Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Xia Guo | + 1496 word(s) | 1496 | 2022-02-09 08:53:57 | | | |
| 2 | Catherine Yang | Meta information modification | 1496 | 2022-02-21 02:12:55 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Guo, X. Abdominal Aortic Aneurysm. Encyclopedia. Available online: https://encyclopedia.pub/entry/19637 (accessed on 07 February 2026).
Guo X. Abdominal Aortic Aneurysm. Encyclopedia. Available at: https://encyclopedia.pub/entry/19637. Accessed February 07, 2026.
Guo, Xia. "Abdominal Aortic Aneurysm" Encyclopedia, https://encyclopedia.pub/entry/19637 (accessed February 07, 2026).
Guo, X. (2022, February 18). Abdominal Aortic Aneurysm. In Encyclopedia. https://encyclopedia.pub/entry/19637
Guo, Xia. "Abdominal Aortic Aneurysm." Encyclopedia. Web. 18 February, 2022.
Copy Citation
Abdominal aortic aneurysm (AAA) is a lethal degenerative vascular disease that affects, mostly, the elder population, with a high mortality rate (>80%) upon rupture. It features a dilation of the aortic diameter to larger than 30 mm or more than 50%. Diverse pathological processes are involved in the development of AAA, including aortic wall inflammation, elastin breakdown, oxidative stress, smooth muscle cell (SMC) phenotypic switching and dysfunction, and extracellular matrix degradation.
abdominal aortic aneurysm
inflammation
vascular smooth muscle cell
1. Introduction
Aneurysm is the term for a dilated blood vessel with a diameter at least 1.5 times its normal size [1]. According to the locations, aneurysms can be divided into three major types, i.e., cerebral aneurysm (brain), aortic aneurysm (AA, aorta), and popliteal aneurysm (popliteal artery) [2][3][4]. The aorta is the largest blood vessel in the body and the AA is categorized into two main types: thoracic aortic aneurysm (TAA, in the chest) and abdominal aortic aneurysm (AAA, in the abdomen) [3]. AAA usually occurs in the infra-renal segment with a diameter exceeding 3.0 cm (Figure 1) [3]. AAA is the most common aneurysm and predominantly affects men aged 65 years and older [5][6].
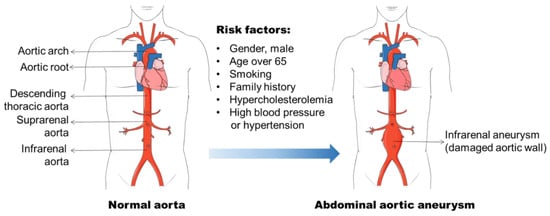
Figure 1. Abdominal aortic aneurysm formation and its risk factors. Abdominal aortic aneurysm (AAA) occurs in the infra-renal segment with a diameter exceeding 3.0 cm. The risk factors, including male gender, aging, smoking, and hypercholesterolemia, etc., have been found to be related to AAA initiation and progression.
AAA diagnosis remains a challenge because it does not present with any symptoms, nor can it be detected by a mere physical examination [7]. With the growing of the aortic diameter, the risk of AAA rupture increases [8]. The rupture of AAA results in profound internal bleeding with a mortality around 80% [9]. The ultrasound screening of the high-risk populations (men of 65-years and older) has been demonstrated to be an effective approach to prevent the AAA related mortality [10][11]. However, it is costly and not appropriate for the assessment of AAA progression. The treatment option for patients with large (≥55 mm), rapidly growing (>10 mm), or symptomatic AAAs remains endovascular exclusion or open surgery [5], although the postsurgical mortality for emergency operations stays at around 50% [12]. Patients who have small AAAs (<55 mm) are not beneficial from surgical repair [7]. Currently, there is no specific drug available to prevent or reverse AAA progression.
The pathophysiology of AAA is complex, involving the increased expression of endothelial cell (EC) adhesion molecules and chemokines, the inflammatory cell infiltration into the aortic wall, vascular smooth muscle cell (VSMC) dysfunction, aortic extracellular matrix (ECM) remodeling, oxidative stress, and the formation of intraluminal thrombus (Figure 2) [3][6][13]. The mechanisms of the AAA initiation and progression remain incompletely understood. The current review discusses the formation and progression of AAA with a focus on VSMC phenotypic switching and dysfunction.
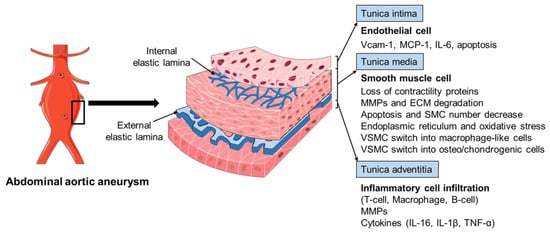
Figure 2. The pathophysiology of abdominal aortic aneurysm. The pathophysiology of abdominal aortic aneurysm (AAA) is a complicated process, involving the endothelial cell (EC) dysfunction with increased expression of adhesion molecules and chemokines, vascular smooth muscle cell (VSMC) phenotypic changes and dysfunction, inflammatory cell infiltration into the aortic wall, oxidative stress, and extracellular matrix (ECM) remodeling. Various mediators are involved in this process, including vascular cell adhesion molecule 1 (Vcam-1), monocyte chemoattractant protein 1 (MCP-1), interleukin-6 (IL-6), interleukin-1β (IL-1β), matrix metalloproteinases (MMPs), and tumor necrosis factor-α (TNF-α).
2. AAA Formation
2.1. Risk Factors for AAA
The identification of risk factors for AAA formation provides strategies for AAA prevention and therapy. In the past few decades, various factors including male gender, aging, cigarette smoking, and elevated blood cholesterol level, etc., have been found to be related to AAA initiation and progression (Figure 1) [6][14][15]. The incidence of AAA is directly proportional to an increase in age, and AAA is a prominent cause of death in older people from 65 years of age [5]. It is often seen in older men, and the ratio of male and female patients ranges 3.5–6:1 based on various screening studies [16][17]. Therefore, the men with an age of more than 65 years old are the high-risk population for AAA development.
Smoking has been recognized as a strong risk factor for AAA in various epidemiological studies [14][16][18]. Approximately 87% of the men with AAA were reported to be current or ex-smokers in a screening study of AAA among 65-year-old Swedish men with an odd ratio of 3.5 (95%CI: 2.4–5.1) [19]. The nicotine in plasma contributes to AAA progression, which has been confirmed in Apolipoprotein E deficient (ApoE−/−) mouse model and elastase-perfusion model of AAA [20][21][22]. The mechanisms underlying smoking induced AAA involve VSMC dysfunction and inflammatory cell function that have been discussed in Norman, P. E. and Curci, J. A. published review [20]. Hypercholesterolemia is a common facilitating cause of AAA, although there is limited knowledge as to its role in the development of AAA [15]. The abdominal aortic region is prone to the atherosclerotic lesion, which is found in virtually all cases of human AAA patients and that atherosclerosis is involved in the process of aortic dilation. Additional evidence to support the important role of hypercholesterolemia in aneurysm formation is the increased AAA formation in angiotensin II (Ang II) infused ApoE−/− mice (hypercholesterolemia mouse model) compared with wild-type (WT) C57BL/6 mice [23]. Genetic analyses of AAA from population screening and gene-associated studies show that different genetic risk factors are involved in the pathogenesis of AAA [24]. A heritability of 70% was reported in a large twin study by Jesper Swedenborg’s research group [25]. Several other studies have found that the risk of AAA in first-degree relatives of affected individuals was almost doubled [26][27][28]. Two loci for AAA have been identified and mapped on chromosome 19q13 and 4q31 [29][30]. The genome wide association studies (GWAS) have identified associations of single nucleotide polymorphisms (SNPs) with AAAs, such as CDKN2BAS1 rs10757274, the low-density lipoprotein receptor (LDLR) rs6511720, and DAB2IP rs10985349 [31][32]. In addition, other factors including DNA methylation, hypertension, and viral infections (e.g., cytomegalovirus, CMV) are also associated with AAA pathogenesis [33][34][35][36][37].
2.2. Histopathology of AAA
The histopathology of AAA is characterized by aortic wall inflammation, EC alteration, SMC dysfunction, oxidative stress, and ECM degradation, which cause a progressive luminal dilation, and finally a rupture (Figure 2) [6][13][38][39]. The aortic wall inflammation with the infiltration of inflammatory cells, including T-cells, B-cells, and macrophages, are essential features of AAA (Figure 2) [40][41][42]. In clinical and experimental AAA, a number of pro-inflammatory cytokines are increased, such as monocyte chemoattractant protein 1 (MCP-1), interleukin-1β (IL-1β), IL-6, and tumor necrosis factor-α (TNF-α). Polymorphisms in inflammatory cytokines may affect the production of these cytokines and, therefore, influence the pathogenesis of AAA [23][43][44]. SNPs (rs1800795 and rs1800796) in the IL-6 promoter have been linked with the development of AAA [44][45]. These inflammatory mediators play a critical role in inflammatory cell infiltration, which promotes an inflammatory response and subsequent SMC dysfunction, ECM degradation (mainly through matrix metalloproteinases, MMPs), and eventual AAA formation [42][46][47][48]. In addition, fragments of the aortic wall degradation serve as attracting agents for macrophage infiltration into the aortic wall to initiate the immune responses and AAA formation [49]. Dysregulation of EC function is another important factor implicated in AAA initiation and/or progression [50][51]. The increased EC expression of MCP-1 and vascular cell adhesion molecule 1 (Vcam-1) recruits macrophages into the aortic wall and leads to ECM degradation and, finally, aneurysm formation (Figure 2). In addition, EC apoptosis with the reduced expression of endothelial nitric oxide synthase (eNOS) also facilitates AAA formation by affecting the activity of NO, which is important in the stability of vascular tone, blood pressure, and SMC relaxation.
3. Application of Single-Cell RNA-Sequencing (scRNA Seq) in AAA Studies
scRNA seq emerges as a powerful approach to study transcriptome profile changes that are useful for identifying cellular clusters and exploring cellular responses in AAA. During AAA development, a myriad of cell types is involved, ranging from circulating immune cells to vascular resident cells (e.g., SMC). Recently, studies using scRNA seq have shed light on the heterogeneity and cellular responses of vascular cells in AAA progression. One recent study identified 17 clusters representing nine-cell lineages. Further Seurat clustering analysis identified four SMC subpopulations and five monocyte/macrophage subpopulations. During AAA progression, three major SMC subpopulations were proportionally decreased, whereas a small subpopulation was increased with downregulated SMC contractile markers and increased pro-inflammatory genes [52], suggesting phenotypic changes. Interestingly, scRNA seq analysis of lesioned aortas has identified macrophage-derived Netrin-1 as a robust inducer of the intracellular calcium flux and MMP3 activity by VSMCs, thereby it mediates the dynamic crosstalk between inflammation and ECM remodeling in AAA [53]. Single-cell analysis of the clinical aortic specimens from Marfan syndrome patients also revealed defective TGF-β signaling, i.e., downregulated TGFBR2 and Smad in a subset of SMCs [54]. Furthermore, an altered subpopulation of dedifferentiated proliferative SMCs was noted in the aortic tissues from Marfan syndrome patients but not from control subjects. These studies underscore the importance of the selective targeting of subgroups of VSMCs based on their transcriptome profiles. The scRNA seq analysis of AAA tissues are useful for dissecting the heterogeneity of cell subpopulations, deregulated signaling pathways, and cellular responses, as well as their interactions during AAA development. It also holds the key for identifying disease-relevant transcriptional signatures in VSMC-lineage cells, which might provide clues for disease predication, diagnosis, and prevention.
References
- Johnston, K.W.; Rutherford, R.B.; Tilson, M.D.; Shah, D.M.; Hollier, L.; Stanley, J.C. Suggested standards for reporting on arterial aneurysms. Subcommittee on Reporting Standards for Arterial Aneurysms, Ad Hoc Committee on Reporting Standards, Society for Vascular Surgery and North American Chapter, International Society for Cardiovascular Surgery. J. Vasc. Surg. 1991, 13, 452–458.
- Jersey, A.M.; Foster, D.M. Cerebral Aneurysm; StatPearls Publishing LLC: Treasure Island, FL, USA, 2021.
- Quintana, R.A.; Taylor, W.R. Cellular Mechanisms of Aortic Aneurysm Formation. Circ. Res. 2019, 124, 607–618.
- Kainth, A.; Smeds, M.R. Popliteal Aneurysm Repair; StatPearls Publishing LLC: Treasure Island, FL, USA, 2021.
- Anderson, P.L.; Arons, R.R.; Moskowitz, A.J.; Gelijns, A.; Magnell, C.; Faries, P.L.; Clair, D.; Nowygrod, R.; Kent, K.C. A statewide experience with endovascular abdominal aortic aneurysm repair: Rapid diffusion with excellent early results. J. Vasc. Surg. 2004, 39, 10–18.
- Nordon, I.M.; Hinchliffe, R.J.; Loftus, I.M.; Thompson, M.M. Pathophysiology and epidemiology of abdominal aortic aneurysms. Nat. Rev. Cardiol. 2011, 8, 92–102.
- Eickhoff, J. Incidence of diagnosis, operation and death from abdominal aortic aneurysms in Danish hospitals: Results from a nation-wide survey, 1977–1990. Eur. J. Surg. Acta Chir. 1993, 159, 619–623.
- Moll, F.L.; Powell, J.T.; Fraedrich, G.; Verzini, F.; Haulon, S.; Waltham, M.; van Herwaarden, J.A.; Holt, P.J.; van Keulen, J.W.; Rantner, B. Management of abdominal aortic aneurysms clinical practice guidelines of the European society for vascular surgery. Eur. J. Vasc. Endovasc. Surg. 2011, 41, S1–S58.
- Kantonen, I.; Lepantalo, M.; Brommels, M.; Luther, M.; Salenius, J.P.; Ylonen, K. Mortality in ruptured abdominal aortic aneurysms. The Finnvasc Study Group. Eur. J. Vasc. Endovasc. Surg. 1999, 17, 208–212.
- Earnshaw, J.J.; Lees, T. Update on Screening for Abdominal Aortic Aneurysm. Eur. J. Vasc. Endovasc. Surg. 2017, 54, 1–2.
- O’Donnell, T.F.X.; Landon, B.E.; Schermerhorn, M.L. AAA Screening Should Be Expanded. Circulation 2019, 140, 889–890.
- Hallin, A.; Bergqvist, D.; Holmberg, L. Literature review of surgical management of abdominal aortic aneurysm. Eur. J. Vasc. Endovasc. Surg. 2001, 22, 197–204.
- Kuivaniemi, H.; Ryer, E.J.; Elmore, J.R.; Tromp, G. Understanding the pathogenesis of abdominal aortic aneurysms. Expert Rev. Cardiovasc. 2015, 13, 975–987.
- Altobelli, E.; Rapacchietta, L.; Profeta, V.F.; Fagnano, R. Risk factors for abdominal aortic aneurysm in population-based studies: A systematic review and meta-analysis. Int. J. Environ. Res. Public Health 2018, 15, 2805.
- Tilson, M. Aortic aneurysms and atherosclerosis. Circulation 1992, 85, 378–379.
- Bonamigo, T.P.; Siqueira, I. Screening for abdominal aortic aneurysms. Rev. Hosp. Clin. Fac. Med. Sao Paulo 2003, 58, 63–68.
- Kim, L.G.; Thompson, S.G.; Marteau, T.M.; Scott, R.A.; Multicentre Aneurysm Screening Study, G. Screening for abdominal aortic aneurysms: The effects of age and social deprivation on screening uptake, prevalence and attendance at follow-up in the MASS trial. J. Med. Screen. 2004, 11, 50–53.
- Saratzis, A.; Dattani, N.; Brown, A.; Shalhoub, J.; Bosanquet, D.; Sidloff, D.; Stather, P.; Vascular, T.; Endovascular Research, N. Multi-Centre Study on Cardiovascular Risk Management on Patients Undergoing AAA Surveillance. Eur. J. Vasc. Endovasc. Surg. 2017, 54, 116–122.
- Svensjo, S.; Bjorck, M.; Gurtelschmid, M.; Djavani Gidlund, K.; Hellberg, A.; Wanhainen, A. Low prevalence of abdominal aortic aneurysm among 65-year-old Swedish men indicates a change in the epidemiology of the disease. Circulation 2011, 124, 1118–1123.
- Norman, P.E.; Curci, J.A. Understanding the effects of tobacco smoke on the pathogenesis of aortic aneurysm. Arter. Thromb. Vasc. Biol. 2013, 33, 1473–1477.
- Stolle, K.; Berges, A.; Lietz, M.; Lebrun, S.; Wallerath, T. Cigarette smoke enhances abdominal aortic aneurysm formation in angiotensin II-treated apolipoprotein E-deficient mice. Toxicol. Lett. 2010, 199, 403–409.
- Maegdefessel, L.; Azuma, J.; Toh, R.; Deng, A.; Merk, D.R.; Raiesdana, A.; Leeper, N.J.; Raaz, U.; Schoelmerich, A.M.; McConnell, M.V.; et al. MicroRNA-21 blocks abdominal aortic aneurysm development and nicotine-augmented expansion. Sci. Transl. Med. 2012, 4, 122ra122.
- Daugherty, A.; Manning, M.W.; Cassis, L.A. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J. Clin. Investig. 2000, 105, 1605–1612.
- Kuivaniemi, H.; Platsoucas, C.D.; Tilson III, M.D. Aortic aneurysms: An immune disease with a strong genetic component. Circulation 2008, 117, 242–252.
- Wahlgren, C.M.; Larsson, E.; Magnusson, P.K.; Hultgren, R.; Swedenborg, J. Genetic and environmental contributions to abdominal aortic aneurysm development in a twin population. J. Vasc. Surg. 2010, 51, 3–7; discussion 7.
- van Vlijmen-van Keulen, C.J.; Pals, G.; Rauwerda, J.A. Familial abdominal aortic aneurysm: A systematic review of a genetic background. Eur. J. Vasc. Endovasc. Surg. 2002, 24, 105–116.
- Cole, C.W.; Barber, G.G.; Bouchard, A.G.; McPhail, N.V.; Roberge, C.; Waddell, W.G.; Wellington, J.L. Abdominal aortic aneurysm: Consequences of a positive family history. Can. J. Surg. 1989, 32, 117–120.
- Larsson, E.; Granath, F.; Swedenborg, J.; Hultgren, R. A population-based case-control study of the familial risk of abdominal aortic aneurysm. J. Vasc. Surg. 2009, 49, 47–50; discussion 51.
- Vaughan, C.J.; Casey, M.; He, J.; Veugelers, M.; Henderson, K.; Guo, D.; Campagna, R.; Roman, M.J.; Milewicz, D.M.; Devereux, R.B. Identification of a chromosome 11q23. 2-q24 locus for familial aortic aneurysm disease, a genetically heterogeneous disorder. Circulation 2001, 103, 2469–2475.
- Shibamura, H.; Olson, J.M.; van Vlijmen-Van Keulen, C.; Buxbaum, S.G.; Dudek, D.M.; Tromp, G.; Ogata, T.; Skunca, M.; Sakalihasan, N.; Pals, G.; et al. Genome scan for familial abdominal aortic aneurysm using sex and family history as covariates suggests genetic heterogeneity and identifies linkage to chromosome 19q13. Circulation 2004, 109, 2103–2108.
- Jones, G.T.; Tromp, G.; Kuivaniemi, H.; Gretarsdottir, S.; Baas, A.F.; Giusti, B.; Strauss, E.; Van’t Hof, F.N.; Webb, T.R.; Erdman, R.; et al. Meta-Analysis of Genome-Wide Association Studies for Abdominal Aortic Aneurysm Identifies Four New Disease-Specific Risk Loci. Circ. Res. 2017, 120, 341–353.
- Singh, T.P.; Field, M.A.; Bown, M.J.; Jones, G.T.; Golledge, J. Systematic review of genome-wide association studies of abdominal aortic aneurysm. Atherosclerosis 2021, 327, 39–48.
- Vats, S.; Sundquist, K.; Wang, X.; Zarrouk, M.; Agren-Witteschus, S.; Sundquist, J.; Gottsater, A.; Memon, A.A. Associations of global DNA methylation and homocysteine levels with abdominal aortic aneurysm: A cohort study from a population-based screening program in Sweden. Int. J. Cardiol. 2020, 321, 137–142.
- Toghill, B.J.; Saratzis, A.; Harrison, S.C.; Verissimo, A.R.; Mallon, E.B.; Bown, M.J. The potential role of DNA methylation in the pathogenesis of abdominal aortic aneurysm. Atherosclerosis 2015, 241, 121–129.
- Takagi, H.; Umemoto, T.; Group, A. Association of Hypertension with Abdominal Aortic Aneurysm Expansion. Ann. Vasc. Surg. 2017, 39, 74–89.
- Nyberg, A.; Skagius, E.; Englund, E.; Nilsson, I.; Ljungh, A.; Henriksson, A.E. Abdominal aortic aneurysm and the impact of infectious burden. Eur. J. Vasc. Endovasc. Surg. 2008, 36, 292–296.
- Jablonska, A.; Zagrapan, B.; Paradowska, E.; Neumayer, C.; Eilenberg, W.; Brostjan, C.; Klinger, M.; Nanobachvili, J.; Huk, I. Abdominal aortic aneurysm and virus infection: A potential causative role for cytomegalovirus infection? J. Med. Virol. 2021, 93, 5017–5024.
- Wolinsky, H.; Glagov, S. A lamellar unit of aortic medial structure and function in mammals. Circ. Res. 1967, 20, 99–111.
- Crawford, E.S.; Cohen, E.S. Aortic aneurysm: A multifocal disease: Presidential address. Arch. Surg. 1982, 117, 1393–1400.
- Koch, A.E.; Haines, G.K.; Rizzo, R.J.; Radosevich, J.A.; Pope, R.M.; Robinson, P.G.; Pearce, W.H. Human abdominal aortic aneurysms. Immunophenotypic analysis suggesting an immune-mediated response. Am. J. Pathol. 1990, 137, 1199.
- Gregory, A.K.; Yin, N.X.; Capella, J.; Xia, S.; Newman, K.M.; Tilson, M.D. Features of autoimmunity in the abdominal aortic aneurysm. Arch. Surg. 1996, 131, 85–88.
- Yuan, Z.; Lu, Y.; Wei, J.; Wu, J.; Yang, J.; Cai, Z. Abdominal Aortic Aneurysm: Roles of Inflammatory Cells. Front. Immunol. 2020, 11, 609161.
- Marculescu, R.; Sodeck, G.; Domanovits, H.; Hobusch, G.; Exner, M.; Heinzl, H.; Huber, K.; Mannhalter, C.; Minar, E.; Wagner, O. Interleukin-1 gene cluster variants and abdominal aortic aneurysms. Thromb. Haemost. 2005, 94, 646–650.
- Bown, M.J.; Burton, P.R.; Horsburgh, T.; Nicholson, M.L.; Bell, P.R.; Sayers, R.D. The role of cytokine gene polymorphisms in the pathogenesis of abdominal aortic aneurysms: A case-control study. J. Vasc. Surg. 2003, 37, 999–1005.
- Jones, K.G.; Brull, D.J.; Brown, L.C.; Sian, M.; Greenhalgh, R.M.; Humphries, S.E.; Powell, J.T. Interleukin-6 (IL-6) and the prognosis of abdominal aortic aneurysms. Circulation 2001, 103, 2260–2265.
- Anidjar, S.; Dobrin, P.B.; Eichorst, M.; Graham, G.P.; Chejfec, G. Correlation of inflammatory infiltrate with the enlargement of experimental aortic aneurysms. J. Vasc. Surg. 1992, 16, 139–147.
- Juvonen, J.; Surcel, H.-M.; Satta, J.; Teppo, A.-M.; Bloigu, A.; Syrjälä, H.; Airaksinen, J.; Leinonen, M.; Saikku, P.; Juvonen, T. Elevated circulating levels of inflammatory cytokines in patients with abdominal aortic aneurysm. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 2843–2847.
- Colonnello, J.S.; Hance, K.A.; Shames, M.L.; Wyble, C.W.; Ziporin, S.J.; Leidenfrost, J.E.; Ennis, T.L.; Upchurch Jr, G.R.; Thompson, R.W. Transient exposure to elastase induces mouse aortic wall smooth muscle cell production of MCP-1 and RANTES during development of experimental aortic aneurysm. J. Vasc. Surg. 2003, 38, 138–146.
- Hance, K.A.; Tataria, M.; Ziporin, S.J.; Lee, J.K.; Thompson, R.W. Monocyte chemotactic activity in human abdominal aortic aneurysms: Role of elastin degradation peptides and the 67–kD cell surface elastin receptor. J. Vasc. Surg. 2002, 35, 254–261.
- Sun, J.; Deng, H.; Zhou, Z.; Xiong, X.; Gao, L. Endothelium as a Potential Target for Treatment of Abdominal Aortic Aneurysm. Oxid. Med. Cell. Longev. 2018, 2018, 6306542.
- Spartalis, E.; Spartalis, M.; Athanasiou, A.; Paschou, S.A.; Patelis, N.; Voudris, V.; Iliopoulos, D.C. Endothelium in Aortic Aneurysm Disease: New Insights. Curr. Med. Chem. 2020, 27, 1081–1088.
- Zhao, G.; Lu, H.; Chang, Z.; Zhao, Y.; Zhu, T.; Chang, L.; Guo, Y.; Garcia-Barrio, M.T.; Chen, Y.E.; Zhang, J. Single-cell RNA sequencing reveals the cellular heterogeneity of aneurysmal infrarenal abdominal aorta. Cardiovasc. Res. 2021, 117, 1402–1416.
- Hadi, T.; Boytard, L.; Silvestro, M.; Alebrahim, D.; Jacob, S.; Feinstein, J.; Barone, K.; Spiro, W.; Hutchison, S.; Simon, R.; et al. Macrophage-derived netrin-1 promotes abdominal aortic aneurysm formation by activating MMP3 in vascular smooth muscle cells. Nat. Commun. 2018, 9, 5022.
- Dawson, A.; Li, Y.; Li, Y.; Ren, P.; Vasquez, H.G.; Zhang, C.; Rebello, K.R.; Ageedi, W.; Azares, A.R.; Mattar, A.B.; et al. Single-Cell Analysis of Aneurysmal Aortic Tissue in Patients with Marfan Syndrome Reveals Dysfunctional TGF-β Signaling. Genes 2021, 13, 95.
More
Information
Subjects:
Agriculture, Dairy & Animal Science
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.6K
Revisions:
2 times
(View History)
Update Date:
21 Feb 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




