Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Maria Teresa Vietri | + 1721 word(s) | 1721 | 2022-02-16 06:50:05 | | | |
| 2 | Rita Xu | Meta information modification | 1721 | 2022-02-22 03:27:52 | | | | |
| 3 | Rita Xu | Meta information modification | 1721 | 2022-02-22 03:29:11 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Vietri, M.T. Pancreatic Cancer with Mutation. Encyclopedia. Available online: https://encyclopedia.pub/entry/19653 (accessed on 13 January 2026).
Vietri MT. Pancreatic Cancer with Mutation. Encyclopedia. Available at: https://encyclopedia.pub/entry/19653. Accessed January 13, 2026.
Vietri, Maria Teresa. "Pancreatic Cancer with Mutation" Encyclopedia, https://encyclopedia.pub/entry/19653 (accessed January 13, 2026).
Vietri, M.T. (2022, February 19). Pancreatic Cancer with Mutation. In Encyclopedia. https://encyclopedia.pub/entry/19653
Vietri, Maria Teresa. "Pancreatic Cancer with Mutation." Encyclopedia. Web. 19 February, 2022.
Copy Citation
Pancreatic ductal adenocarcinoma (PDAC) is the seventh leading cause of cancer death worldwide; most of cases are sporadic, however about 5% to 10% report a hereditary predisposition. Several hereditary syndromes have been associated with familial pancreatic cancer (FPC) onset, including hereditary breast and ovarian cancer syndrome (HBOC), Lynch syndrome (LS), Familial atypical multiple mole melanoma (FAMMM), Familial adenomatous polyposis (FAP), Li–Fraumeni syndrome (LFS), Peutz–Jeghers syndrome (PJS), and Hereditary pancreatitis (HP).
pancreatic ductal adenocarcinoma
hereditary breast and ovarian cancer syndrome
hereditary nonpolyposis colon cancer syndrome
1. Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the twelfthmost common tumor and the seventh main cause of cancer death worldwide [1]. It represents about 5% of all malignancy cases, with the overall survival (OS) 5 years worse than other cancers [2]. Recently, a 2.3-fold increase has been seen in the global number of cases and deaths from PDAC, with a 3 times higher incidence in most developed countries [3]. Most of the PDAC cases are sporadic, but about 5% to 10% report a hereditary predisposition [4].
Pancreatic cancer arises in several hereditary syndromes, including Hereditary breast and ovarian cancer (HBOC), Lynch syndrome (LS), Familial atypical multiple mole melanoma (FAMMM), Familial adenomatous polyposis (FAP), Li–Fraumeni syndrome (LFS), Peutz–Jeghers syndrome (PJS), and Hereditary pancreatitis (HP) [5][6][7][8]. Most familial pancreatic cancer (FPC) is attributable to HBOC syndrome that results from germline mutations in BRCA1/2 genes and other genes such as ATM, BRIP1, CHEK2, RAD50, and RAD51C [9][10]. In FPC belonging to HBOC, BRCA2 mutations are the most common genetic alteration, observed in about 5–10% of cases [11].
It was shown that about half of the patients with PDAC report pathogenic germline variants, even if they do not have a family history of pancreatic carcinoma. Moreover, the mutation presence could have therapeutic implications [12]. The American Society of Clinical Oncology (ASCO) and National Comprehensive Cancer Network (NCCN) guidelines to date recommend universal germline tests for all patients with PDAC [13] in order to identify germline mutation in patients that may benefit from alternative treatments. Moreover, identification of germline mutations in a patient will allow for testing family members at risk ofthe same or other associated cancers.
Immunetherapies or molecularly targeted agents have been recently introduced into cancer clinical practice [14][15][16], but it is still unclear whether they promote benefits in PDAC patients. For patients with BRCA mutation, positive results data have been shown in clinical trials on poly (ADP-ribose) polymerase (PARP) inhibitors, as in use of anti-PD-1 antibodies for patients with mismatch repair (MMR) mutation [17]. In a retrospective analysis, patients with PDAC, with a BRCA mutation, after treatment with the combination regimen of leucovorin calcium, fluorouracil, irinotecan hydrochloride, and oxaliplatin (FOLFIRINOX), had a longer OS than those without the mutations [18]. No differences in survival outcomes were observed in patients treated with Gemcitabine/nab-paclitaxel and FOLFIRINOX, so alternative treatments could be considered in patients with PDAC [19].
2. HBOC Families
Out of 56 HBOC probands, 16 (28.6%) reported a pathogenic mutation, particularly 6/56 (10.7%) in BRCA1, 9/56 (16.1%) in BRCA2, and 1/56 (1.8%) reported a double mutation (DM), both in BRCA2.
The number and percentage of cancers type that occur in mutated HBOC families are reported in Figure 1A; PDAC is the second recurrent cancer, resulting in 13% of cases. Table 1 shows the frequency of cancer types that occur in mutated and non-mutated HBOC families. A statistically significant difference is observed in OC onset.
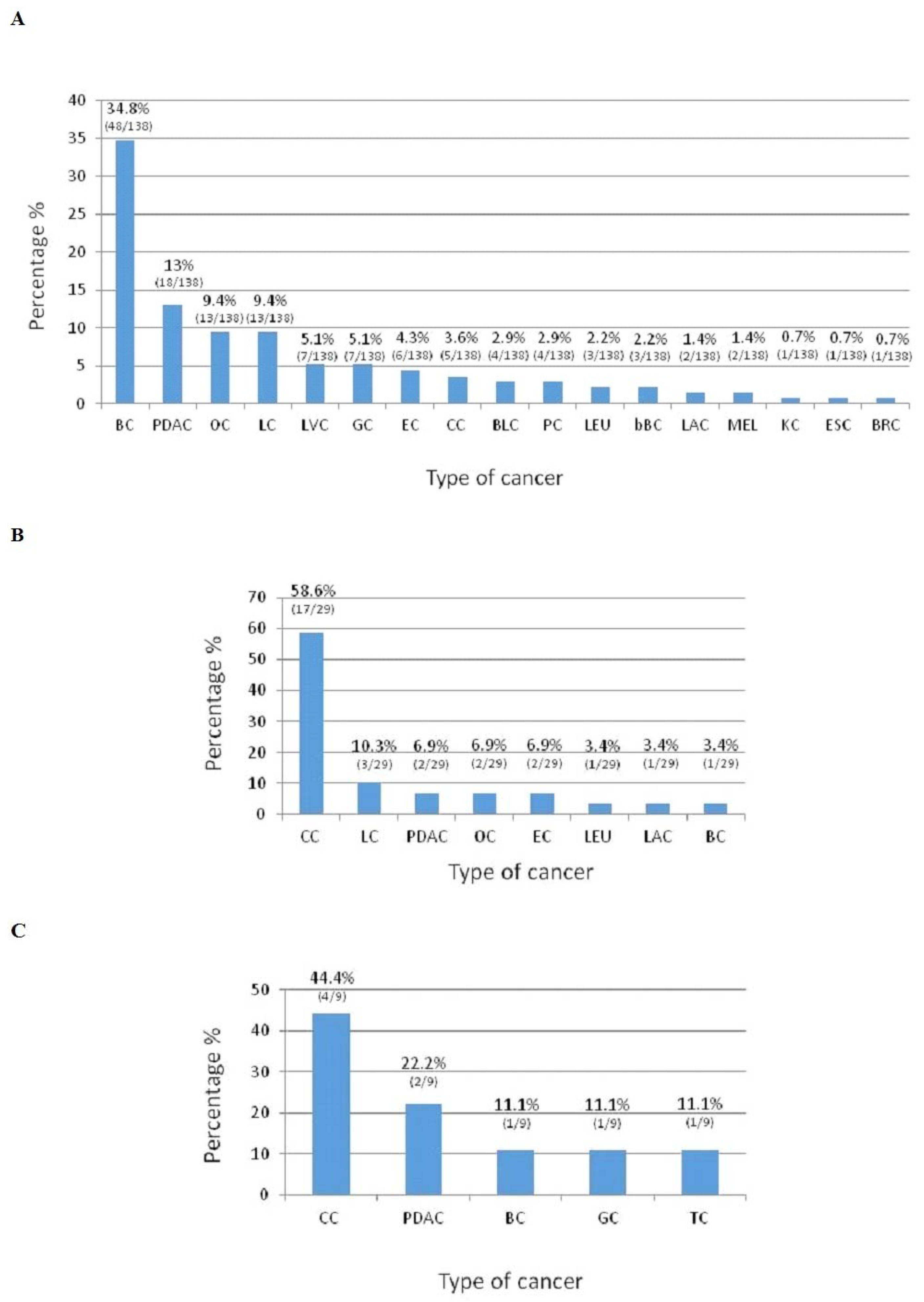
Figure 1. Cancer types that occur in HBOC mutated families (A). Cancer types that occur in LS mutated families (B). Cancer types that occur in FAP mutated family(C). BC: Breast cancer; PDAC: Pancreatic ductal adenocarcinoma; OC: Ovarian cancer; LC: Lung cancer; LVC: Liver cancer; GC: Gastric cancer; EC: Endometrial cancer; CC: Colon cancer; BLC: Bladder cancer; PC: Prostate cancer; LEU: Leukemia; bBC: Bilateral Breast cancer; LAC: Laryngeal cancer; MEL: Melanoma; KC: Kidney cancer; ESC: Esophageal cancer; BRC: Brain cancer; and TC: Thyroid cancer.
Table 1. Number and percentage of cancers type that occur in mutated and non-mutated HBOC families.
| Type of Cancer | Cancer Number in Family | Non Mutated PDAC Patients | Mutated PDAC Patients | p-Value |
|---|---|---|---|---|
| BC | 0 | 2 (4%) | 2 (11%) | 0.27 |
| 1 | 15 (33%) | 2 (11%) | ||
| 2 | 13 (28%) | 4 (22%) | ||
| 3 | 10 (22%) | 5 (28%) | ||
| ≥4 | 6 (13%) | 5 (28%) | ||
| PDAC | 1 | 34 (74%) | 14 (78%) | 0.75 |
| 2 | 12 (26%) | 4 (22%) | ||
| OC | 0 | 33 (72%) | 8 (44%) | 0.042 |
| 1 | 11 (24%) | 6 (33%) | ||
| 2 | 2 (4%) | 4 (22%) | ||
| LC | 0 | 23 (50%) | 10 (56%) | 0.30 |
| 1 | 18 (39%) | 4 (22%) | ||
| 2 | 4 (9%) | 3 (17%) | ||
| 3 | 1 (2%) | 0 (0%) | ||
| 4 | 0 (0%) | 1 (6%) | ||
| LVC | 0 | 34 (74%) | 13 (72%) | 0.34 |
| 1 | 10 (22%) | 4 (22%) | ||
| 2 | 2 (4%) | 0 (0%) | ||
| 3 | 0 (0%) | 1 (6%) | ||
| GC | 0 | 27 (59%) | 11 (61%) | 0.93 |
| 1 | 15 (33%) | 6 (33%) | ||
| 2 | 3 (7%) | 1 (6%) | ||
| 4 | 1 (2%) | 0 (0%) | ||
| EC | 0 | 33 (72%) | 15 (83%) | 0.71 |
| 1 | 7 (15%) | 1 (6%) | ||
| 2 | 4 (9%) | 1 (6%) | ||
| 3 | 2 (4%) | 1 (6%) | ||
| CC | 0 | 34 (74%) | 14 (78%) | 0.78 |
| 1 | 6 (13%) | 3 (17%) | ||
| 2 | 4 (9%) | 1 (6%) | ||
| 3 | 2 (4%) | 0 (0%) | ||
| BLC | 0 | 37 (80%) | 15 (83%) | 0.35 |
| 1 | 8 (17%) | 2 (11%) | ||
| 2 | 0 (0%) | 1 (6%) | ||
| 3 | 1 (2%) | 0 (0%) | ||
| PC | 0 | 40 (87%) | 14 (78%) | 0.62 |
| 1 | 5 (11%) | 3 (17%) | ||
| 2 | 1 (2%) | 1 (6%) | ||
| LEU | 0 | 41 (89%) | 15 (83%) | 0.53 |
| 1 | 5 (11%) | 3 (17%) | ||
| bBC | 0 | 44 (96%) | 15 (83%) | 0.099 |
| 1 | 2 (4%) | 3 (17%) | ||
| LAC | 0 | 39 (85%) | 15 (83%) | 0.77 |
| 1 | 6 (13%) | 3 (17%) | ||
| 2 | 1 (2%) | 0 (0%) | ||
| MEL | 0 | 43 (93%) | 16 (89%) | 0.54 |
| 1 | 3 (7%) | 2 (11%) | ||
| KC | 0 | 43 (93%) | 17 (94%) | 0.89 |
| 1 | 3 (7%) | 1 (6%) | ||
| ESC | 0 | 45 (98%) | 16 (89%) | 0.13 |
| 1 | 1 (2%) | 2 (11%) | ||
| BRC | 0 | 43 (93%) | 17 (94%) | 0.81 |
| 1 | 2 (4%) | 1 (6%) | ||
| 2 | 1 (2%) | 0 (0%) | ||
| TC | 0 | 42 (91%) | 18 (100%) | 0.20 |
| 1 | 4 (9%) | 0 (0%) |
BC: Breast cancer; PDAC: Pancreatic ductal adenocarcinoma; OC: Ovarian cancer; LC: Lung cancer; LVC: Liver cancer; GC: Gastric cancer; EC: Endometrial cancer; CC: Colon cancer; BLC: Bladder cancer; PC: Prostate cancer; LEU: Leukemia; bBC: Bilateral Breast cancer; LAC: Laryngeal cancer; MEL: Melanoma; KC: Kidney cancer; ESC: Esophageal cancer; BRC: Brain cancer; and TC: Thyroid cancer.
One of the BRCA2 mutations, specifically c.5511delT (p.Phe1837LeufsX3), was a novel germline mutation, not previously reported in any database, previously observedas somatic mutation in tissue of mucoepidermoid carcinoma [20]. This mutation, localized in exon 11, consists of a thymine deletion at nucleotide 5511, which results in the introduction of a premature stop codon at position 1840 (Figure 2A). It was identified in a 46-year-old woman affected with breast cancer diagnosed at the age of 45. Figure 2B reports the pedigree of the proband; her mother was affected with pancreatic cancer. Moreover, the analysis was extended in the sisters of 48 and 43 years, that carried the mutation. Finally, the analysis was conducted to daughters of 23 and 19 years and it revealed the presence of mutation only in the daughter of 19 years.
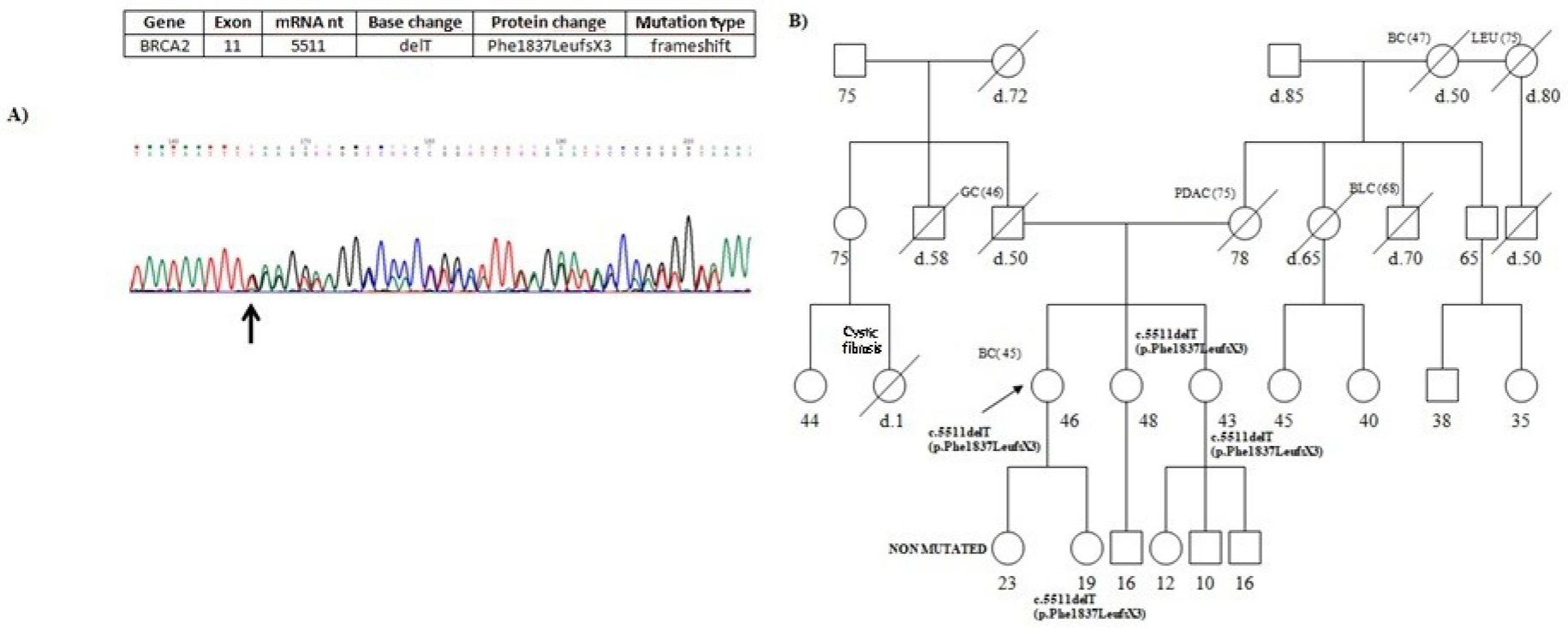
Figure 2. (A) Partial electropherogram of BRCA2 exon 11. The novel germline mutation c.5511delT (p.Phe1837LeufsX3) results in chain termination at codon 1840. (B) Pedigree of the proband carrying the BRCA2 novel mutation c.5511delT (p.Phe1837LeufsX3). The ages at diagnosis are indicated in brackets. Her mother died at 80 years old, was affected with PDAC.
Figure 3 shows the location through BRCA1/2 genes of the mutations found. The mutations are located throughout the length of gene, in BRCA2 most of them fall in exon 11.
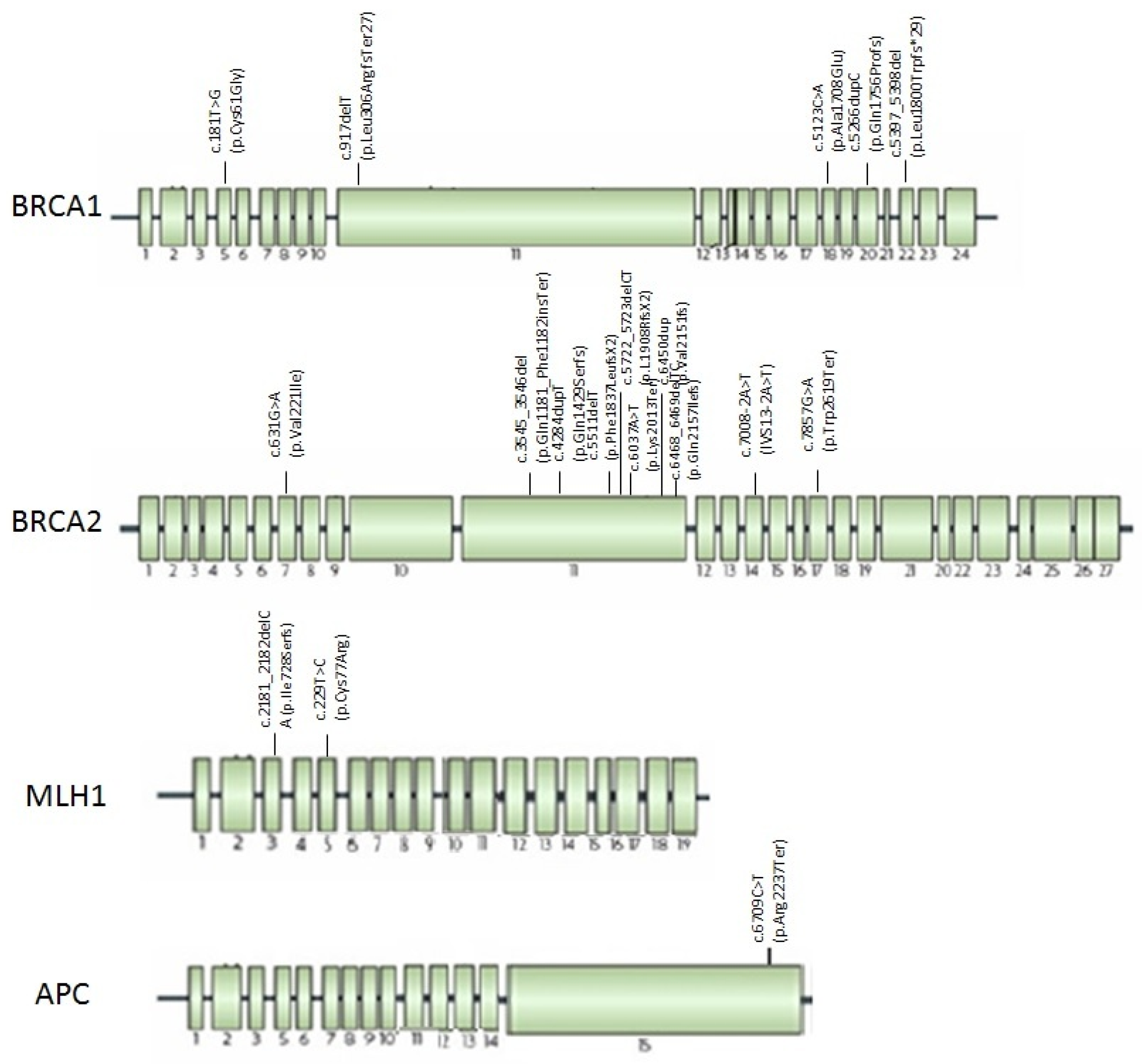
Figure 3. Mutations found in HBOC, LS, and FAP families with PDAC localized trough BRCA1, BRCA2, MLH1, and APC genes.
Mutational analysis was extended to 60 family members of 16 mutated patients, both healthy and cancer affected. The results of genetic test were summarized in Table 2. Twenty-sixfamily members resulted wild-type and 34 inherited the proband’s mutation, of these 11 with cancer and 23 unaffected.
Table 2. Mutations identified and results of mutational analysis conducted in family members of 16HBOC mutated patients, 2LS mutated patients, and 1 FAP mutated patient. Name of mutation was reported in bold.
| Family | Gene | Mutation | Exon | Family Members (Diagnosis) |
Age | Mutational Analysis |
|---|---|---|---|---|---|---|
| 1 | BRCA1 | c.181T>G (p.Cys61Gly) |
5 | Proband (Ovarian Cancer) | 72 | Mutated |
| Sister (Breast Cancer) | 64 | Mutated | ||||
| Daughter (Unaffected) | 48 | Wild Type | ||||
| 2 | BRCA1 | c.917delT (p.Leu306ArgfsTer27) |
11 | Proband (Breast/Ovarian Cancer) | 56 | Mutated |
| Son (Unaffected) | 28 | Mutated | ||||
| Brother (Unaffected) | 61 | Mutated | ||||
| Sister (Unaffected) | 65 | Wild Type | ||||
| 3 | BRCA1 | c.5123C>A (p.Ala1708Glu) |
18 | Proband (Pancreatic cancer) | 67 | Mutated |
| Sister (Ovarian cancer) | 63 | Mutated | ||||
| Nephew (Unaffected) | 38 | Wild Type | ||||
| Niece (Unaffected) | 40 | Mutated | ||||
| 4 | BRCA1 | c.5266dupC (p.Gln1756Profs) |
20 | Proband (Breast and Ovarian cancer) | 64 | Mutated |
| Daugheter (Unaffected) | 62 | Wild Type | ||||
| Son (Unaffected) | 38 | Mutated | ||||
| Brother (Unaffected) | 66 | Mutated | ||||
| Niece (Breast cancer) | 32 | Mutated | ||||
| Niece (Unaffected) | 41 | Mutated | ||||
| 5 | BRCA1 | c.5266dupC (p.Gln1756Profs) |
20 | Proband (Pancreatic Cancer) | 73 | Mutated |
| Sister (Breast Cancer) | 72 | Mutated | ||||
| 6 | BRCA1 | c.5397_5398del (p.Leu1800Trpfs*29) |
22 | Proband (Ovarian Cancer) | 63 | Mutated |
| Son (Unaffected) | 33 | Wild Type | ||||
| Daughter (Unaffected) | 41 | Wild Type | ||||
| 7 | BRCA2 | c.3545_3546del (p.Gln1181_Phe1182insTer) |
11 | Proband (Ovarian cancer) | 57 | Mutated |
| Daugheter (Unaffected) | 32 | Wild Type | ||||
| Daugheter (Unaffected) | 23 | Wild Type | ||||
| Son (Unaffected) | 28 | Wild Type | ||||
| Brother (Unaffected) | 58 | Wild Type | ||||
| Sister (Ovarian cancer) | 62 | Mutated | ||||
| Niece (Unaffected) | 34 | Mutated | ||||
| Nephew (Unaffected) | 30 | Mutated | ||||
| 8 | BRCA2 | c.4284dupT (p.Gln1429Serfs) |
11 | Proband (Breast cancer) | 85 | Mutated |
| Daugheter (Unaffected) | 57 | Wild Type | ||||
| Daugheter (Unaffected) | 56 | Mutated | ||||
| Son(Unaffected) | 52 | Wild Type | ||||
| Son(Unaffected) | 50 | Mutated | ||||
| Sister (Unaffected) | 83 | Mutated | ||||
| Niece (Unaffected) | 48 | Mutated | ||||
| Nephew (Unaffected) | 42 | Mutated | ||||
| Nephew (Unaffected) | 45 | Mutated | ||||
| Nephew (Unaffected) | 36 | Wild Type | ||||
| 9 | BRCA2 | c.5511delT (p.Phe1837LeufsX3) |
11 | Proband (Breast cancer) | 46 | Mutated |
| Sister (Unaffected) | 48 | Mutated | ||||
| Sister (Unaffected) | 43 | Mutated | ||||
| Daughter (Unaffected) | 23 | Wild Type | ||||
| Daughter (Unaffected) | 19 | Mutated | ||||
| 10 | BRCA2 | c.5722_5723delCT (p.L1908RfsX2) |
11 | Proband (Breast cancer) | 62 | Mutated |
| Brother (Unaffected) | 61 | Wild Type | ||||
| 11 | BRCA2 | c.6037A>T (p.Lys2013Ter) |
11 | Proband (Ovarian cancer) | 52 | Mutated |
| Daugheter (Unaffected) | 25 | Mutated | ||||
| Son (Unaffected) | 21 | Wild type | ||||
| Sister (Unaffected) | 58 | Mutated | ||||
| Niece (Unaffected) | 32 | Wild type | ||||
| Niece (Unaffected) | 28 | Wild type | ||||
| 12 | BRCA2 | c.6450dup (p.Val2151fs) |
11 | Proband (Breast cancer) | 54 | Mutated |
| Son (Unaffected) | 24 | Wild Type | ||||
| Daughter (Unaffected) | 21 | Mutated | ||||
| 13 | BRCA2 | c.6468_6469delTC (p.Gln2157Ilefs) |
11 | Proband (Breast Cancer) | 47 | Mutated |
| Father (Unaffected) | 75 | Mutated | ||||
| Sister (Unaffected) | 46 | Mutated | ||||
| Sister (Unaffected) | 41 | Wild type | ||||
| 14 | BRCA2 | c.6468_6469delTC (p.Gln2157Ilefs) |
11 | Proband (Pancreatic Cancer) | 61 | Mutated |
| Sister (Breast and EndometrialCancer) | 62 | Mutated | ||||
| Sister (Ovarian Cancer) | 54 | Mutated | ||||
| Nephew (Unaffected) | 26 | Wild type | ||||
| 15 | BRCA2 | c.7857G>A (p.Trp2619Ter) |
17 | Proband (Breast Cancer) | 45 | Mutated |
| Sister (Unaffected) | 48 | Wild Type | ||||
| 16 | BRCA2 BRCA2 |
c.631G>A (p.Val221Ile) c.7008-2A>T (IVS13-2A>T) |
7 14 |
Proband (Breast Cancer) | 62 | Mutated |
| Daugheter (Unaffected) | 41 | Wild type | ||||
| Daugheter (Unaffected) | 35 | Mutated | ||||
| Brother (Unaffected) | 59 | Wild Type | ||||
| Sister (Bilateral Breast Cancer) | 56 | Mutated | ||||
| Niece (Unaffected) | 35 | Wild Type | ||||
| Niece (Unaffected) | 30 | Wild Type | ||||
| Niece (Breast cancer) | 33 † | Mutated | ||||
| Cousin (Breast cancer) | 70 | Mutated | ||||
| 1 | MLH1 | c.2181_2182delCA (p.Ile728Serfs) |
5 | Proband (Colon Cancer) | 25 | Mutated |
| Brother (Unaffected) | 29 | Wild Type | ||||
| Aunt (Breast Cancer) | 58 | Wild Type | ||||
| Aunt (Unaffected) | 54 | Wild Type | ||||
| 2 | MLH1 | c.229T>C (p.Cys77Arg) |
3 | Proband (Colon Cancer) | 48 | Mutated |
| No family members | - | |||||
| 1 | APC | c.6709C>T (p.Arg2237Ter) |
Proband (Pancreatic Cancer) | 52 † | Mutated | |
| Brother (Cerebral angiomas) | 65 | Mutated | ||||
| Sister (Pancreatic Cancer) | 48 † | Mutated | ||||
| Sister (Colon Cancer) | 55 | Mutated | ||||
| Sister (Colon Cancer) | 57 | Mutated | ||||
| Sister (Unaffected) | 67 | Wild Type | ||||
| Sister (Unaffected) | 66 | Wild Type |
3. LS Families
Out of sevenprobands, two (28.6%) reported a pathogenic mutation in MLH1 gene. The mean age of pancreatic cancer onset was 62.5 years in mutated patients and 57.3 years in non-mutated patients.
The number and percentage of cancers type that occur in LS mutated families were reported in Figure 1B. PDAC is the third recurrent cancer and happen in 6.9% of cases.
The mutations found were in exon 3 and 5 of MLH1 gene, as shown in Figure 3.
Mutational analysis was extended to three family members of one mutated patient, both healthy and cancer affected; in the second family, no family member was available for genetic testing. The results of genetic test were summarized in Table 2; no family member inherited the mutation.
4. FAP Families
Out of three probands, one (33.3%) reported a pathogenic mutation in APC gene. The mean age of pancreatic cancer onset was 47.5 years in mutated patient and 49.6 years in non-mutated patients.
The number and percentage of cancers type that occur in APC mutated family were reported in Figure 1C. PDAC is the second recurrent cancer and occurred in 44.4% of cases.
The mutation found was in exon 15 of APC gene, as showed in Figure 3.
Mutational analysis was extended to six family members, both healthy and cancer affected, of mutated patient; four inherited the proband’s mutation (Table 2).
References
- Simoes, P.K.; Olson, S.H.; Saldia, A.; Kurtz, R.C. Epidemiology of pancreatic adenocarcinoma. Chin. Clin. Oncol. 2017, 6, 24.
- Saad, A.M.; Turk, T.; Al-Husseini, M.J.; Abdel-Rahman, O. Trends in pancreatic adenocarcinoma incidence and mortality in the United States in the last four decades; a SEER-based study. BMC Cancer 2018, 18, 688.
- Collaborators GBDPC. The global, regional, and national burden of pancreatic cancer and its attributable risk factors in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2019, 4, 934–947.
- Pilarski, R. The Role of BRCA Testing in Hereditary Pancreatic and Prostate Cancer Families. Am. Soc. Clin. Oncol. Educ. Book 2019, 39, 79–86.
- Shimmura, H.; Kuramochi, H.; Jibiki, N.; Katagiri, S.; Nishino, T.; Araida, T. Dramatic response of FOLFIRINOX regimen in a collision pancreatic adenocarcinoma patient with a germline BRCA2 mutation: A case report. Jpn. J. Clin. Oncol. 2019, 49, 1049–1054.
- Petersen, G.M. Familial pancreatic cancer. Semin. Oncol. 2016, 43, 548–553.
- Vietri, M.T.; D’Elia, G.; Caliendo, G.; Casamassimi, A.; Resse, M.; Passariello, L.; Cioffi, M.; Molinari, A.M. Double mutation of APC and BRCA1 in an Italian family. Cancer Genet. 2020, 244, 32–35.
- Vietri, M.T.; D’Elia, G.; Caliendo, G.; Casamassimi, A.; Federico, A.; Passariello, L.; Cioffi, M.; Molinari, A.M. Prevalence of mutations in BRCA and MMR genes in patients affected with hereditary endometrial cancer. Med. Oncol. 2021, 38, 13.
- Vietri, M.T.; Molinari, A.M.; De Paola, M.; Cantile, F.; Fasano, M.; Cioffi, M. Identification of a novel in-frame deletion in BRCA2 and analysis of variants of BRCA1/2 in Italian patients affected with hereditary breast and ovarian cancer. Clin. Chem. Lab. Med. 2012, 50, 2171–2180.
- Vietri, M.T.; D’Elia, G.; Benincasa, G.; Ferraro, G.; Caliendo, G.; Nicoletti, G.F.; Napoli, C. DNA methylation and breast cancer: A way forward (Review). Int. J. Oncol. 2021, 59, 98.
- Lai, E.; Ziranu, P.; Spanu, D.; Dubois, M.; Pretta, A.; Tolu, S.; Camera, S.; Liscia, N.; Mariani, S.; Persano, M.; et al. BRCA-mutant pancreatic ductal adenocarcinoma. Br. J. Cancer 2021, 125, 1321–1332.
- Benzel, J.; Fendrich, V. Familial Pancreatic Cancer. Oncol. Res. Treat. 2018, 41, 611–618.
- Stoffel, E.M.; McKernin, S.E.; Brand, R.; Canto, M.; Goggins, M.; Moravek, C.; Nagarajan, A.; Petersen, G.M.; Simeone, D.M.; Yurgelun, M.; et al. Evaluating susceptibility to pancreatic cancer: ASCO Provisional Clinical Opinion. J. Clin. Oncol. 2019, 37, 153–164.
- Colao, A.; de Nigris, F.; Modica, R.; Napoli, C. Clinical Epigenetics of Neuroendocrine Tumors: The Road Ahead. Front. Endocrinol. 2020, 11, 604341.
- de Nigris, F.; Ruosi, C.; Napoli, C. Clinical efficiency of epigenetic drugs therapy in bone malignancies. Bone 2021, 143, 115605.
- Scognamiglio, G.; De Chiara, A.; Parafioriti, A.; Armiraglio, E.; Fazioli, F.; Gallo, M.; Aversa, L.; Camerlingo, R.; Cacciatore, F.; Colella, G.; et al. Patient-derived organoids as a potential model to predict response to PD-1/PD-L1 checkpoint inhibitors. Br. J. Cancer 2019, 121, 979–982.
- Ohmoto, A.; Yachida, S.; Morizane, C. Genomic Features and Clinical Management of Patients with Hereditary Pancreatic Cancer Syndromes and Familial Pancreatic Cancer. Int. J. Mol. Sci. 2019, 20, 561.
- Sehdev, A.; Gbolahan, O.; Hancock, B.A.; Stanley, M.; Shahda, S.; Wan, J.; Wu, H.H.; Radovich, M.; O’Neil, B.H. Germline and Somatic DNA Damage Repair Gene Mutations and Overall Survival in Metastatic Pancreatic Adenocarcinoma Patients Treated with FOLFIRINOX. Clin. Cancer Res. 2018, 24, 6204–6211.
- Williet, N.; Petrillo, A.; Roth, G.; Ghidini, M.; Petrova, M.; Forestier, J.; Lopez, A.; Thoor, A.; Weislinger, L.; De Vita, F.; et al. Gemcitabine/Nab-Paclitaxel versus FOLFIRINOX in Locally Advanced Pancreatic Cancer: A European Multicenter Study. Cancers 2021, 13, 2797.
- Wang, F.; Xi, S.Y.; Hao, W.W.; Yang, X.H.; Deng, L.; Xu, Y.X.; Wu, X.Y.; Zeng, L.; Guo, K.H.; Wang, H.Y. Mutational landscape of primary pulmonary salivary gland-type tumors through targeted next-generation sequencing. Lung Cancer 2021, 160, 1–7.
- Vietri, M.T.; Caliendo, G.; Schiano, C.; Casamassimi, A.; Molinari, A.M.; Napoli, C.; Cioffi, M. Analysis of PALB2 in a cohort of Italianbreastcancerpatients: Identification of a novel PALB2 truncatingmutation. Fam. Cancer2015, 14, 341–348. https://doi.org/10.1007/s10689-015-9786-z.
- Vietri, M.T.; Caliendo, G.; Casamassimi, A.; Cioffi, M.; De Paola, M.L.; Napoli, C.; Molinari, A.M. A novel PALB2 truncatingmutation in an Italian family with male breastcancer. Oncol. Rep. 2015, 33, 1243–1247. https://doi.org/10.3892/or.2014.3685.
- Vietri, M.T.; Caliendo, G.; D’Elia, G.; Resse, M.; Casamassimi, A.; Minucci, P.B.; Dello Ioio, C.; Cioffi, M.; Molinari, A.M. FiveItalian Families with TwoMutations in BRCA Genes. Genes 2020, 11, 1451. https://doi.org/10.3390/genes11121451.
- Vietri, M.T.; Caliendo, G.; D’Elia, G.; Resse, M.; Casamassimi, A.; Minucci, P.B.; Cioffi, M.; Molinari, A.M. BRCA and PALB2 mutations ina cohort of male breastcancer with onebilateral case. Eur. J. MedGenet. 2020, 63, 103883. https://doi.org/10.1016/j.ejmg.2020.103883.
More
Information
Subjects:
Genetics & Heredity; Oncology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
974
Revisions:
3 times
(View History)
Update Date:
22 Feb 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




