
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Esedullah AKARAS | + 1854 word(s) | 1854 | 2022-02-18 10:08:10 | | | |
| 2 | Vicky Zhou | Meta information modification | 1854 | 2022-02-18 10:19:33 | | | | |
| 3 | Vicky Zhou | -18 word(s) | 1836 | 2022-02-21 04:21:20 | | |
Video Upload Options
High-intensity bench press training (g = 1.03) and 12 RM bench press exercises (g = 1.21) showed a large effect size on increasing pectoralis major muscle size. In the elbow extensors, large effects were reported for an increase in muscle size with isometric maximal voluntary co-contraction training (g = 1.97), lying triceps extension exercise (g = 1.25), and nonlinear periodised resistance training (g = 2.07). In addition, further large effects were achieved in the elbow flexors via traditional elbow flexion exercises (g = 0.93), concentric low-load forearm flexion-extension training (g = 0.94, g = 1), isometric maximal voluntary co-contraction training (g = 1.01), concentric low-load forearm flexion-extension training with blood flow restriction (g = 1.02, g = 1.07), and nonlinear periodised resistance training (g = 1.13, g = 1.34). Regarding the forearm muscles, isometric ulnar deviation training showed a large effect (g = 2.22) on increasing the flexor carpi ulnaris and radialis muscle size. Results show that these training modalities are suitable for gaining hypertrophy in the relevant muscles with at least four weeks of training duration.
1. Introduction
2. The Effects of Resistance Training on Architecture and Volume of the Upper Extremity Muscles
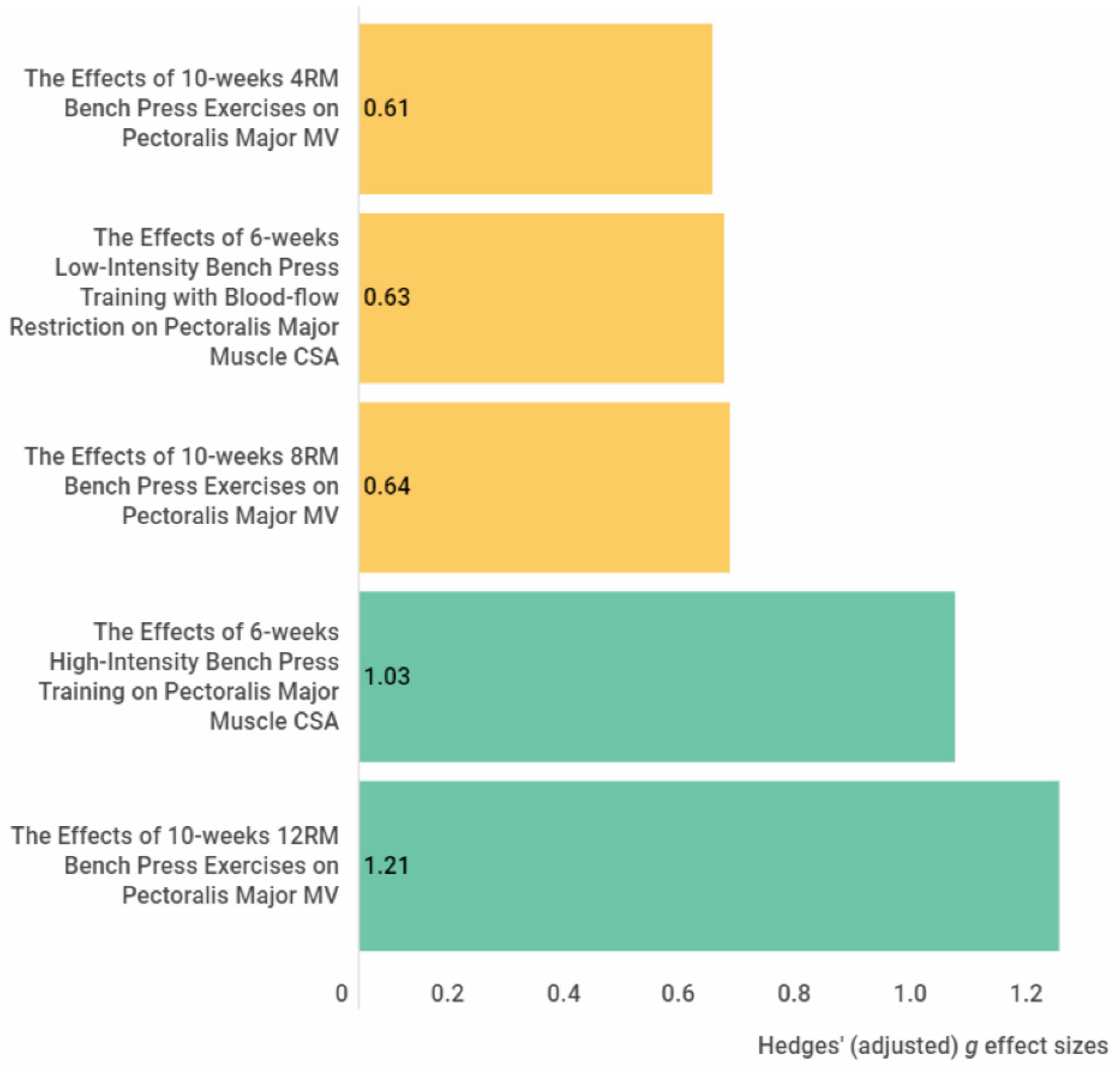 Figure 1. The effect size of the exercise interventions on the pectoralis major muscle cross-sectional area and volume. The yellow colour indicates a medium effect size, and the green colour indicates a large effect size. Abbreviations: CSA, cross-sectional area, MV, muscle volume.
Figure 1. The effect size of the exercise interventions on the pectoralis major muscle cross-sectional area and volume. The yellow colour indicates a medium effect size, and the green colour indicates a large effect size. Abbreviations: CSA, cross-sectional area, MV, muscle volume.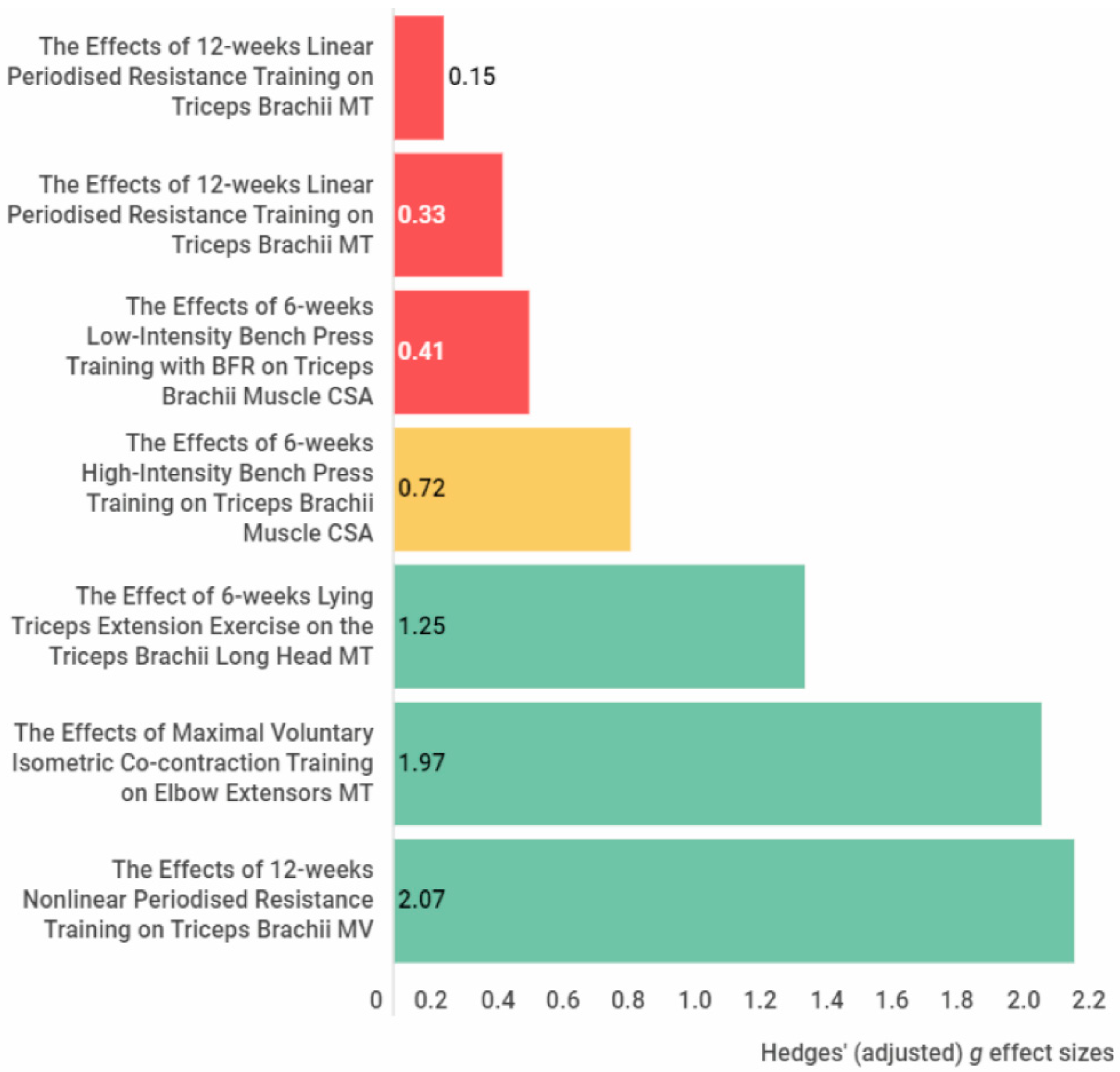 Figure 2. The effect size of the exercise interventions on the elbow extensor muscle thickness, cross-sectional muscle area and muscle volume. The red colour indicates a small or trivial effect size, the yellow colour indicates a medium effect size, and the green colour indicates a large effect size. Abbreviations: CSA, cross-sectional area; MT, muscle thickness; MV, muscle volume.
Figure 2. The effect size of the exercise interventions on the elbow extensor muscle thickness, cross-sectional muscle area and muscle volume. The red colour indicates a small or trivial effect size, the yellow colour indicates a medium effect size, and the green colour indicates a large effect size. Abbreviations: CSA, cross-sectional area; MT, muscle thickness; MV, muscle volume.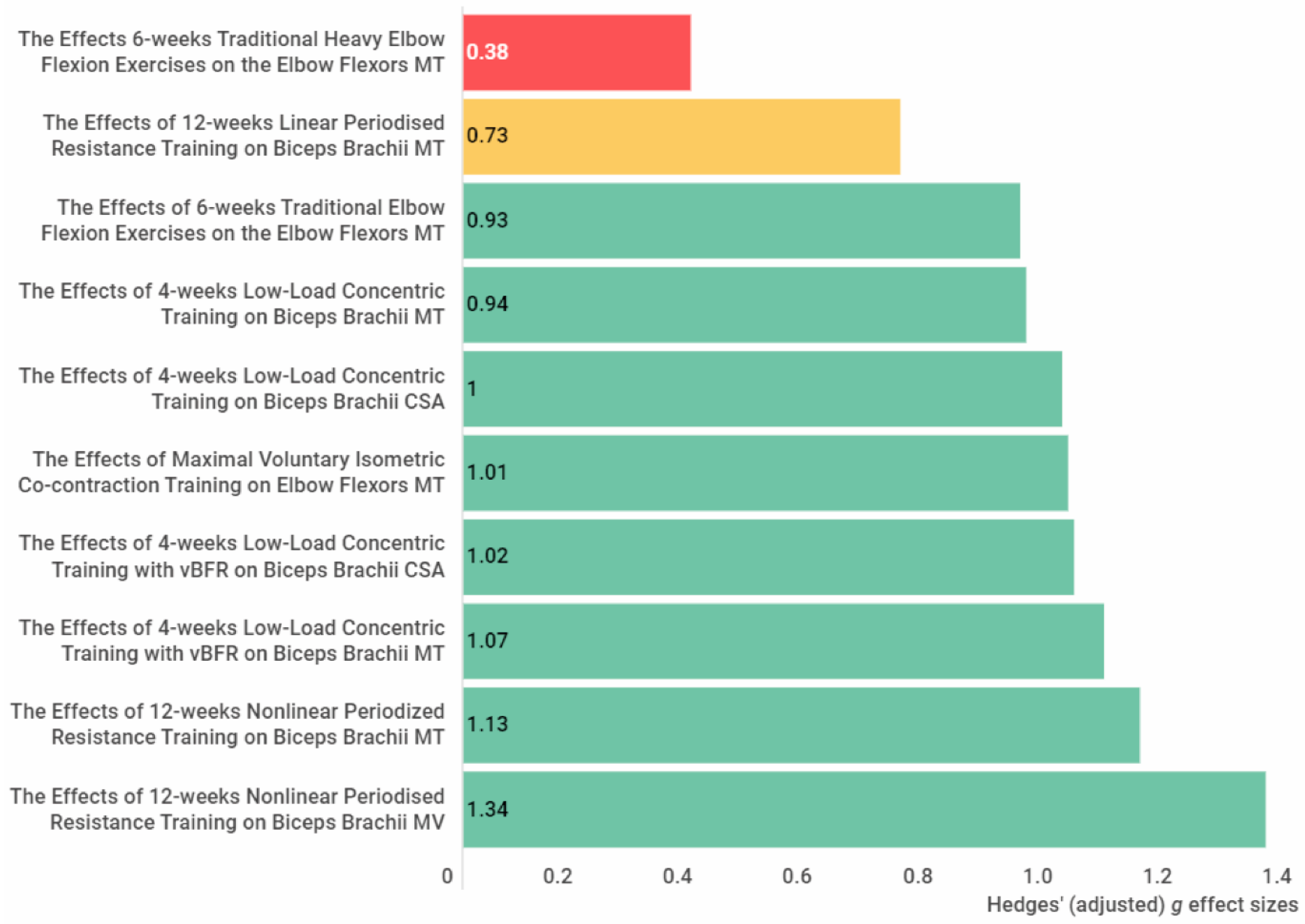 Figure 3. The effect size of the exercise interventions on the elbow flexors muscle thickness, muscle cross-sectional area and muscle volume. The red colour indicates a small or trivial effect size, the yellow colour indicates a medium effect size, and the green colour indicates a large effect size. Abbreviations: CSA, cross-sectional area; MT, muscle thickness; MV, muscle volume.
Figure 3. The effect size of the exercise interventions on the elbow flexors muscle thickness, muscle cross-sectional area and muscle volume. The red colour indicates a small or trivial effect size, the yellow colour indicates a medium effect size, and the green colour indicates a large effect size. Abbreviations: CSA, cross-sectional area; MT, muscle thickness; MV, muscle volume. Figure 4. The effect size of the exercise interventions on the forearm flexors muscle thickness. The green colour indicates a large effect size. Abbreviations: MT, muscle thickness.
Figure 4. The effect size of the exercise interventions on the forearm flexors muscle thickness. The green colour indicates a large effect size. Abbreviations: MT, muscle thickness.3. Conclusions
References
- Blazevich, A. Effects of Physical Training and Detraining, Immobilisation, Growth and Aging on Human Fascicle Geometry. Sports Med. 2006, 36, 1003–1017.
- Lieber, R.L.; Fridén, J. Functional and clinical significance of skeletal muscle architecture. Muscle Nerve 2000, 23, 1647–1666.
- Abe, T.; Kojima, K.; Stager, J.M. Skeletal muscle mass and muscular function in master swimmers is related to training distance. Rejuvenation Res. 2014, 17, 415–421.
- Abe, T.; Loenneke, J.P.; Thiebaud, R.S. Morphological and functional relationships with ultrasound measured muscle thickness of the lower extremity: A brief review. Ultrasound 2015, 23, 166–173.
- Akima, H.; Kano, Y.; Enomoto, Y.; Ishizu, M.; Okada, M.; Oishi, Y.; Katsuta, S.; Kuno, S. Muscle function in 164 men and women aged 20–84 year. Med. Sci. Sports Exerc. 2001, 33, 220–226.
- Freilich, R.J.; Kirsner, R.L.; Byrne, E. Isometric strength and thickness relationships in human quadriceps muscle. Neuromuscul. Disord. 1995, 5, 415–422.
- Fukunaga, T.; Roy, R.R.; Shellock, F.G.; Hodgson, J.A.; Edgerton, V.R. Specific tension of human plantar flexors and dorsiflexors. J. Appl. Physiol. 1996, 80, 158–165.
- Ikai, M.; Fukunaga, T. Calculation of muscle strength per unit cross-sectional area of human muscle by means of ultrasonic measurement. Int. Z. Angew. Physiol. 1968, 26, 26–32.
- Lieber, R.L. Skeletal Muscle Structure and Function; Lippincott Williams & Wilkins: Baltimore, MD, USA, 1992.
- Maughan, R.J.; Watson, J.S.; Weir, J. Strength and cross-sectional area of human skeletal muscle. J. Physiol. 1983, 338, 37–49.
- Moreau, N.G.; Simpson, K.N.; Teefey, S.A.; Damiano, D.L. Muscle architecture predicts maximum strength and is related to activity levels in cerebral palsy. Phys. Ther. 2010, 90, 1619–1630.
- Narici, M.V.; Landoni, L.; Minetti, A.E. Assessment of human knee extensor muscles stress from in vivo physiological cross-sectional area and strength measurements. Eur. J. Appl. Physiol. Occup. Physiol. 1992, 65, 438–444.
- Shephard, R.J.; Bouhlel, E.; Vandewalle, H.; Monod, H. Muscle mass as a factor limiting physical work. J. Appl. Physiol. 1988, 64, 1472–1479.
- Strasser, E.M.; Draskovits, T.; Praschak, M.; Quittan, M.; Graf, A. Association between ultrasound measurements of muscle thickness, pennation angle, echogenicity and skeletal muscle strength in the elderly. Age 2013, 35, 2377–2388.
- Abe, T.; Fukashiro, S.; Harada, Y.; Kawamoto, K. Relationship between sprint performance and muscle fascicle length in female sprinters. J. Physiol. Anthropol. Appl. Hum. Sci. 2001, 20, 141–147.
- Anousaki, E.; Zaras, N.; Stasinaki, A.N.; Panidi, I.; Terzis, G.; Karampatsos, G. Effects of a 25-Week Periodized Training Macrocycle on Muscle Strength, Power, Muscle Architecture, and Performance in Well-Trained Track and Field Throwers. J. Strength Cond. Res. 2021, 35, 2728–2736.
- Brechue, W.F.; Abe, T. The role of FFM accumulation and skeletal muscle architecture in powerlifting performance. Eur. J. Appl. Physiol. 2002, 86, 327–336.
- Ikebukuro, T.; Kubo, K.; Okada, J.; Yata, H.; Tsunoda, N. The relationship between muscle thickness in the lower limbs and competition performance in weightlifters and sprinters. Jpn. J. Phys. Fit. Sports Med. 2011, 60, 401–411.
- Kumagai, K.; Abe, T.; Brechue, W.; Ryushi, T.; Takano, S.; Mizuno, M. Sprint performance is related to muscle fascicle length in male 100-m sprinters. J. Appl. Physiol. 2000, 88, 811–816.
- Mangine, G.T.; Fukuda, D.H.; LaMonica, M.B.; Gonzalez, A.M.; Wells, A.J.; Townsend, J.R.; Jajtner, A.R.; Fragala, M.S.; Stout, J.R.; Hoffman, J.R. Influence of gender and muscle architecture asymmetry on jump and sprint performance. J. Sports Sci. Med. 2014, 13, 904–911.
- Mangine, G.T.; Fukuda, D.H.; Townsend, J.R.; Wells, A.J.; Gonzalez, A.M.; Jajtner, A.R.; Bohner, J.D.; LaMonica, M.; Hoffman, J.R.; Fragala, M.S.; et al. Sprinting performance on the Woodway Curve 3.0TM is related to muscle architecture. Eur. J. Sport Sci. 2015, 15, 606–614.
- Nasirzade, A.; Ehsanbakhsh, A.; Argavani, H.; Sobhkhiz, A.; Aliakbari, M. Selected anthropometrical, muscular architecture, and biomechanical variables as predictors of 50-m performance of front crawl swimming in young male swimmers. Sci. Sports 2014, 29, e75–e81.
- Nasirzade, A.; Ehsanbakhsh, A.; Ilbeygi, S.; Sobhkhiz, A.; Argavani, H.; Aliakbari, M. Relationship between sprint performance of front crawl swimming and muscle fascicle length in young swimmers. J. Sports Sci. Med. 2014, 13, 550–556.
- Nimphius, S.; McGuigan, M.R.; Newton, R.U. Changes in muscle architecture and performance during a competitive season in female softball players. J. Strength Cond. Res. 2012, 26, 2655–2666.
- Zaras, N.; Stasinaki, A.-N.; Terzis, G. Biological Determinants of Track and Field Throwing Performance. J. Funct. Morphol. Kinesiol. 2021, 6, 40.
- Zaras, N.D.; Stasinaki, A.N.; Methenitis, S.K.; Krase, A.A.; Karampatsos, G.P.; Georgiadis, G.V.; Spengos, K.M.; Terzis, G.D. Rate of Force Development, Muscle Architecture, and Performance in Young Competitive Track and Field Throwers. J. Strength Cond. Res. 2016, 30, 81–92.
- Hides, J.; Frazer, C.; Blanch, P.; Grantham, B.; Sexton, C.; Mendis, M.D. Clinical utility of measuring the size of the lumbar multifidus and quadratus lumborum muscles in the Australian football league setting: A prospective cohort study. Phys. Ther. Sport 2020, 46, 186–193.
- Hides, J.A.; Brown, C.T.; Penfold, L.; Stanton, W.R. Screening the lumbopelvic muscles for a relationship to injury of the quadriceps, hamstrings, and adductor muscles among elite Australian Football League players. J. Orthop. Sports Phys. Ther. 2011, 41, 767–775.
- Hides, J.A.; Stanton, W.R. Can motor control training lower the risk of injury for professional football players? Med. Sci. Sports Exerc. 2014, 46, 762–768.
- Hides, J.A.; Stanton, W.R. Predicting football injuries using size and ratio of the multifidus and quadratus lumborum muscles. Scand. J. Med. Sci. Sports 2017, 27, 440–447.
- Hides, J.A.; Stanton, W.R.; Mendis, M.D.; Franettovich Smith, M.M.; Sexton, M.J. Small Multifidus Muscle Size Predicts Football Injuries. Orthop. J. Sports Med. 2014, 2, 2325967114537588.
- Jeon, J.Y.; Kang, H.W.; Kim, D.Y.; Kim, Y.T.; Lee, D.Y.; Lee, D.-O. Relationship between calf muscle cross-sectional area and ankle fracture. Foot Ankle Surg. 2020, 27, 860–864.
- Mangine, G.T.; Hoffman, J.R.; Gonzalez, A.M.; Jajtner, A.R.; Scanlon, T.; Rogowski, J.P.; Wells, A.J.; Fragala, M.S.; Stout, J.R. Bilateral differences in muscle architecture and increased rate of injury in national basketball association players. J. Athl. Train. 2014, 49, 794–799.
- Timmins, R.G.; Bourne, M.N.; Shield, A.J.; Williams, M.D.; Lorenzen, C.; Opar, D.A. Short biceps femoris fascicles and eccentric knee flexor weakness increase the risk of hamstring injury in elite football (soccer): A prospective cohort study. Br. J. Sports Med. 2016, 50, 1524–1535.
- Netter, F.H. Atlas of Human Anatomy; Elsevier: Amsterdam, The Netherlands, 2014.
- Nasirzadeh, A.; Sadeghi, H.; Sobhkhiz, A.; Mohammadian, K.; Nikouei, A.; Baghaian, M.; Fatahi, A. Multivariate analysis of 200-m front crawl swimming performance in young male swimmers. Acta Bioeng. Biomech. Wroc. Univ. Technol. 2015, 17, 137–143.
- Tachibana, K.; Yashiro, K.; Miyazaki, J.; Ikegami, Y.; Higuchi, M. Muscle cross-sectional areas and performance power of limbs and trunk in the rowing motion. Sports Biomech. 2007, 6, 44–58.
- Terzis, G.; Georgiadis, G.; Vassiliadou, E.; Manta, P. Relationship between shot put performance and triceps brachii fiber type composition and power production. Eur. J. Appl. Physiol. 2003, 90, 10–15.
- Fukunaga, T.; Miyatani, M.; Tachi, M.; Kouzaki, M.; Kawakami, Y.; Kanehisa, H. Muscle volume is a major determinant of joint torque in humans. Acta Physiol. Scand. 2001, 172, 249–255.
- Moss, B.M.; Refsnes, P.E.; Abildgaard, A.; Nicolaysen, K.; Jensen, J. Effects of maximal effort strength training with different loads on dynamic strength, cross-sectional area, load-power and load-velocity relationships. Eur. J. Appl. Physiol. Occup. Physiol. 1997, 75, 193–199.
- Ichinose, Y.; Kanehisa, H.; Ito, M.; Kawakami, Y.; Fukunaga, T. Morphological and functional differences in the elbow extensor muscle between highly trained male and female athletes. Eur. J. Appl. Physiol. Occup. Physiol. 1998, 78, 109–114.
- Brorsson, S.; Nilsdotter, A.; Hilliges, M.; Sollerman, C.; Aurell, Y. Ultrasound evaluation in combination with finger extension force measurements of the forearm musculus extensor digitorum communis in healthy subjects. BMC Med. Imaging 2008, 8, 6.
- Akagi, R.; Tohdoh, Y.; Hirayama, K.; Kobayashi, Y. Relationship of pectoralis major muscle size with bench press and bench throw performances. J. Strength Cond. Res. 2014, 28, 1778–1782.
- Thomas, S.J.; Cobb, J.; Sheridan, S.; Rauch, J.; Paul, R.W. Chronic Adaptations of the Posterior Rotator Cuff in Professional Pitchers. Am. J. Sports Med. 2021, 49, 892–898.
- Wakahara, T.; Kanehisa, H.; Kawakami, Y.; Fukunaga, T.; Yanai, T. Relationship between muscle architecture and joint performance during concentric contractions in humans. J. Appl. Biomech. 2013, 29, 405–412.
- Adams, G.R.; Cheng, D.C.; Haddad, F.; Baldwin, K.M. Skeletal muscle hypertrophy in response to isometric, lengthening, and shortening training bouts of equivalent duration. J. Appl. Physiol. 2004, 96, 1613–1618.
- Franchi, M.V.; Reeves, N.D.; Narici, M.V. Skeletal Muscle Remodeling in Response to Eccentric vs. Concentric Loading: Morphological, Molecular, and Metabolic Adaptations. Front. Physiol. 2017, 8, 447.
- Kawakami, Y.; Abe, T.; Fukunaga, T. Muscle-fiber pennation angles are greater in hypertrophied than in normal muscles. J. Appl. Physiol. 1993, 74, 2740–2744.
- Pincheira, P.A.; Boswell, M.A.; Franchi, M.V.; Delp, S.L.; Lichtwark, G.A. Biceps femoris long head sarcomere and fascicle length adaptations after 3 weeks of eccentric exercise training. J. Sport Health Sci. 2021, 24, S29.
- Schünemann, H.; Brożek, J.; Guyatt, G.; Oxman, A. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations; GRADE Working Group: Oslo, Norway, 2013.
- Akagi, R.; Shikiba, T.; Tanaka, J.; Takahashi, H. A Six-Week Resistance Training Program Does Not Change Shear Modulus of the Triceps Brachii. J. Appl. Biomech. 2016, 32, 373–378.
- Hill, E.C.; Housh, T.J.; Keller, J.L.; Smith, C.M.; Schmidt, R.J.; Johnson, G.O. Early phase adaptations in muscle strength and hypertrophy as a result of low-intensity blood flow restriction resistance training. Eur. J. Appl. Physiol. 2018, 118, 1831–1843.
- Krentz, J.R.; Chilibeck, P.D.; Farthing, J.P. The effects of supramaximal versus submaximal intensity eccentric training when performed until volitional fatigue. Eur. J. Appl. Physiol. 2017, 117, 2099–2108.
- Kubo, K.; Ikebukuro, T.; Yata, H. Effects of 4, 8, and 12 Repetition Maximum Resistance Training Protocols on Muscle Volume and Strength. J. Strength Cond. Res. 2021, 35, 879–885.
- Radaelli, R.; Fleck, S.J.; Leite, T.; Leite, R.D.; Pinto, R.S.; Fernandes, L.; Simão, R. Dose-response of 1, 3, and 5 sets of resistance exercise on strength, local muscular endurance, and hypertrophy. J. Strength Cond. Res. 2015, 29, 1349–1358.
- Matta, T.; Simão, R.; de Salles, B.F.; Spineti, J.; Oliveira, L.F. Strength training’s chronic effects on muscle architecture parameters of different arm sites. J. Strength Cond. Res. 2011, 25, 1711–1717.
- Stasinaki, A.N.; Zaras, N.; Methenitis, S.; Tsitkanou, S.; Krase, A.; Aggeliki, K.; Terzis, G. Triceps Brachii Muscle Strength and Architectural Adaptations with Resistance Training Exercises at Short or Long Fascicle Length. J. Funct. Morphol. Kinesiol. 2018, 3, 28.
- Winnard, A.; Scott, J.; Waters, N.; Vance, M.; Caplan, N. Effect of Time on Human Muscle Outcomes During Simulated Microgravity Exposure Without Countermeasures—Systematic Review. Front. Physiol. 2019, 10, 1046.
- Šimunič, B.; Koren, K.; Rittweger, J.; Lazzer, S.; Reggiani, C.; Rejc, E.; Pišot, R.; Narici, M.; Degens, H. Tensiomyography detects early hallmarks of bed-rest-induced atrophy before changes in muscle architecture. J. Appl. Physiol. 2019, 126, 815–822.
- Wall, B.T.; Dirks, M.L.; Snijders, T.; Senden, J.M.G.; Dolmans, J.; van Loon, L.J.C. Substantial skeletal muscle loss occurs during only 5 days of disuse. Acta Physiol. 2014, 210, 600–611.
- Yasuda, T.; Ogasawara, R.; Sakamaki, M.; Bemben, M.G.; Abe, T. Relationship between limb and trunk muscle hypertrophy following high-intensity resistance training and blood flow-restricted low-intensity resistance training. Clin. Physiol. Funct. Imaging 2011, 31, 347–351.
- Maeo, S.; Yoshitake, Y.; Takai, Y.; Fukunaga, T.; Kanehisa, H. Neuromuscular adaptations following 12-week maximal voluntary co-contraction training. Eur. J. Appl. Physiol. 2014, 114, 663–673.
- Maeo, S.; Yoshitake, Y.; Takai, Y.; Fukunaga, T.; Kanehisa, H. Effect of short-term maximal voluntary co-contraction training on neuromuscular function. Int. J. Sports Med. 2014, 35, 125–134.
- Spineti, J.; de Salles, B.F.; Rhea, M.R.; Lavigne, D.; Matta, T.; Miranda, F.; Fernandes, L.; Simão, R. Influence of exercise order on maximum strength and muscle volume in nonlinear periodized resistance training. J. Strength Cond. Res. 2010, 24, 2962–2969.
- Dankel, S.J.; Bell, Z.W.; Spitz, R.W.; Wong, V.; Viana, R.B.; Chatakondi, R.N.; Buckner, S.L.; Jessee, M.B.; Mattocks, K.T.; Mouser, J.G.; et al. Assessing differential responders and mean changes in muscle size, strength, and the crossover effect to 2 distinct resistance training protocols. Appl. Physiol. Nutr. Metab. 2020, 45, 463–470.
- Hill, E.C.; Housh, T.J.; Keller, J.L.; Smith, C.M.; Anders, J.V.; Schmidt, R.J.; Johnson, G.O.; Cramer, J.T. Low-load blood flow restriction elicits greater concentric strength than non-blood flow restriction resistance training but similar isometric strength and muscle size. Eur. J. Appl. Physiol. 2020, 120, 425–441.
- Simão, R.; Spineti, J.; de Salles, B.F.; Matta, T.; Fernandes, L.; Fleck, S.J.; Rhea, M.R.; Strom-Olsen, H.E. Comparison between nonlinear and linear periodized resistance training: Hypertrophic and strength effects. J. Strength Cond. Res. 2012, 26, 1389–1395.
- Spineti, J.; Figueiredo, T.; Miranda, H.; de Salles, B.; Oliveira, L.; Simão, R. The effects of exercise order and periodized resistance training on maximum strength and muscle thickness. Int. SportMed J. 2014, 15, 374–390.
- Farthing, J.P.; Chilibeck, P.D.; Binsted, G. Cross-education of arm muscular strength is unidirectional in right-handed individuals. Med. Sci. Sports Exerc. 2005, 37, 1594–1600.




