under a volume-controlled (VC) setting. The ventilator’s tidal volume (TV) is set to 5–10 mL/kg, respiratory rate (RR) at 60–100/min, positive end-expiratory pressure (PEEP) at 2–4 cm H2O, and fraction of inspiration O₂ (FiO2) at 0.5–1.0 [1][2][3].
The laparotomy is performed, the xyphoid is dissected and retracted cephalad to expose the diaphragm. The chest wall is flipped outward with forceps and fixed on both sides to expose the whole thorax (Figure 1A). The inferior vena cava is injected with 500–1000 IU/kg heparin. After heparinization, the inferior vena cava and left atrium are incised for exsanguination. Perfusion is performed with 10–20 mL 4 °C low-potassium dextran-glucose solution into the pulmonary artery (PA) at a constant pressure of 20–30 cm H2O (similar to the physiologic PA systolic pressure in rats) [4][5][6]. Perfusion needs to take place while the lungs are ventilated as this ensures flushing of the small capillaries. The chest cavity should be filled with blood during perfusion to avoid air emboli going through the pulmonary valve. After the perfusion, the trachea is clamped while the lung remains inflated with 50% O2. A whole heart–lung bloc extraction is recommended when harvesting the donor. At this moment, the heart–lung bloc can be dissociated from surrounding tissue and esophagus. The lungs are placed on a petri dish with gauze soaked in cold low-potassium dextran-glucose solution.
2.2. Cuff Technique for Anastomosis
To maintain the operation in a hypothermic humid environment and to reduce the organ’s warm ischemia time, the petri dish is placed on ice, and the organ is covered with wet gauze. The left hilum is dissected carefully to dissociate the PA, PV, and bronchus. Then, the heart and right lung are removed from the donor’s left lung. A needle holder is used to secure the cuff (Figure 1B,C). The vessel is passed through the cuff, and tissue is folded over the cuff body and secured with a 7-0 nylon suture. The bronchial cuff is inserted the same way as the vessels (Figure 1D,E). Moreover, there is a technique by using a petri dish with carved foam blocks and a bulldog clamp. The bulldog clamp is set into foam blocks as a stabilizer for cuffing vessels and bronchus, which brings convenience during the cuffing procedure (Figure 1F) [7]. The average duration of these steps is approximately 20 min. The donor lung should be preserved in the low-potassium dextran glucose solution at 4 °C while keeping the bronchus clamped until the transplantation.
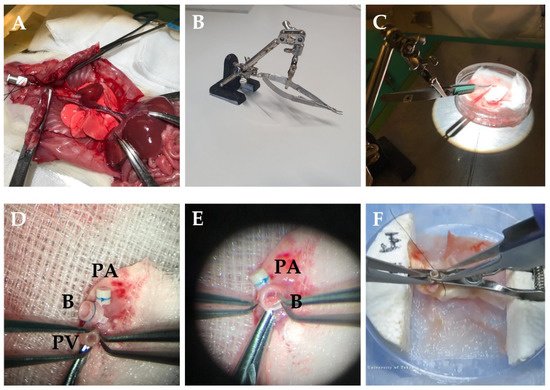
Figure 1. Donor procedure in rats LTx. (A) The chest wall is flipped outward with forceps and fixed on both sides. (B) Stabilizer with needle holder. (C) Benchwork for preparing donor lung. (D) The vessel is passed through the cuff, and tissue is folded over the cuff body and secured with a 7-0 nylon suture. (E) The bronchus is cuffed the same way as the vessels. (F) The bulldog clamp is set into foam blocks as a stabilizer for cuffing vessels and bronchus [7]. PV—pulmonary vein; B—bronchus; PA—pulmonary artery.
2.3. Transplantation
It is recommended to use inhalation anesthesia for the recipient as the depth of anesthesia is easy to control by adjusting the anesthetic dose. Isoflurane 1.5–2% or sevoflurane 2.5–3% can be used with 0.01–0.05 mg/kg buprenorphine subcutaneous injection [1][8][9]. Previous studies have also supported the protective effect of pretreatment with inhalation halothane-kind anesthetics, which can attenuate direct severe lung injury [9]. The alternative way is to inject a mixture of 100 mg/kg ketamine and 4–10 mg/kg xylazine intraperitoneal, which requires fewer facilities and costs [10][11][12]. However, it may result in a less stable model because of ongoing low-flow ischemia during the experiment [13].
The depth of anesthesia and analgesia must be checked before incision to reduce the intraoperative usage of isoflurane and its adverse effect on cardiac function. The anesthesia is maintained with isoflurane or sevoflurane or a combination of 15 mg/kg/h ketamine and 1.5 mg/kg/h xylazine to the corresponding anesthesia method. 1.5 mL/h saline is administered subcutaneously or intraperitoneally for volume replacement if the procedure continues for an extended period [12].
The recipient is placed in the right decubitus position on a heating pad. The thoracotomy is performed layer by layer from the area of the cardiac apex impulse, extended dorsally along the ribs. The thorax is entered through the third or fourth intercostal space, the ribs are spread with the retractor, and the left lung is carefully retracted. After dissecting the hilum, the vessels and bronchus are separately clipped near the heart with two clamps or slipknots to expose the anastomosis area. Two aneurysm clips can be used to clamp the PA and PV separately. A T-shape or V-shape incision instead of a simple lateral incision could enlarge the entrance of the cuffed tissues, thereby reducing the tangential friction and the risk of lacerations. All manipulations should be made with fine instruments (e.g., mosquito clamp and eye scissors). To avoid over-inflation of the contralateral lung, one has to adjust TV and RR once the hilum of the left lung is clipped.
After reperfusion, the native lung is removed, and the deflated transplanted donor lung is placed back into the chest cavity with sufficient irrigation. The thorax is closed by placing one interrupted 4-0 Prolene suture around the ribs [14]. The muscles and subcutaneous tissue are sewn interruptedly layer by layer using 4-0 Prolene and Vicryl suture, and the anesthesia is gradually stopped [15]. When the last stitch is placed, one large breath is administrated to recruit the transplanted lung and to eliminate the remaining air and fluid from the chest; hereafter, the chest can be closed completely. The ETT can be removed once the rat breathes spontaneously and is starting to recover. From 0.01 to 0.05 mg/kg buprenorphine should be injected subcutaneously every eight hours until two days after surgery. The subcutaneous application of non-steroid anti-inflammatory drugs such as 5 mg/kg/d ketorolac or 2 mg/kg/d meloxicam relieves postoperative pain and decreases the incidence of anastomotic thrombosis. Antibiotics are not necessary unless the aim is long-term observation [16][17].
2.4. LTx in Mice—Similarities and Differences to the Rat Model
The procedure for mice transplantation is comparable to rats due to the similar anatomy. However, drug dosage and instruments size should be adjusted according to the weight. When extracting the heart-lung bloc, the donor lung is perfused with 2–3 mL 4 °C low-potassium dextran glucose solution at 10cm H2O pressure [18]. The PA cuff is made of a 26–24-gauge intravenous catheter, while the PV and B cuff are constructed with a 22–20-gauge catheter [19][20]. Preferably, the tails are removed after cuffing. A 20-gauge ETT is used to intubate mice, and the ventilator settings are TV 0.3–1 mL, RR 120–130/min, and PEEP 0.5 cm H2O [20][21][22][23].
The thoracotomy is similarly performed in mice as in rats. After the left lung is retracted, a curved micro serrefine is placed on the recipient‘s left lung (Figure 2A,B). The pulmonary artery and vein are closed using 9-0 sutures by making a slipknot (Figure 2C). As the pulmonary vein is most prone to rupture, it might be considered to occlude one of the two branches of the vein to create a larger segment of the recipient’s vein to introduce the donor cuff. To secure the anastomosis, circumferential 10-0 nylon sutures are used for the vessels and 9-0 sutures for bronchus (Figure 2D). After the implantation, the slipknot of the vein is released first, followed by the slipknot of the artery (Figure 2E).
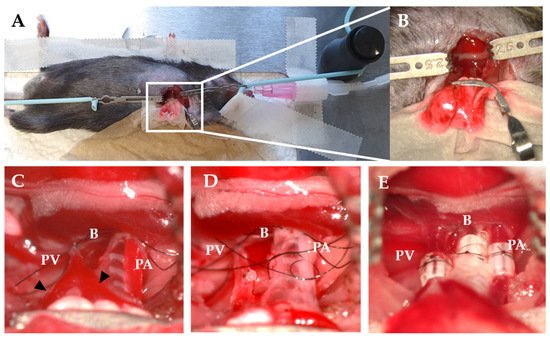
Figure 2. Recipient procedure in mice LTx. (A) Right decubitus position of recipient. (B) The hilum is exposed using a curved micro serrefine. (C) Occlusion of PV and PA using a slipknot. Triangles indicate the branches of the PV. (D) B and PA are ligated with a circumferential suture. (E) Cuffs after implantation and reperfusion. PV—pulmonary vein; B—bronchus; PA—pulmonary artery.
3. Recent Findings in LTx on Rodent Models
3.1. Ischemia–Reperfusion Injury (IRI) and Ex Vivo Lung Perfusion (EVLP)
IRI induces diffuse endothelial and epithelial damage and pulmonary edema. The injury further causes damage-associated molecular patterns (DAMPs) expression and activates the local innate and adaptive immune system, injuring the graft severely [24].
The traditional cold organ preservation method shuts down cell energy consumption but also, at the same time, its metabolism and potential for self-repairment. EVLP can maintain the ventilation and circulation of the donor lung ex vivo. Additionally, EVLP also provides opportunities for organ quality assessment and repairing therapies, which is another hot topic in this research field. Wang et al. [25] reported that 3-Aminobenzamide (3-AB) attenuates the IL-6/IL-10 ratio in plasma and bronchoalveolar lavage and maintains the balance between pro- and anti-inflammatory mediators within rat lungs during EVLP. Lonati et al. [26] reported that synthetic α-melanocyte-stimulating hormone analog [Nle4,D-Phe7]-α-MSH (NDP-MSH) during EVLP could exert positive influences in rat lungs exposed to different injuries. On the other hand, Arni and colleagues focused on the setting parameters of EVLP. They proved that EVLP under subnormothermic temperature (28 °C) could improve the quality of rat donor lungs [3].
3.2. Immune Rejection and Immunosuppression Regimens
Innate and adaptive immune cells can induce rejection through indirect methods such as secreting cytokines and activating the complement system or directly by cytotoxicity. In addition, T lymphocytes can help B lymphocytes to produce antibodies and aggravate immune rejection [27]. The rat model is usually adopted in allotransplantation histology research by strain-mismatch such as allotransplantation from Brown Norway rat (BN) to Lewis (LEW) rat or from LEW to F344 rats [28][29][30]. While studies using the mouse model pay more attention to the immune cells and cytokines, e.g., Th17, γδ T cells, NK cells, and IL-17 [31][32][33]. Fan et al. established a reproducible model of chronic rejection by LTx from C57BL/10 mice to C57BL/6 (minor HLA mismatch model). They also found that neutralizing IL-17 can prevent chronic rejection, reduce acute rejection, and upregulate systemic IL-10 [34].
The classic immunosuppression regimen combination includes calcineurin inhibitors (e.g., tacrolimus or cyclosporin A), steroids (e.g., prednisone), and antimetabolites (e.g., mycophenolate) [35]. As mentioned in the introduction, the long-term usage of immunosuppression drugs could cause complications, including infection, nephrotoxicity, and solid organ tumors. Small animal models can also be used for research in this field for novel drugs research and improvement of adverse effects. Trametinib, a MEK pathway inhibitor, is usually used in cancer treatment such as malignant melanoma. In rats, LTx trametinib has been shown to attenuate graft rejection by suppressing T cell infiltration into the graft and its functional differentiation and B cell activation [36]. In addition, it has been demonstrated that the combined intravenous injection of adipose-derived mesenchymal stem cells and tacrolimus can improve histopathological evaluation, which could be a beneficial therapeutic approach after LTx [37].
References
- Zhai, W.; Ge, J.; Inci, I.; Hillinger, S.; Markus, C.; Korom, S.; Weder, W. Simplified Rat Lung Transplantation by Using a Modified Cuff Technique. J. Investig. Surg. 2008, 21, 33–37.
- Saito, M.; Chen-Yoshikawa, T.F.; Takahashi, M.; Kayawake, H.; Yokoyama, Y.; Kurokawa, R.; Hirano, S.I.; Date, H. Protective Effects of a Hydrogen-Rich Solution During Cold Ischemia in Rat Lung Transplantation. J. Thorac. Cardiovasc. Surg. 2020, 159, 2110–2118.
- Arni, S.; Maeyashiki, T.; Citak, N.; Opitz, I.; Inci, I. Subnormothermic Ex Vivo Lung Perfusion Temperature Improves Graft Preservation in Lung Transplantation. Cells 2021, 10, 748.
- Miyamoto, E.; Motoyama, H.; Sato, M.; Aoyama, A.; Menju, T.; Shikuma, K.; Sowa, T.; Yoshizawa, A.; Saito, M.; Takahagi, A.; et al. Association of Local Intrapulmonary Production of Antibodies Specific to Donor Major Histocompatibility Complex Class I with the Progression of Chronic Rejection of Lung Allografts. Transplantation 2017, 101, e156–e165.
- Rajab, T.K.; Okamoto, T.; Ren, X.; Mathisen, D.J.; Ott, H.C. Intralipid Improves Oxygenation after Orthotopic Rat Lung Transplantation. J. Heart Lung Transplant. 2019, 38, 225–227.
- Kenkel, D.; Yamada, Y.; Weiger, M.; Jungraithmayr, W.; Wurnig, M.C.; Boss, A. Magnetization Transfer as a Potential Tool for the Early Detection of Acute Graft Rejection after Lung Transplantation in Mice. J. Magn. Reson. Imaging 2016, 44, 1091–1098.
- Tian, D.; Shiiya, H.; Sato, M.; Nakajima, J. Rat Lung Transplantation Model: Modifications of the Cuff Technique. Ann. Transl. Med. 2020, 8, 407.
- Curtin, L.I.; Grakowsky, J.A.; Suarez, M.; Thompson, A.C.; DiPirro, J.M.; Martin, L.B.; Kristal, M.B. Evaluation of Buprenorphine in a Postoperative Pain Model in Rats. Comp. Med. 2009, 59, 60–71.
- Ohsumi, A.; Marseu, K.; Slinger, P.; McRae, K.; Kim, H.; Guan, Z.; Hwang, D.M.; Liu, M.; Keshavjee, S.; Cypel, M. Sevoflurane Attenuates Ischemia-Reperfusion Injury in a Rat Lung Transplantation Model. Ann. Thorac. Surg. 2017, 103, 1578–1586.
- Schossleitner, K.; Habertheuer, A.; Finsterwalder, R.; Friedl, H.P.; Rauscher, S.; Groger, M.; Kocher, A.; Wagner, C.; Wagner, S.N.; Fischer, G.; et al. A Peptide to Reduce Pulmonary Edema in a Rat Model of Lung Transplantation. PLoS ONE 2015, 10, e0142115.
- Hsiao, H.M.; Fernandez, R.; Tanaka, S.; Li, W.; Spahn, J.H.; Chiu, S.; Akbarpour, M.; Ruiz-Perez, D.; Wu, Q.; Turam, C.; et al. Spleen-Derived Classical Monocytes Mediate Lung Ischemia-Reperfusion Injury through Il-1beta. J. Clin. Investig. 2018, 128, 2833–2847.
- Ohsumi, A.; Kanou, T.; Ali, A.; Guan, Z.; Hwang, D.M.; Waddell, T.K.; Juvet, S.; Liu, M.; Keshavjee, S.; Cypel, M. A Method for Translational Rat Ex Vivo Lung Perfusion Experimentation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 319, L61–L70.
- Clarysse, M.; Accarie, A.; Gunst, J.; Farré, R.; Vanuytsel, T.; Ceulemans, L.; Pirenne, J. P-01: Rat Model of Intestinal Ischemia-Reperfusion Injury: Impact of Anesthetic Method. Transplantation 2021, 105, S48.
- Rajab, T.K. Techniques for Lung Transplantation in the Rat. Exp. Lung Res. 2019, 45, 267–274.
- Habertheuer, A.; Kocher, A.; Laufer, G.; Petzelbauer, P.; Andreas, M.; Aharinejad, S.; Ehrlich, M.; Wiedemann, D. Innovative, Simplified Orthotopic Lung Transplantation in Rats. J. Surg. Res. 2013, 185, 419–425.
- Ruiz-Pérez, D.; Largo, C.; García-Río, F. Technical Aspects and Benefits of Experimental Mouse Lung Transplantation. Arch. Bronconeumol. 2016, 52, 596–604.
- Richardson, J.; Sabanathan, S.; Mearns, A.J.; Evans, C.S.; Bembridge, J.; Fairbrass, M. Efficacy of Pre-Emptive Analgesia and Continuous Extrapleural Intercostal Nerve Block on Post-Thoracotomy Pain and Pulmonary Mechanics. J. Cardiovasc. Surg. 1994, 35, 219–228.
- Krupnick, A.S.; Lin, X.; Li, W.; Okazaki, M.; Lai, J.; Sugimoto, S.; Richardson, S.B.; Kornfeld, C.G.; Garbow, J.R.; Patterson, G.A.; et al. Orthotopic Mouse Lung Transplantation as Experimental Methodology to Study Transplant and Tumor Biology. Nat. Protoc. 2009, 4, 86–93.
- Li, W.; Sugimoto, S.; Lai, J.; Patterson, G.A.; Gelman, A.E.; Krupnick, A.S.; Kreisel, D. Orthotopic Vascularized Right Lung Transplantation in the Mouse. J. Thorac. Cardiovasc. Surg. 2010, 139, 1637–1643.
- Jungraithmayr, W.M.; Korom, S.; Hillinger, S.; Weder, W. A Mouse Model of Orthotopic, Single-Lung Transplantation. J. Thorac. Cardiovasc. Surg. 2009, 137, 486–491.
- Kawamura, T.; Momozane, T.; Sanosaka, M.; Sugimura, K.; Iida, O.; Fuchino, H.; Funaki, S.; Shintani, Y.; Inoue, M.; Minami, M.; et al. Carnosol Is a Potent Lung Protective Agent: Experimental Study on Mice. Transplant. Proc. 2015, 47, 1657–1661.
- Oishi, H.; Juvet, S.C.; Martinu, T.; Sato, M.; Medin, J.A.; Liu, M.; Keshavjee, S. A Novel Combined Ex Vivo and in Vivo Lentiviral Interleukin-10 Gene Delivery Strategy at the Time of Transplantation Decreases Chronic Lung Allograft Rejection in Mice. J. Thorac. Cardiovasc. Surg. 2018, 156, 1305–1315.
- Jungraithmayr, W.; Weder, W. The Technique of Orthotopic Mouse Lung Transplantation as a Movie-Improved Learning by Visualization. Am. J. Transplant. 2012, 12, 1624–1626.
- Kaczorowski, D.J.; Tsung, A.; Billiar, T.R. Innate Immune Mechanisms in Ischemia/Reperfusion. Front. Biosci. 2009, 1, 91–98.
- Wang, X.; Parapanov, R.; Debonneville, A.; Wang, Y.; Abdelnour-Berchtold, E.; Gonzalez, M.; Gronchi, F.; Perentes, J.Y.; Ris, H.B.; Eckert, P.; et al. Treatment with 3-Aminobenzamide During Ex Vivo Lung Perfusion of Damaged Rat Lungs Reduces Graft Injury and Dysfunction after Transplantation. Am. J. Transplant. 2020, 20, 967–976.
- Lonati, C.; Battistin, M.; Dondossola, D.E.; Bassani, G.A.; Brambilla, D.; Merighi, R.; Leonardi, P.; Carlin, A.; Meroni, M.; Zanella, A.; et al. Ndp-Msh Treatment Recovers Marginal Lungs During Ex Vivo Lung Perfusion (Evlp). Peptides 2021, 141, 170552.
- Liu, A.B.; Zhang, C.Y.; Zhang, L.Y. Cellular Immune Rejection Response of Lung Xenotransplantation. Int. J. Immunol. 2018, 41, 105–107.
- Pezzuto, F.; Lunardi, F.; Vadori, M.; Zampieri, D.; Casiraghi, F.; Azzollini, N.; Vuljan, S.E.; Mammana, M.; Vedovelli, L.; Schiavon, M.; et al. Chronic Lung Allograft Pathology Lesions in Two Rat Strain Combinations. J. Thorac. Dis. 2021, 13, 2833–2843.
- Hirschburger, M.; Greschus, S.; Kuchenbuch, T.; Plotz, C.; Obert, M.; Traupe, H.; Padberg, W.; Grau, V. Lung Transplantation in the Fischer 344 → Wistar Kyoto Rat Strain Combination Is Not Suitable to Study Bronchiolitis Obliterans. J. Heart Lung Transplant. 2007, 26, 390–398.
- Yamane, M.; Sano, Y.; Nagahiro, I.; Aoe, M.; Date, H.; Ando, A.; Shimizu, N. Humoral Immune Responses During Acute Rejection in Rat Lung Transplantation. Transpl. Immunol. 2003, 11, 31–37.
- Neujahr, D.C.; Larsen, C.P. Regulatory T Cells in Lung Transplantation—An Emerging Concept. Semin. Immunopathol. 2011, 33, 117–127.
- Sharma, A.K.; LaPar, D.J.; Zhao, Y.; Li, L.; Lau, C.L.; Kron, I.L.; Iwakura, Y.; Okusa, M.D.; Laubach, V.E. Natural Killer T Cell-Derived Il-17 Mediates Lung Ischemia-Reperfusion Injury. Am. J. Respir. Crit. Care Med. 2011, 183, 1539–1549.
- Naohiko, F.; Ramachandran, S.; Saini, D.; Walter, M.; Chapman, W.; Patterson, G.A.; Mohanakumar, T. Antibodies to Mhc Class I Induce Autoimmunity: Role in the Pathogenesis of Chronic Rejection. J. Immunol. 2009, 182, 309–318.
- Fan, L.; Benson, H.L.; Vittal, R.; Mickler, E.A.; Presson, R.; Fisher, A.J.; Cummings, O.W.; Heidler, K.M.; Keller, M.R.; Burlingham, W.J.; et al. Neutralizing Il-17 Prevents Obliterative Bronchiolitis in Murine Orthotopic Lung Transplantation. Am. J. Transplant. 2011, 11, 911–922.
- Bharat, A. A Need for Targeted Immunosuppression after Lung Transplantation. Am. J. Respir. Cell. Mol. Biol. 2019, 61, 279–280.
- Takahagi, A.; Shindo, T.; Chen-Yoshikawa, T.F.; Yoshizawa, A.; Gochi, F.; Miyamoto, E.; Saito, M.; Tanaka, S.; Motoyama, H.; Aoyama, A.; et al. Trametinib Attenuates Delayed Rejection and Preserves Thymic Function in Rat Lung Transplantation. Am. J. Respir. Cell. Mol. Biol. 2019, 61, 355–366.
- Watanabe, H.; Tsuchiya, T.; Shimoyama, K.; Shimizu, A.; Akita, S.; Yukawa, H.; Baba, Y.; Nagayasu, T. Adipose-Derived Mesenchymal Stem Cells Attenuate Rejection in a Rat Lung Transplantation Model. J. Surg. Res. 2018, 227, 17–27.
More
Information
Subjects:
Transplantation
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
755
Revisions:
2 times
(View History)
Update Date:
21 Feb 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




