Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sandra Rayego-Mateos | + 1935 word(s) | 1935 | 2022-02-11 04:40:34 | | | |
| 2 | Rita Xu | + 2 word(s) | 1937 | 2022-02-22 02:44:37 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Rayego-Mateos, S. Cellular Communication Network-2. Encyclopedia. Available online: https://encyclopedia.pub/entry/19638 (accessed on 08 February 2026).
Rayego-Mateos S. Cellular Communication Network-2. Encyclopedia. Available at: https://encyclopedia.pub/entry/19638. Accessed February 08, 2026.
Rayego-Mateos, Sandra. "Cellular Communication Network-2" Encyclopedia, https://encyclopedia.pub/entry/19638 (accessed February 08, 2026).
Rayego-Mateos, S. (2022, February 18). Cellular Communication Network-2. In Encyclopedia. https://encyclopedia.pub/entry/19638
Rayego-Mateos, Sandra. "Cellular Communication Network-2." Encyclopedia. Web. 18 February, 2022.
Copy Citation
Cellular communication network-2 (CCN2), also called connective tissue growth factor (CTGF), is considered a fibrotic biomarker and has been suggested as a potential therapeutic target for kidney pathologies. CCN2 is a matricellular protein with four distinct structural modules that can exert a dual function as a matricellular protein and as a growth factor.
CCN2
CTGF
EGFR
kidney damage
1. Introduction
Cellular communication network-2 (CCN2), also called connective tissue growth factor (CTGF), is a member of the CCN family and shares many properties with other CCN proteins [1][2]. Intensive research in the last years has unraveled the complexity of the CCN family. All CCN family members are matricellular proteins, key components of the extracellular matrix (ECM), that contain multiple ligand and receptor binding sites and are actively involved in cellular communication and signaling. However, key differences between them have been described, with CCN2 being especially relevant in renal diseases [3][4].
CCN2 is a complex matricellular protein that exerts multiple cellular actions based on its special structure and cellular localization [3][4][5][6]. CCN2 has four functional modules that can be cleaved by proteases, releasing active fragments. Among them, the carboxyl-terminal cysteine knot module 4 (here named CCN2(IV)) shares many responses with the full-length CCN2 [3][4]. Numerous preclinical studies using the recombinant CCN2 complete protein, or some of its fragments, have described a wide diversity of cellular responses and activation of molecular pathways [7]. One of these cellular responses is cell growth, in which CCN2 participates in the regulation of proliferation, apoptosis, and migration [8]. In addition, CCN2 can also promote phenotype changes, as described in T lymphocytes differentiation towards Th17 subtype [9], in the induction of cellular senescence [10][11], and in the processes of epithelial/endothelial to mesenchymal transitions (EMT/EndMT, respectively) [12][13]. CCN2 is also involved in angiogenesis, cancer, inflammation, and fibrogenesis [14][15][16][17][18]. In fact, CCN2 is considered a key fibrotic marker, commonly used in preclinical models [18][19]. Thus, based on these investigations, functions of CCN2 have been widely studied, finally according to it as a growth factor and a proinflammatory cytokine. However, more recent studies using mainly conditional CCN2 knockout mice have remarked the importance of CCN2 as a matricellular protein implicated in cell-to-cell communication and response to micro-environmental signaling [3][17]. These studies have shown that CCN2 exerts key cellular responses in physiological and pathological conditions. Thus, CCN2 acts as an ECM component, involved in the regulation of other ECM components and therefore contributing to matrix remodeling [20], tissue stiffness [1][17], and pathological ECM accumulation in fibrotic disorders, including kidney fibrosis [21], and more recently, participating in vascular wall homeostasis or adaptive remodeling for prevention of structural damage, especially in the context of hypertension and inflammation [22].
In human chronic kidney disease (CKD) of diverse etiology, kidney CCN2 overexpression has been found correlated with cellular proliferation and ECM accumulation, both in the glomerular and interstitial areas [23][24][25][26]. Initial studies done in cultured renal cells demonstrated that CCN2 expression is upregulated by many factors involved in renal damage, including transforming growth factor-β (TGF-β), angiotensin II, elevated glucose concentrations, and cellular stress [18][24][25][27]. Interestingly, in several independent studies, circulating or urinary levels of CCN2 have been proposed as a risk biomarker of human diabetic nephropathy and other forms of CKD [19][28][29]. Additionally, CCN2 has also been proposed as a potential therapeutic target for renal diseases. Preclinical studies have shown that inhibition of endogenous CCN2 by antisense oligonucleotides or gene silencing slows disease progression in experimental diabetic nephropathy, unilateral ureteral obstruction, and nephrectomized transgenic mice overexpressing TGF-β1 [19][30][31][32]. More recently, studies in CCN2 conditional deficient mice have demonstrated the role of CCN2 in renal fibrosis, cell growth arrest, oxidative stress, and senescence [11][21].
The CCN2 signaling receptor is still controversial. Different studies have shown that integrins are involved in CCN2 downstream signaling responses [7], which turns out to be relevant in some disorders such as cancer. In this regard, CCN2 can also bind to non-integrin receptors [16][33], such as low-density lipoprotein receptor-like protein 1 (LRP-1), receptor activator of NF-κB (RANK), osteoprotegerin, fibroblast growth factor receptor, tyrosine kinase receptor of nerve growth factor (TrkA), cation-independent mannose-6-phosphate (M6P) receptor, and epidermal growth factor receptor (EGFR) [1][33][34], all of them multi-ligand receptors. In addition, CCN2 can bind to several growth factors, being able to modify their responses [7][27]. These multiple receptors and ligand binding sites are a characteristic shared with matricellular proteins and other CCN proteins. However, the downstream functional consequences depend on the environment and physiopathological situation. In the kidney context, two potential CCN2 receptors have been described in in vitro studies. TrkA mediates CCN2 responses in cultured mesangial [35] and tubular epithelial [34] cells, whereas EGFR is involved in tubular epithelial cells responses, including partial EMT phenotype changes [21].
The EGFR pathway activation regulates several cellular functions, including development, angiogenesis, migration, and ECM production [7][36]. EGFR participates in cancer development and progression in many human malignancies, where it is overexpressed, dysregulated, or mutated [36][37][38]. In the kidney, EGFR signaling participates in embryonic development and controls renal electrolyte homeostasis [39]. Experimental studies have shown that genetic or pharmacological EGFR inhibition ameliorates renal damage progression [40][41][42], whereas in acute kidney injury, EGFR activation can accelerate renal recovery [43].
2. CCN2 Binds to Tubular Epithelial Cells, but Not to Glomerular Cells, in a Model of Cy5-CCN2(IV) Intra-Renal Injection
To explore the CCN2 binding sites in vivo in the kidney, the researchers developed an experimental murine model by direct renal injection of the recombinant CCN2(IV) protein, previously labeled with a fluorochrome. For these experiments, labeled Cy5-CCN2(IV) was injected in the right kidney, using its corresponding contralateral kidney as a control. The mice were sacrificed at different times, and then renal tissue was embedded in OCT, and the localization of CCN2(IV) binding to renal structures was done by confocal microscopy. In Cy5-CCN2 (IV)-injected kidneys, a red fluorescent signal was observed, mainly in tubular epithelial cells (Figure 1). The maximal fluorescence intensity signal was reached at 30 min, and no red signal was found in the contralateral kidneys (not shown).
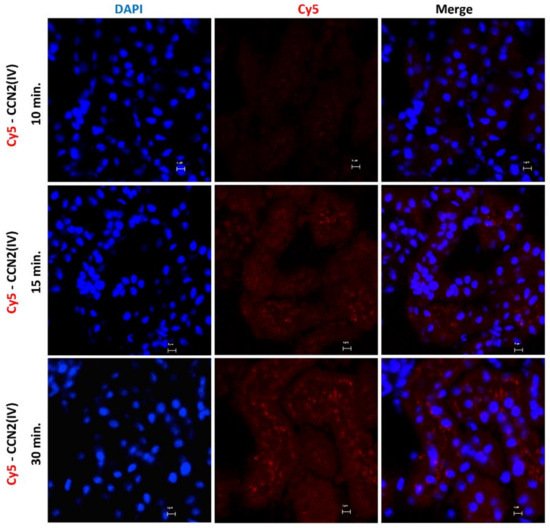
Figure 1. Evaluation of CCN2(IV) binding to renal tissue in the model of intra-renal injection. C57BL/6 mice were injected with Cy5-CCN2(IV) (dose of 2.5 ng/g of body weight, n = 3 mice per group) in the right kidney and sacrificed at different times (from 10 to 30 min). Frozen OCT-embedded renal samples were used for confocal microscopy. Red immunostaining (corresponding to CCN2(IV) labeled with Cy5 fluorophore) was found in tubular epithelial cells, showing CCN2(IV) binding to renal cells. Nuclei were stained with DAPI to distinguish renal structures. The overlaid images in red and blue (merge) indicated CCN2(IV) localization mainly in the membrane of tubuloepithelial cells. Figures show one representative mouse per group, and scale bar represents 5 μM.
Interestingly, red immunofluorescence was only observed in the tubuli but not in other renal structures, such as the glomeruli (Figure 2).
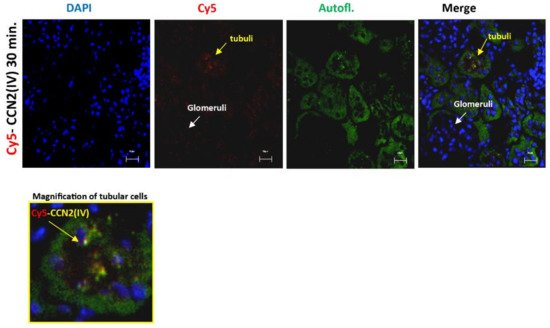
Figure 2. CCN2 (IV) binding was located in the tubuli but not in other renal structures in the kidney. Mice were injected with Cy5-CCN2(IV) in the right kidney and studied 30 min later. Red immunostaining was only found in the tubuli but was not present in the glomeruli. Renal tissue autofluorescence (green) and DAPI staining (blue) show renal structures. The overlaid images indicate CCN2(IV) localization only in the tubuli. Figures show a representative mouse per group, and scale bar represents 20 μM.
3. CCN2 Binds to Tubular Epithelial Cells, but Not to Glomerular Cells, in a Model of Systemic Administration of Cy5-CCN2(IV) into Mice
To evaluate whether there are some differences between local and systemic administration of the recombinant CCN2(IV), a mice model of Cy5-CCN2(IV) i.p. injection was conducted. In the kidneys of Cy5-CCN2(IV)-i.p injected mice, a red immunofluorescence signal was found, showing the maximal intensity at 30 min (Figure 3).
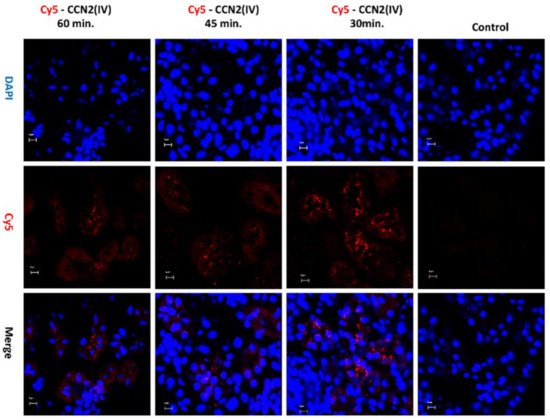
Figure 3. Evaluation of CCN2(IV) binding to the kidney in the model of systemic administration in mice. C57BL/6 mice were i.p. injected with 2.5 ng/g of body weight of recombinant Cy5- CCN2(IV) and sacrificed 30, 45, and 60 min later. In the kidneys from Cy5-CCN2(IV)-injected mice, red immunostaining corresponding to CCN2(IV) labeled with Cy5 fluorophore (red staining) was observed by confocal microscopy. Nuclei were stained with DAPI to locate renal structures. The overlaid images in red and blue (merge) indicated CCN2 (IV) localization mainly in the membrane of tubular epithelial cells. Figures show a representative mouse per group of 3 done, and scale bar represents 5 μM.
The Cy5-CCN2(IV) binding was mainly located in proximal tubular epithelial cells (Figure 4), as demonstrated using specific markers of proximal (LotusTetragonolobus Lectin, green) or distal (Dolichos Biflorus Lectin, green) tubuli.
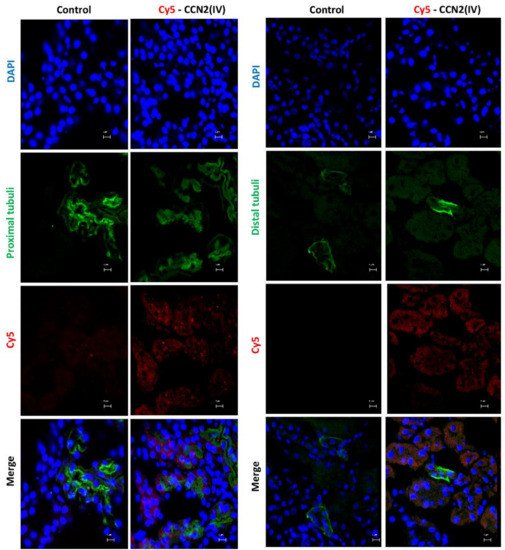
Figure 4. Determination of CCN2(IV) binding to proximal or distal tubuli in the kidney in the model of systemic administration of CCN2(IV). Co-localization of Cy5-CCN2(VI) (red) staining with markers of proximal (LotusTetragonolobus Lectin, green) or distal (Dolichos Biflorus Lectin, green) tubuli in CCN2(IV)-injected mice. Proximal tubuli are the main sites of Cy5-CCN2 binding sites. The scale bar represents 5 μM.
4. CCN2 Binding Is Linked to EGFR Pathway Activation in the Kidney
Our second aim was to evaluate whether intra-renal injection of CCN2(IV) could activate the EGFR signaling pathway. The researchers have previously demonstrated that CCN2 is an EGFR ligand [34]. Ligand binding to EGFR induces a conformational change leading to the formation of receptor homo- or heterodimers and subsequent activation (phosphorylation of specific tyrosine (Tyr) residues) of the tyrosine kinase domain located in the cytoplasmic tail of the receptor [44]. In the model of renal Cy5-CCN2(IV) injection, they found that cells with positive Cy5-CCN2(IV) binding also presented phosphorylated-EGFR immunostaining (Figure 5). Moreover, co-localization of CCN2(IV) binding and EGFR activation in the same tubular cells were also observed in the model of systemic CCN2(IV) administration (not shown). All these data demonstrate that CCN2(IV) binds to EGFR and activates its downstream signaling pathway in tubular epithelial cells in vivo.
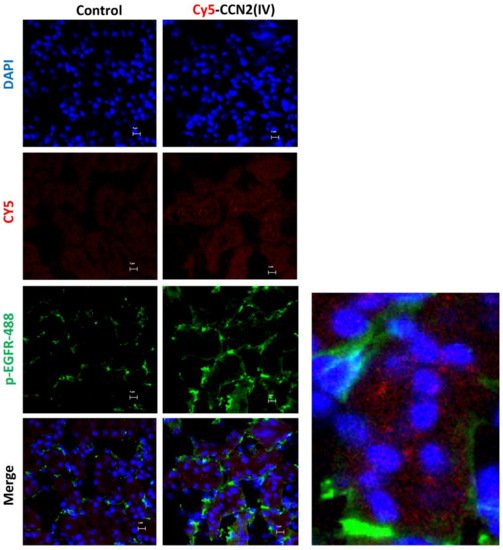
Figure 5. Localization of CCN2(IV) binding and EGFR activation in tubuloepithelial cells in vivo. C57BL/6 mice were injected with CCN2(IV)-Cy5 (2.5 ng/g of body weight, n = 3 mice per group) in the right kidney, using its corresponding contralateral kidney as control and sacrificed at 30 min. Figure shows Cy5-CCN2(IV) binding in red, activated EGFR in green (corresponding to phosphorylated EGFR (Alexa fluor® 488)), and nuclei in blue (DAPI). The overlaid images show the colocalization of CCN2(IV) with EGFR activated in several tubule epithelial cells. A magnification is shown on the right. Figures show one representative mouse per group, and scale bar represents 5 μM.
5. CCN2 Binding Is Linked to EGFR Pathway Activation in the Kidney
To further demonstrate CCN2(IV) binding to EGFR, cultured human tubular epithelial live-cell imaging was performed by confocal time-lapse microscopy. The involvement of EGFR was evaluated by gene silencing. HK2 cells were transfected with a siRNA against EGFR or its corresponding scrambled control siRNA (both FAM-labeled). After adding Cy5-CCN2(IV) to siRNA-transfected control HK2 cells, a red immunofluorescent signal was rapidly located in the cellular membrane (Figure 6), whereas in EGFR-silenced cells, the signal was decreased (Figure 6). As a control of the technique, Cy5-fluorochrome was added to the medium, showing no cellular binding (not shown). These data show that CCN2(IV) in vivo binds to tubular epithelial cells and activates the EGFR pathway in these cells.
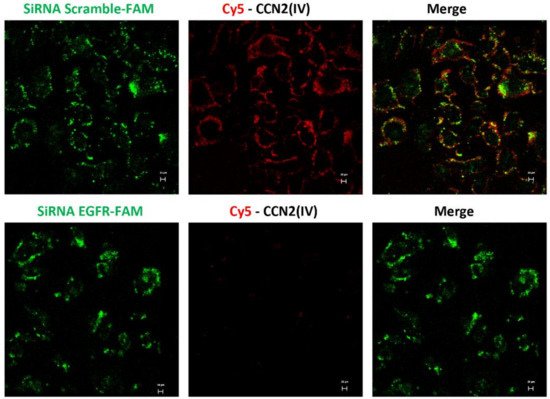
Figure 6. In vitro binding of Cy5-CCN2(IV) to tubular epithelial cells via EGFR. Serum-starved human tubular epithelial cells were transfected with a siRNA against EGFR or its corresponding scramble control siRNA, both FAM-labeled (green) and then stimulated with Cy5-CCN2(IV) for 10 min. Only cells expressing EGFR can bind to Cy5-CCN2(IV) (red signal), mainly located in the cellular membrane, whereas this signal is not found in EGFR-silenced cells. The scale bar represents 20 μM.
References
- Takigawa, M. An early history of CCN2/CTGF research: The road to CCN2 via hcs24, ctgf, ecogenin, and regenerin. J. Cell Commun. Signal. 2018, 12, 253–264.
- Bedore, J.; Leask, A.; Séguin, C.A. Targeting the extracellular matrix: Matricellular proteins regulate cell-extracellular matrix communication within distinct niches of the intervertebral disc. Matrix Biol. 2014, 37, 124–130.
- Rayego-Mateos, S.; Campillo, S.; Rodrigues-Diez, R.R.; Tejera-Muñoz, A.; Marquez-Exposito, L.; Goldschmeding, R.; Rodríguez-Puyol, D.; Calleros, L.; Ruiz-Ortega, M. Interplay between extracellular matrix components and cellular and molecular mechanisms in kidney fibrosis. Clin. Sci. 2021, 135, 1999–2029.
- Feng, D.; Ngov, C.; Henley, N.; Boufaied, N.; Gerarduzzi, C. Characterization of matricellular protein expression signatures in mechanistically diverse mouse models of kidney injury. Sci. Rep. 2019, 9, 16736.
- Perbal, B. CCN proteins: Multifunctional signalling regulators. Lancet 2004, 363, 62–64.
- de Winter, P.; Leoni, P.; Abraham, D. Connective tissue growth factor: Structure-function relationships of a mosaic, multifunctional protein. Growth Factors 2008, 26, 80–91.
- Rayego-Mateos, S.; Rodrigues-Diez, R.; Morgado-Pascual, J.L.; Valentijn, F.; Valdivielso, J.M.; Goldschmeding, R.; Ruiz-Ortega, M. Role of epidermal growth factor receptor (EGFR) and its ligands in kidney inflammation and damage. Mediat. Inflamm. 2018, 2018, 8739473.
- Markiewicz, M.; Nakerakanti, S.S.; Kapanadze, B.; Ghatnekar, A.; Trojanowska, M. Connective tissue growth factor (CTGF/CCN2) mediates angiogenic effect of S1P in human dermal microvascular endothelial cells. Microcirculation 2011, 18, 1–11.
- Rodrigues-Díez, R.; Rodrigues-Díez, R.R.; Rayego-Mateos, S.; Suarez-Alvarez, B.; Lavoz, C.; Stark Aroeira, L.; Sánchez-López, E.; Orejudo, M.; Alique, M.; Lopez-Larrea, C.; et al. The C-terminal module IV of connective tissue growth factor is a novel immune modulator of the Th17 response. Lab. Investig. 2013, 93, 812–824.
- Jun, J.-I.; Lau, L.F. CCN2 induces cellular senescence in fibroblasts. J. Cell Commun. Signal. 2017, 11, 15–23.
- Valentijn, F.A.; Knoppert, S.N.; Pissas, G.; Rodrigues-Diez, R.R.; Marquez-Exposito, L.; Broekhuizen, R.; Mokry, M.; Kester, L.A.; Falke, L.L.; Goldschmeding, R.; et al. CCN2 Aggravates the immediate oxidative stress-DNA damage response following renal ischemia-reperfusion injury. Antioxidants 2021, 10, 2020.
- Liu, B.-C.; Zhang, J.-D.; Zhang, X.-L.; Wu, G.-Q.; Li, M.-X. Role of connective tissue growth factor (CTGF) module 4 in regulating epithelial mesenchymal transition (EMT) in HK-2 cells. Clin. Chim. Acta 2006, 373, 144–150.
- He, M.; Chen, Z.; Martin, M.; Zhang, J.; Sangwung, P.; Woo, B.; Tremoulet, A.H.; Shimizu, C.; Jain, M.K.; Burns, J.C.; et al. miR-483 targeting of CTGF suppresses endothelial-to-mesenchymal transition: Therapeutic implications in kawasaki disease. Circ. Res. 2017, 120, 354–365.
- Sánchez-López, E.; Rayego, S.; Rodrigues-Díez, R.; Rodriguez, J.S.; Rodrigues-Díez, R.; Rodríguez-Vita, J.; Carvajal, G.; Aroeira, L.S.; Selgas, R.; Mezzano, S.A.; et al. CTGF promotes inflammatory cell infiltration of the renal interstitium by activating NF-kappaB. J. Am. Soc. Nephrol. 2009, 20, 1513–1526.
- Sakai, N.; Nakamura, M.; Lipson, K.E.; Miyake, T.; Kamikawa, Y.; Sagara, A.; Shinozaki, Y.; Kitajima, S.; Toyama, T.; Hara, A.; et al. Inhibition of CTGF ameliorates peritoneal fibrosis through suppression of fibroblast and myofibroblast accumulation and angiogenesis. Sci. Rep. 2017, 7, 5392.
- Leask, A.; Abraham, D.J. All in the CCN family: Essential matricellular signaling modulators emerge from the bunker. J. Cell Sci. 2006, 119, 4803–4810.
- Chaqour, B. Caught between a “Rho” and a hard place: Are CCN1/CYR61 and CCN2/CTGF the arbiters of microvascular stiffness? J. Cell Commun. Signal. 2020, 14, 21–29.
- Sánchez-López, E.; Rodrigues Díez, R.; Rodríguez Vita, J.; Rayego Mateos, S.; Rodrigues Díez, R.R.; Rodríguez García, E.; Lavoz Barria, C.; Mezzano, S.; Egido, J.; Ortiz, A.; et al. Connective tissue growth factor (CTGF): A key factor in the onset and progression of kidney damage. Nefrologia 2009, 29, 382–391.
- Phanish, M.K.; Winn, S.K.; Dockrell, M.E.C. Connective tissue growth factor-(CTGF, CCN2)--a marker, mediator and therapeutic target for renal fibrosis. Nephron. Exp. Nephrol. 2010, 114, e83–e92.
- Ivkovic, S.; Yoon, B.S.; Popoff, S.N.; Safadi, F.F.; Libuda, D.E.; Stephenson, R.C.; Daluiski, A.; Lyons, K.M. Connective tissue growth factor coordinates chondrogenesis and angiogenesis during skeletal development. Development 2003, 130, 2779–2791.
- Rayego-Mateos, S.; Morgado-Pascual, J.L.; Rodrigues-Diez, R.R.; Rodrigues-Diez, R.; Falke, L.L.; Mezzano, S.; Ortiz, A.; Egido, J.; Goldschmeding, R.; Ruiz-Ortega, M. Connective tissue growth factor induces renal fibrosis via epidermal growth factor receptor activation. J. Pathol. 2018, 244, 227–241.
- Rodrigues-Díez Raul, R.; Tejera-Muñoz, A.; Esteban, V.; Steffensen Lasse, B.; Rodrigues-Díez, R.; Orejudo, M.; Rayego-Mateos, S.; Falke Lucas, L.; Cannata-Ortiz, P.; Ortiz, A.; et al. CCN2 (Cellular communication network factor 2) deletion alters vascular integrity and function predisposing to aneurysm formation. Hypertension 2021.
- Gupta, S.; Clarkson, M.R.; Duggan, J.; Brady, H.R. Connective tissue growth factor: Potential role in glomerulosclerosis and tubulointerstitial fibrosis. Kidney Int. 2000, 58, 1389–1399.
- Riser, B.L.; Denichilo, M.; Cortes, P.; Baker, C.; Grondin, J.M.; Yee, J.; Narins, R.G. Regulation of connective tissue growth factor activity in cultured rat mesangial cells and its expression in experimental diabetic glomerulosclerosis. J. Am. Soc. Nephrol. 2000, 11, 25–38.
- Rupérez, M.; Ruiz-Ortega, M.; Esteban, V.; Lorenzo, O.; Mezzano, S.; Plaza, J.J.; Egido, J. Angiotensin II increases connective tissue growth factor in the kidney. Am. J. Pathol. 2003, 163, 1937–1947.
- Toda, N.; Mukoyama, M.; Yanagita, M.; Yokoi, H. CTGF in kidney fibrosis and glomerulonephritis. Inflamm. Regen. 2018, 38, 14.
- Shi-Wen, X.; Leask, A.; Abraham, D. Regulation and function of connective tissue growth factor/CCN2 in tissue repair, scarring and fibrosis. Cytokine Growth Factor Rev. 2008, 19, 133–144.
- Slagman, M.C.J.; Nguyen, T.Q.; Waanders, F.; Vogt, L.; Hemmelder, M.H.; Laverman, G.D.; Goldschmeding, R.; Navis, G. Effects of antiproteinuric intervention on elevated connective tissue growth factor (CTGF/CCN-2) plasma and urine levels in nondiabetic nephropathy. Clin. J. Am. Soc. Nephrol. 2011, 6, 1845–1850.
- Tam, F.W.K.; Riser, B.L.; Meeran, K.; Rambow, J.; Pusey, C.D.; Frankel, A.H. Urinary monocyte chemoattractant protein-1 (MCP-1) and connective tissue growth factor (CCN2) as prognostic markers for progression of diabetic nephropathy. Cytokine 2009, 47, 37–42.
- Guha, M.; Xu, Z.-G.; Tung, D.; Lanting, L.; Natarajan, R. Specific down-regulation of connective tissue growth factor attenuates progression of nephropathy in mouse models of type 1 and type 2 diabetes. FASEB J. 2007, 21, 3355–3368.
- Okada, H.; Kikuta, T.; Kobayashi, T.; Inoue, T.; Kanno, Y.; Takigawa, M.; Sugaya, T.; Kopp, J.B.; Suzuki, H. Connective tissue growth factor expressed in tubular epithelium plays a pivotal role in renal fibrogenesis. J. Am. Soc. Nephrol. 2005, 16, 133–143.
- Yokoi, H.; Mukoyama, M.; Nagae, T.; Mori, K.; Suganami, T.; Sawai, K.; Yoshioka, T.; Koshikawa, M.; Nishida, T.; Takigawa, M.; et al. Reduction in connective tissue growth factor by antisense treatment ameliorates renal tubulointerstitial fibrosis. J. Am. Soc. Nephrol. 2004, 15, 1430–1440.
- Lau, L.F. Cell surface receptors for CCN proteins. J. Cell Commun. Signal. 2016, 10, 121–127.
- Rayego-Mateos, S.; Rodrigues-Díez, R.; Morgado-Pascual, J.L.; Rodrigues Díez, R.R.; Mas, S.; Lavoz, C.; Alique, M.; Pato, J.; Keri, G.; Ortiz, A.; et al. Connective tissue growth factor is a new ligand of epidermal growth factor receptor. J. Mol. Cell Biol. 2013, 5, 323–335.
- Wahab, N.A.; Weston, B.S.; Mason, R.M. Connective tissue growth factor CCN2 interacts with and activates the tyrosine kinase receptor TrkA. J. Am. Soc. Nephrol. 2005, 16, 340–351.
- Sibilia, M.; Kroismayr, R.; Lichtenberger, B.M.; Natarajan, A.; Hecking, M.; Holcmann, M. The epidermal growth factor receptor: From development to tumorigenesis. Differentiation 2007, 75, 770–787.
- Bronte, G.; Terrasi, M.; Rizzo, S.; Sivestris, N.; Ficorella, C.; Cajozzo, M.; Di Gaudio, F.; Gulotta, G.; Siragusa, S.; Gebbia, N.; et al. EGFR genomic alterations in cancer: Prognostic and predictive values. Front. Biosci. 2011, 3, 879–887.
- Martinez-Useros, J.; Garcia-Foncillas, J. The challenge of blocking a wider family members of EGFR against head and neck squamous cell carcinomas. Oral Oncol. 2015, 51, 423–430.
- Zeng, F.; Singh, A.B.; Harris, R.C. The role of the EGF family of ligands and receptors in renal development, physiology and pathophysiology. Exp. Cell Res. 2009, 315, 602–610.
- Lautrette, A.; Li, S.; Alili, R.; Sunnarborg, S.W.; Burtin, M.; Lee, D.C.; Friedlander, G.; Terzi, F. Angiotensin II and EGF receptor cross-talk in chronic kidney diseases: A new therapeutic approach. Nat. Med. 2005, 11, 867–874.
- Terzi, F.; Burtin, M.; Hekmati, M.; Federici, P.; Grimber, G.; Briand, P.; Friedlander, G. Targeted expression of a dominant-negative EGF-R in the kidney reduces tubulo-interstitial lesions after renal injury. J. Clin. Investig. 2000, 106, 225–234.
- Flamant, M.; Bollée, G.; Hénique, C.; Tharaux, P.-L. Epidermal growth factor: A new therapeutic target in glomerular disease. Nephrol. Dial. Transplant. 2012, 27, 1297–1304.
- Tang, J.; Liu, N.; Zhuang, S. Role of epidermal growth factor receptor in acute and chronic kidney injury. Kidney Int. 2013, 83, 804–810.
- Sweeney, C.; Carraway, K.L. Ligand discrimination by ErbB receptors: Differential signaling through differential phosphorylation site usage. Oncogene 2000, 19, 5568–5573.
More
Information
Subjects:
Urology & Nephrology; Allergy
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
740
Revisions:
2 times
(View History)
Update Date:
19 Apr 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




