Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Anna Cecchele | + 4072 word(s) | 4072 | 2022-02-08 07:36:30 | | | |
| 2 | Vivi Li | Meta information modification | 4072 | 2022-02-18 03:10:41 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Cecchele, A. Fragmentation of Human Embryos. Encyclopedia. Available online: https://encyclopedia.pub/entry/19594 (accessed on 08 March 2026).
Cecchele A. Fragmentation of Human Embryos. Encyclopedia. Available at: https://encyclopedia.pub/entry/19594. Accessed March 08, 2026.
Cecchele, Anna. "Fragmentation of Human Embryos" Encyclopedia, https://encyclopedia.pub/entry/19594 (accessed March 08, 2026).
Cecchele, A. (2022, February 17). Fragmentation of Human Embryos. In Encyclopedia. https://encyclopedia.pub/entry/19594
Cecchele, Anna. "Fragmentation of Human Embryos." Encyclopedia. Web. 17 February, 2022.
Copy Citation
Embryo fragmentation represents a phenomenon generally characterized by the presence of membrane-bound extracellular cytoplasm into the perivitelline space.
embryo
fragmentation
micronuclei
vesicles
apoptosis
1. Introduction
Embryo fragmentation represents a phenomenon generally characterized by the presence of membrane-bound extracellular cytoplasm into the perivitelline space. In vitro, human embryo fragmentation has been reported since the 1980s [1], but it has been described also in human embryos conceived in vivo [2], indicating that it is not an artifact of the in vitro culture. Many diverse terms have been used to refer to these cytoplasmic fragments, including corpse, cytoplasmic pinching, micronuclei, debris, and shedding microvesicles. Highlights from the current literature support the cellular and molecular heterogeneity of embryo fragments: they can vary in size, kinetics, and organelle and molecular content [3]. Importantly, during assisted reproduction technology (ART) procedures, fragmentation and cell debris are considered important prognostic factors in the static morphologic assessment of human embryo quality, along with cell number, size, and symmetry. In this context, the presence of cytoplasmic fragments is suggestive of a poor prognosis embryo development and poor ART outcomes. On the basis of this idea but without strong supporting backgrounds, some groups have proposed to remove these cellular structures from the embryos before the transfer [4]. More recently, time-lapse microscopy (TLM) documented that cellular fragments can be extruded or reabsorbed into blastomeres, highlighting a dynamism in the process [5].
2. Different Fragment-like Cellular Structures: Characterization, Timing, and Cargo
2.1 Fragment size
Embryo fragments are heterogeneous in size: they can vary from normal-size blastomeres to simple cellular debris. Johansson et al. classified 44 cleavage embryos according to fragment size: entities smaller than 45 µm in day 2 and smaller than 40 µm in day 3, respectively, have been considered as anucleated cytoplasmic fragments, while larger structures as blastomeres [6]. In addition, human embryo can naturally release extracellular vesicles (EVs) that, on the basis of cellular origin, size, and release mechanism, can be categorized into exosomes (30–150 nm in diameter) [7], microvesicles (50–1000 nm) [8], and apoptotic bodies (50 nm–5 µm) [9][10][11][12] (Figure 1).
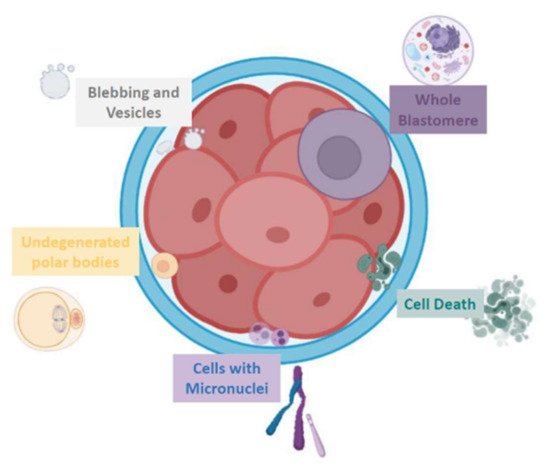
Figure 1. Schematic representation of the various fragment-like cellular structures detected in embryos.
2.2 Timing of cytoplasmic fragments formation
Fragmentation may occur from the first embryo division of pre-implantation development when the maternal genome drives the development; this phenomenon has been initially suggested to be less common after the embryonic genome activation [13]. Interestingly, representative time-lapse frames exhibited dissimilar temporal and spatial patterns of fragmentation among various steps of pre-implantation embryo development [3]. Fragments formed in some embryos during the pronuclear or early cleavage stages, but were no longer detectable at later stages. For other embryos, some of the cellular fragments occurring during early cleavage were still detectable in blastomeres during late cleavage [3] (Figure 2). Direct evidence of the dynamic nature of the phenomena was subsequently reported. Handarson and colleagues described by time-lapse sequence imaging the internalization/reabsorption of cellular fragments into neighboring blastomeres, while others disappear, leaving behind only debris [5]. A more recent suggestion is that an embryo can be capable of excluding any unwanted cell and/or cellular fragment from the remaining viable cells during the morula-to-blastocyst transition [14] (Figure 3). The complete exclusion of fragments or entire blastomeres can be observed in compacted embryos, morulae and blastocysts (Figure 4). Commonly, fragments do not take part in the blastocyst formation. This phenomenon is usually not very visible when the blastocoel inside blastocyst increases causing the progressive thinning of the zona pellucida (ZP). It is more visible when the blastocyst shows collapse or contraction episodes: some cells or fragments or debris become clearly visible in the perivitelline space (Figure 3).
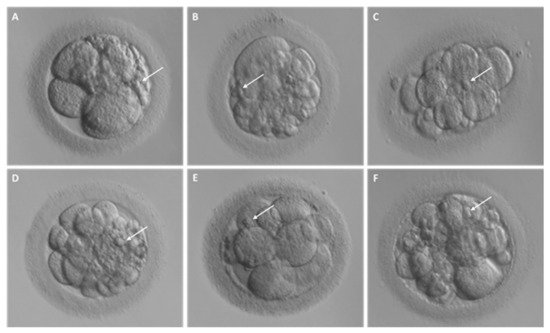
Figure 2. Different degrees of fragmentation in cleavage stage human embryos. (A–F) Representative images of day 3 embryos characterized by cellular fragments of different sizes and positions. For each embryo, arrows indicate a representative cellular fragment.
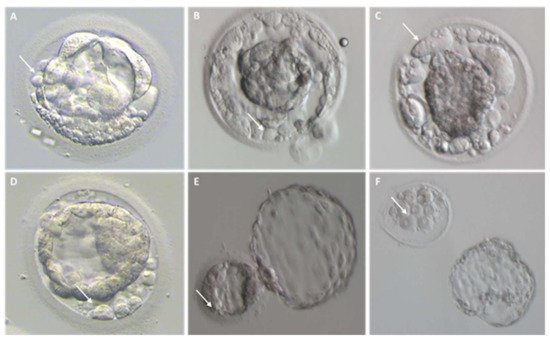
Figure 3. Cellular debris/fragments in the zona pellucida of day 5, 6, and 7 blastocysts. For each embryo, arrows indicate a representative cellular fragment or blastomere excluded upon blastocyst formation. (A–D) Representative images of collapsing blastocysts presenting different degree of fragmentation; (E) hatching blastocyst characterized by several cellular fragments within the zona pellucida; (F) hatched blastocyst on the right and its original zona pellucida containing leftovers of cell debris on the left.
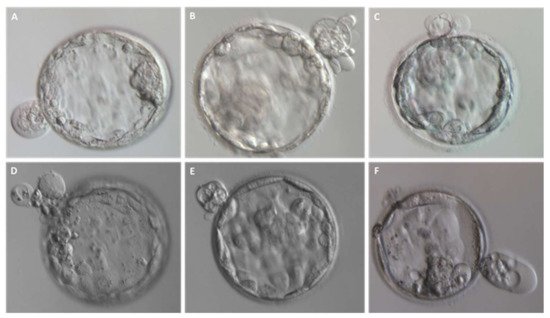
Figure 4. Entire blastomeres are excluded upon blastocyst formation. (A–F) Representative images of expanded blastocysts expelling one or more cells.
Similarly, the release of human embryo-derived EVs has been reported from the zygote stage up to expanded blastocyst stage [15][16]. Indeed, EVs were detected in the zona pellucida of human zygotes and in embryo conditioned culture media, but not in the ZP of metaphase II oocytes, suggesting that EV release begins shortly after fertilization [16].
2.3 Fragment cargo
The cargo of embryo fragments (from entire blastomere up to EVs) may contain nucleic acids, proteins, lipids, chromosomes, and entire organelles. Organelles as vacuoles, large mitochondria, vesicle complexes, and lysosomes may be sequestered into fragments [17][18]. Entire or portions of chromosomes, sequestered during fragmentation, can originate from either the mother or the father [19][20]. Evidence reveals that a preferential sequestering of particular chromosomes is unlikely. Chromosomal fragment size was found to range from 6 to 85 Mb [20].
The Different Origins Proposed for Embryo Fragment-like Entities
1. Extruded blastomeres
Thanks to the use of TLM that allows a deeper observation of the events underling embryo development, blastomere exclusion has been observed both in morulae and blastocysts of several mammalian species (i.e., humans, rhesus macaques, cattle, and mice) [21][22][23][24][25][26][27][28][29] (Figure 3). Blastomeres could be excluded during the first phases of embryo compaction, while others could be extruded from the compacted morula after a transient involvement during this process [14]. Lagalla et al. retrospectively evaluated 791 embryos obtained in 145 ART cycles by time-lapse morphokinetics analysis. Array-CGH analyses performed on both trophoectoderm cells and those excluded during morula compaction demonstrated that the latter have a higher incidence of aneuploidies as compared to the former ones. Those extruded cells are unable to flatten and establish tight intercellular contacts. Several factors may be involved in the failure of the compaction process: an abnormal formation of tight junctions [29] or the inability to express proteins involved in cell adhesion [13]. The frequency of this phenomenon is not a well-documented process, but it is clearly known that pre-implantation embryos tolerate it well.
2. Chromosome-containing micronuclei
Although initially embryonic fragments were considered as anucleate cytoplasmic components, subsequently, the presence of nuclear DNA started to be demonstrated [19][30][31]. By performing immunofluorescence analysis using antibodies against the centromere protein-A (CENP-A) and the nuclear envelope marker, LAMIN-B1, Chavez and colleagues demonstrated the presence of micronuclei in cleavage stage human embryos, suggesting their formation could be a mechanism adopted by embryos to sequester mis-segregated chromosome [19]. Micronuclei are extra-nuclear bodies containing damaged chromosome fragments and/or whole chromosomes that were not incorporated into the nucleus after cell division. They originate from acentric chromatid/chromosome fragments or from entire chromatids/chromosomes that are not correctly included in the main nucleus during the telophase [32]. They are, instead, enwrapped by the nuclear membrane, acquiring the structure of the daughter nucleus, even though smaller in size [33][34]. Fragments containing micronuclei can be reabsorbed by the embryo and fused with neighboring blastomeres, thus possibly resulting in the correction of aneuploid blastomeres or in altering the correct ploidy status if they fused with euploid blastomeres. Thus, this process can have a positive effect since, if tolerated, it can influence karyotype evolution in species, or the result may be deleterious, leading to additional genetic mutation [19].
3. Apoptotic bodies
Since the 1980s, a role of apoptosis process in pre-implantation embryo development has been suggested. The first report demonstrating the presence of ultrastructural features associated with degenerating cells and with a micro-pinocytotic activity in the inner cell mass (ICM) cells of viable/hatched human blastocysts was published in 1982 [35][36]. This latter study confirmed previously published results in primate models [36], suggesting a physiological role of cell death process in ICM development. Nevertheless, the pioneers of ART realized that both viable and arrested embryos may contain a proportion of cytoplasmic apoptotic fragments, suggesting that cellular apoptosis could play a role also in embryonic arrest. A detailed description of the controversial findings on the apoptotic phenomena associated with embryo fragmentation is reported in a subsequent paragraph (Section 2.2 point 1.). A distinctive morphological change of apoptosis is the blebbing and the consequent apoptotic bodies formation. These membrane-bound vesicles contain cytoplasm, organelles, and nuclear fragments, and they are released into the extracellular space. After the release, apoptotic bodies are usually phagocytosed and degraded/digested by professional phagocytes (such as macrophages) or non-professional phagocytes (such as epithelial cells) [37]. The phagocytic competence of early embryonic cells has been proposed following the observation of in vitro internalization of fluorescence microspheres in trophectoderm cells of human blastocysts after overnight co-culture evaluated by transmission electron microscopy (TEM) and fluorescence microscopy [38]. Li et al. did not detect phagocytic activity in the ICM, and an increased ability in blastocysts after 6 days of culture was observed compared to faster ones. However, the authors did not investigate the molecular mediators of phenomenon. Phagocytosis is a very specific process, characterized by several successive steps and mediated by fine interactions between cell surface ligands and cell surface receptors. It is also true that Pisko and colleagues supported this idea in a mouse model where embryonic cells had all the key molecules necessary for the recognition and digestion of damaged blastomeres, undertaking the clearance of the majority of cellular debris in blastocysts [39]. Despite the above assumptions, many cellular fragments mostly persist in the blastocoel and in the perivitelline space, suggesting that other mechanisms may be responsible for fragmentation and debris formation in pre-implantation embryos.
4. Persisting polar bodies
Polar bodies (PBs) are the byproduct of the oocyte meiotic cell divisions. They are small cytoplasmic blebs containing haploid genetic material plus a small amount of cytoplasmic organelles. Generally, they undergo apoptosis in 17–24 h after formation [40]. Evidence suggests that persisting PBs in embryos at the blastocyst stage can give rise to cellular fragments in the sub-zonal space [41]. Ottolini and colleagues performed a genetic analysis of a slow developing embryo; they analyzed the trophectoderm and the excluded cell fragments. Results obtained from the karyotyping of the fragments (47,XX,+19) and of the trophectoderm sample (46,XY) were non-concordant. In order to investigate deeper the cause of these results, DNA fingerprinting analyses using a panel of informative short tandem repeats markers and amelogenin were performed in all the samples. Subsequently, samples were also analyzed for karyomapping. The fragments demonstrated the absence of any paternal alleles and the presence of only a single maternal allele at each locus. The karyomapping revealed that the DNA amplified from fragments was exclusively that of the second polar body corresponding to the fertilized oocyte that gave rise to the embryo from which the trophectoderm had been biopsied [42]. This study demonstrated that a fraction of fragments may derive from the second PB.
5. Extracellular Vesicles
In 2019, Vyas and collaborators demonstrated that EVs could be released from all stages of pre-implantation embryos including 1-cell zygotes, cleavage embryos (2-cell, 4-cell, and 8–10-cell), morulae, and blastocysts. EVs were also detected throughout the ZP from the inner to its external surface, suggesting the capability of these vesicles to pass through ZP. In the same year, Battaglia and colleagues reported the presence of EVs in human blastocoel fluid [42]. Some studied confirmed that embryo-derived EVs are present in embryo-conditioned culture media of both day 3 and day 5 human pre-implantation embryos and, in both cases, with a diameter between 50 and 200 nm, consistent with exosome and microvesicle size [9][16]. Their specific molecular cargo (OCT4 and NANOG gene transcripts, HLA-G protein) suggests that they can arise from both ICM and trophectoderm compartment [9]. Unlike other cytoplasmic fragments, EVs have been shown to act as mediators of active cell-to-cell communication by packaging and transferring molecules from one cell to another both locally and remotely [43].
6. Others
-
Mitochondria
Fragmented embryos displayed a different organization of mitochondrial distribution: a higher concentration of mitochondria has been observed in the center rather than in the periphery of blastomeres in fragmented embryos as compared to non-fragmented ones [44]. This pattern could be linked to reduced adenosine triphosphate (ATP) content and reduced developmental potential [45] that can ultimately result in the disruption of the membrane with subsequent cellular lysis caused by disruption of the ion pump function [3].
-
Perivitelline threads
Perivitelline threads (PVTs) (also defined with the term of trans-zonal projections) are thin filaments that extend across the perivitelline space connecting the ZP with the oolemma or with the blastomere membrane. Their origin and nature are not clear. A theory linked their formation to the corona radiata; indeed, corona radiata cells are characterized by projections that can traverse the ZP with a role in the communication with the oolemma before ovulation [46][47]. After the luteinizing hormone surge, these projections are withdrawn and are thought not to persist beyond the meiotic reactivation stage. Nevertheless, observations during intracytoplasmic sperm injection, demonstrated that remnants of the projections of corona radiata persist, thus resulting in the formation of PVT [48]. Derrick and colleagues demonstrated an association between the presence of PVTs and embryo fragments. Analyzing 525 blastocysts, the authors found that 77% of them were characterized by the presence of PVTs, most appearing at the 2-cell stage (98% of the cases). Almost all of the embryos characterized by PVTs presented fragments (98%). Conversely, fragmentation was significantly less frequently observed in embryos without PVTs [48]. During the first mitotic division, a tight adherence between the PVT and the membrane may cause a strain during movement of the cells, causing fragments to form where there are already some weaknesses, thus explaining the link between PVTs and the generation of fragments [49]. Notably, there is no evidence of a relationship between PVTs and implantation potential (implanted embryos with PVT vs. without PVT: 25 vs. 29%) or with the ploidy status (euploid embryos with PVT vs. without PVT: 40 vs. 49%), suggesting no significant relationship between PVTs and embryo developmental potential [48].
3. Theories on the Origins over the Years
The precise mechanism(s) by which embryo fragmentation occur remains to be clarified. The cellular machineries involved in fragment-like entities formation and release could be unique or various (depending on type of cellular content or the timing of formation). Nevertheless, several hypotheses on the origin of fragments have been proposed, including apoptotic cell death, the effect of reactive oxygen species, cytoskeletal disorders, vesicles and micronuclei formation. All these theories might be valid. In addition, the frequency of this phenomenon in humans is also unknown. Notably, as a general idea, it has to be underlined that, in recent years, the interest in chromosome abnormalities and chromosomal mosaicism in human pre-implantation embryos has increased. As a consequence, the presence of whole chromosomal abnormalities or aneuploidy has been considered to be a primary determinant of whether an embryo arrests or reaches the blastocyst stage [50]. Thanks to the use of high-resolution techniques, it has been estimated that between 50% and 80% of cleavage stage human embryos contain more than one aneuploid cell [10][51][52][53][54][55]. Because of the lack of cell cycle check points during blastulation, different types of mosaicism (i.e., aneuploid/diploid mosaicism and complex aneuploid mosaicism) have been frequently found in embryos [55][56]. Despite the majority of chromosomal errors not being corrected, there are several lines of evidence supporting the existence of “embryo self-corrective” mechanisms that are involved in the extrusion of aneuploid cells during embryo development [57][58]. These mechanisms are thought to be the results of multipolar divisions, blastomere exclusion, and cellular fragmentation. The hypothesis that embryo fragmentation could be a tool of regulation and maintenance of cellular homeostasis in human embryo was supported by reported associations between extensive fragmentation and chromosomal abnormalities [59][60][61][62].
However, different mechanisms were studied over the years linking embryo homeostasis and the release of fragments, uneven cells, or debris and they are described below.
3.1 Apoptotic cell death
Apoptosis involves redistribution of membrane phospholipids within the lipid bilayer, nuclear fragmentation, cytoplasmic shrinkage, and plasma membrane protuberans known as blebs [63][64]. One of the early events of the apoptotic process, before the loss of cell membrane integrity, is the phospholipid phosphatidylserine translocation to the outer leaflet of the membrane bilayer [65]. Phospholipid phosphatidylserine externalization can be easily detected using annexin V, a phosphatidylserine-binding protein. This apoptosis stage is strongly associated with chromatin condensation events on the inner nuclear membrane [66], a process that can be detected by labelling DNA with specific fluorochromes such as propidium iodide (PI) and 4′,6-diamidino-2-phenylindole (DAPI). Another important feature of late phase apoptosis is the DNA fragmentation that can be detected by the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay [67]. Correlation of cellular fragmentation with apoptosis in fragmented or normally developing human cleavage stage embryos represents a controversial finding. Yang and colleagues reported a TUNEL signal in most fragmented cleavage stage embryos (from the two-cell to eight-cell stage) but not in non-fragmented ones [68]. Antczak and Van Blerkom reported no TUNEL signal or annexin V fluorescence in both fragments and in intact blastomeres of living fragmented embryos between 2- and 8-cell stages [69]. Levy and colleagues reported increased annexin V staining and TUNEL assay labelling in arrested and fragmented day 2 embryos but no annexin V staining in cleavage stage embryos normally developing after thawing [70]. Jurisicova and colleagues also proposed cellular fragmentation as a consequence of embryo programmed cell death of blastomeres in human cleavage embryos arrested at different stage of development. Several cellular fragments containing organelles and condensed chromatin within the ZP, associated with apoptosis markers (TUNEL staining) as well as caspase-2 and caspase-3 mRNA expression, were observed in these embryos [71][72]. Studies investigating apoptosis pathways reported that some markers such as BAX and BCL (mRNA and proteins) were even expressed from unfertilized oocyte, while others such as PDCD5, BAD (mRNA), caspases, and Harakiri were expressed mainly at the blastocyst stage [71]. Martinez and colleagues frequently observed positivity for caspase activity in fragments but rarely in normal blastomeres of arrested embryos. No differences were detected in the proportion of caspase-positive cellular fragments between 2-cell and 12-cell stage embryos, thus before and after embryonic gene activation [73]. In 2001, with an integrated approach between retrospective data and mathematical modeling, apoptosis episodes were demonstrated from morula to blastocyst stage in viable embryos of good morphology [74]. Hardy and colleagues assessed morphological and biochemical markers of apoptosis in fixed zona-free embryos at different developmental stages until blastocyst stage by using confocal microscopy. Nuclear morphology was evaluated after treatment of samples with DAPI, and fragmented DNA detection was evaluated by TUNEL. The levels of TUNEL-labelled cells substantially increased at blastocyst stage, while apoptosis markers were absent in cleavage stage embryos. The appearance of apoptotic markers has been associated with important steps of pre-implantation embryogenesis: the activation of the embryonic genome, the development of gap junctions, and the maturation of mitochondria. Cell–cell communication via gap-junctions, in particular, rarely present in cleavage stage embryos, was supported as a molecular requirement for apoptotic signal propagation [75][76]. In line with the idea that selection/correction of aneuploidies are one of the mechanisms for fragmentation of the embryos, Santos and colleagues observed aneuploid blastomeres leaving the blastocyst following the activation of apoptotic pathways [77][78]. Recently, also, the blastocoel fluid was analyzed for the presence of apoptosis markers [43][79]. Caspase-3 protease activity has been detected in this compartment, supporting the idea that a fraction of molecules in blastocoel fluid are products of apoptotic embryonic cells [80]. In general, however, several aspects still need to be elucidated, i.e., factors affecting blastomere apoptosis and the entity of the phenomenon in the human pre-implantation embryo at different stages.
3.2 Reactive oxygen species effect
Generated during the physiological consumption of oxygen, reactive oxygen species (ROS) can be the product of the embryo metabolism, but they may also originate from embryo surroundings [81]. High levels of ROS along with an imbalanced formation of antioxidants is thought to result in oxidative stress, resulting in suboptimal embryos competence [82][83]. Indeed, differently from what occurs in vivo, in which the presence of antioxidants or antioxidative enzymes in the fluid and epithelium of oviduct protects the embryo from ROS, in an in vitro culture system, levels of these compound have been demonstrated to inversely correlate to embryo developmental competence [81][84][85]. Interestingly, several studies have reported a positive correlation between ROS levels in the spent medium and the fragmentation rate in human embryos at cleavage and blastocyst stage [84][86]. Thanks to the use of imaging techniques, such as TEM and other fluorescence assays, ROS have been detected at a higher concentration in embryos with a higher rate of cellular fragmentation [68]. While a certain amount of ROS may benefit the embryo development, as mitochondrial oxidative phosphorylation is an efficient way to produce ATP but at a cost of ROS generation, elevated ROS levels have harmful effects, including DNA damage and alteration of most types of cellular molecules [86]. Nevertheless, a recent study reported a lack of association between ROS levels in media of cultured individually embryos (as evaluated by a chemiluminescence assay using luminol) and embryonic development or high embryo fragmentation [87].
3.3. Membrane compartmentalization of DNA
As mentioned above, micronuclei have been detected in cleavage stage human embryos as a whole chromosome or a fragment of a chromosome that is not incorporated into one of the daughter nuclei during cell division. There is no evidence of a preferential association of aneuploidy with a subtype of chromosomes: both large and small chromosomes can be sequestered [88]. In addition, mis-segregated chromosomes and chromatid fragments encapsulated within micronuclei are dynamic entities: they may persist, rejoin the primary nucleus, or might be definitely eliminated from the embryo, in line with the theory of embryo “self-correction”. Moreover, chromosomes can undergo a specific phenomenon of “chromosome pulverization”, known as chromothripsis, that allows the reduction of one or a few chromosomal fragments into many pieces, randomly reassembled in one unique cellular event during a single-cell division [59]. Not all the fragments are characterized by the presence of sequestered micronuclei; thus, the rearrangement of fragments does not always result in the alteration of the ploidy [5][19][89].
3.4. Abnormal cytokinesis and cytoskeletal disorder
It is well documented that embryos with abnormal duration of cell cycles and cytokinesis (generally, with a delayed first mitosis, an earlier start of the second mitosis, and a longer duration of the third mitosis) are more likely to be fragmented [13]. An incorrect cell cycle may result in genomic alterations because the cell does not have enough time to correct any eventual error during DNA replication. Alikani and colleagues reported that loss of interplay between the spindle complex and cortical microfilaments was associated with blebs and cellular fragments formation. In addition, the authors demonstrated that treatment with cytokinesis inhibitors prevented cytokinesis as well as fragment formation, supporting a cause–effect relationship [13]. Stensen and colleagues also linked the rate of embryo fragmentation with the duration of meiotic process. A delay in the oocyte meiotic division (formation of the meiotic spindle 36.2 h after human chorionic gonadotropin injection) was associated with higher fragmentation rates [50–100%] in resultant embryos [90]. The reason underlying this observation may be related to cell cycle defects implicated in oocyte aneuploidy involving alterations in chromosome pairing, recombination, and spindle assembly, resulting in a delayed meiotic cell cycle [91][92][93]. No correlation, instead, was found between fragmentation and other spindle characteristics (i.e., a delay in its formation and the angle calculated between the first polar body and the meiotic spindle). Lastly, according to the same group, the process of fragmentation was more pronounced during the early phases of cell division, when the maternal genome is still active. After the activation of the embryonic genome, the tendency of human blastomeres to fragment would be lost. Extruded blastomeres from these embryos would express maternal instead of embryonic transcripts, during an inappropriate timing for the developmental stage [90].
3.5. Extracellular vesicles formation
Human embryos can secrete EVs in their culture media that can be easily taken up by endometrial cells [15]. They are formed through multiple biogenetic pathways: (i) Exosomes are generated from the endosomal system by the formation of late endosomes, which are formed by inward budding of the multivesicular body (MVB) membrane. Invagination of late endosomal membranes results in the formation of intraluminal vesicles (ILVs) within large MVBs [94]. In the next step, MVBs have two fates: most ILVs are released into the extracellular space upon fusion with the plasma membrane or, alternatively, these components are trafficked to lysosomes for degradation [95][96]. (ii) Microvesicles, instead, are formed through the outward budding and fission from plasma membranes. In contrast to exosome formation, the secretion of microvesicles requires the lipid microdomains at the membrane and a reorganization of the actin–myosin cytoskeletal network [97][98]. A possible association between the EV quantity in the spent culture media and embryo quality and competence has been suggested [99][100][101]. Specifically, fewer EVs have been reported in spent culture media of embryos leading to successful pregnancy than in those who failed, suggesting that a good quality and competent embryo releases different amounts/types of EVs compared to a low-quality embryo [102][103][104][105].
References
- Trounson, A.; Sathananthan, A.H. The application of electron microscopy in the evaluation of two—to four-cell human embryos cultured in vitro for embryo transfer. J. Vitr. Fertil. Embryo Transf. 1984, 1, 153–165.
- Pereda, J.; Croxatto, H.B. Ultrastructure of a Seven-Cell Human Embryo. Biol. Reprod. 1978, 18, 481–489.
- Van Blerkom, J.; Davis, P.; Alexander, S. A microscopic and biochemical study of fragmentation phenotypes in stage-appropriate human embryos. Hum. Reprod. 2001, 16, 719–729.
- Kim, S.-G.; Kim, Y.-Y.; Park, J.-Y.; Kwak, S.-J.; Yoo, C.-S.; Park, I.-H.; Sun, H.-G.; Kim, J.-W.; Lee, K.-H.; Park, H.-D.; et al. Early fragment removal on in vitro fertilization day 2 significantly improves the subsequent development and clinical outcomes of fragmented human embryos. Clin. Exp. Reprod. Med. 2018, 45, 122–128.
- Hardarson, T.; Löfman, C.; Coull, G.; Sjögren, A.; Hamberger, L.; Edwards, R. Internalization of cellular fragments in a human embryo: Time-lapse recordings. Reprod. Biomed. Online 2002, 5, 36–38.
- Johansson, M.; Hardarson, T.; Lundin, K. There Is a Cutoff Limit in Diameter Between a Blastomere and a Small Anucleate Fragment. J. Assist. Reprod. Genet. 2003, 20, 309–313.
- Barile, L.; Vassalli, G. Exosomes: Therapy delivery tools and biomarkers of diseases. Pharmacol. Ther. 2017, 174, 63–78.
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750.
- Giacomini, E.; Vago, R.; Sanchez, A.M.; Podini, P.; Zarovni, N.; Murdica, V.; Rizzo, R.; Bortolotti, D.; Candiani, M.; Viganã, P. Secretome of in vitro cultured human embryos contains extracellular vesicles that are uptaken by the maternal side. Sci. Rep. 2017, 7, 5210.
- Borges, F.T.; Reis, L.A.; Schor, N. Extracellular vesicles: Structure, function, and potential clinical uses in renal diseases. Braz. J. Med. Biol. Res. 2013, 46, 824–830.
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727.
- Yáñez-Mó, M.; Siljander, P.R.-M.; Andreu, Z.; Zavec, A.B.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066.
- Alikani, M. Epithelial cadherin distribution in abnormal human pre-implantation embryos. Hum. Reprod. 2005, 20, 3369–3375.
- Lagalla, C.; Coticchio, G.; Sciajno, R.; Tarozzi, N.; Zacà, C.; Borini, A. Alternative patterns of partial embryo compaction: Prevalence, morphokinetic history and possible implications. Reprod. Biomed. Online 2020, 40, 347–354.
- Giacomini, E.; Alleva, E.; Fornelli, G.; Quartucci, A.; Privitera, L.; Vanni, V.S.; Viganò, P. Embryonic extracellular vesicles as informers to the immune cells at the maternal–fetal interface. Clin. Exp. Immunol. 2019, 198, 15–23.
- Vyas, P.; Balakier, H.; Librach, C. Ultrastructural identification of CD9 positive extracellular vesicles released from human embryos and transported through the zona pellucida. Syst. Biol. Reprod. Med. 2019, 65, 273–280.
- Chi, H.-J.; Koo, J.-J.; Choi, S.-Y.; Jeong, H.-J.; Roh, S.-I. Fragmentation of embryos is associated with both necrosis and apoptosis. Fertil. Steril. 2011, 96, 187–192.
- Halvaei, I.; Khalili, M.A.; Esfandiari, N.; Safari, S.; Talebi, A.R.; Miglietta, S.; Nottola, S.A. Ultrastructure of cytoplasmic fragments in human cleavage stage embryos. J. Assist. Reprod. Genet. 2016, 33, 1677–1684.
- Chavez, S.; Loewke, K.E.; Han, J.; Moussavi, F.; Colls, P.; Munne, S.; Behr, B.; Pera, R.A.R. Dynamic blastomere behaviour reflects human embryo ploidy by the four-cell stage. Nat. Commun. 2012, 3, 1251.
- Daughtry, B.L.; Chavez, S.L. Time-Lapse Imaging for the Detection of Chromosomal Abnormalities in Primate Preimplantation Embryos. Methods Mol. Biol. 2018, 1769, 293–317.
- Calarco, P.G.; Pedersen, R.A. Ultrastructural observations of lethal yellow (Ay/Ay) mouse embryos. J. Embryol. Exp. Morphol. 1976, 35, 73–80.
- Lindner, G.M.; Wright, R.W. Bovine embryo morphology and evaluation. Theriogenology 1983, 20, 407–416.
- Alikani, M.; Cohen, J.; Tomkin, G.; Garrisi, G.J.; Mack, C.; Scott, R.T. Human embryo fragmentation in vitro and its implications for pregnancy and im-plantation. Fertil. Steril. 1999, 71, 836–842.
- Alikani, M.; Willadsen, S.M. Human blastocysts from aggregated mononucleated cells of two or more non-viable zygote-derived embryos. Reprod. Biomed. Online 2002, 5, 56–58.
- Hardy, K. Apoptosis in the human embryo. Rev. Reprod. 1999, 4, 125–134.
- Van Soom, A.; Mateusen, B.; Leroy, J.; de Kruif, A. Assessment of mammalian embryo quality: What can we learn from embryo morphology? Reprod. Biomed. Online 2003, 7, 664–670.
- Prados, F.J.; Debrock, S.; Lemmen, J.G.; Agerholm, I. The cleavage stage embryo. Hum. Reprod. 2012, 27, i50–i71.
- Chavez, S.L.; McElroy, S.L.; Bossert, N.L.; De Jonge, C.J.; Rodriguez, M.V.; Leong, D.E.; Behr, B.; Westphal, L.M.; Pera, R.A.R. Comparison of epigenetic mediator expression and function in mouse and human embryonic blastomeres. Hum. Mol. Genet. 2014, 23, 4970–4984.
- Watson, A.J. The cell biology of blastocyst development. Mol. Reprod. Dev. 1992, 33, 492–504.
- Eftekhari-Yazdi, P.; Valojerdi, M.R.; Ashtiani, S.K.; Eslaminejad, M.B.; Karimian, L. Effect of fragment removal on blastocyst formation and quality of human embryos. Reprod. Biomed. Online 2006, 13, 823–832.
- Keltz, M.D.; Skorupski, J.C.; Bradley, K.; Stein, D. Predictors of embryo fragmentation and outcome after fragment removal in in vitro fertilization. Fertil. Steril. 2006, 86, 321–324.
- Luzhna, L.; Kathiria, P.; Kovalchuk, O. Micronuclei in genotoxicity assessment: From genetics to epigenetics and beyond. Front. Genet. 2013, 4, 131.
- Sedelnikova, O.A.; Nakamura, A.; Kovalchuk, O.; Koturbash, I.; Mitchell, S.A.; Marino, S.A.; Brenner, D.J.; Bonner, W.M. DNA Double-Strand Breaks Form in Bystander Cells after Microbeam Irradiation of Three-dimensional Human Tissue Models. Cancer Res. 2007, 67, 4295–4302.
- Fenech, M.; Kirsch-Volders, M.; Natarajan, A.T.; Surralles, J.; Crott, J.W.; Parry, J.; Norppa, H.; Eastmond, D.A.; Tucker, J.D.; Thomas, P. Molecular mechanisms of micronucleus, nucleoplasmic bridge and nuclear bud formation in mammalian and human cells. Mutagenesis 2010, 26, 125–132.
- Mohr, L.R.; Trounson, A.O. Comparative ultrastructure of hatched human, mouse and bovine blastocysts. J. Reprod. Fertil. 1982, 66, 499–504.
- Hurst, P.R.; Jefferies, K.; Eckstein, P.; Wheeler, A.G. An ultrastructural study of preimplantation uterine embryos of the rhesus monkey. J. Anat. 1978, 126, 209–220.
- Blander, J.M. The many ways tissue phagocytes respond to dying cells. Immunol. Rev. 2017, 277, 158–173.
- Li, Y.; Xu, J.; Zhou, C.-Q.; Zhang, C.-L.; Zhuang, G.-L. Nonprofessional phagocytosis in trophectoderm cells of human preimplantation blastocysts. Syst. Biol. Reprod. Med. 2016, 62, 243–248.
- Pisko, J.; Špirková, A.; Čikoš, Š.; Olexiková, L.; Kovaříková, V.; Šefčíková, Z.; Fabian, D. Apoptotic cells in mouse blastocysts are eliminated by neighbouring blastomeres. Sci. Rep. 2021, 11, 9228.
- Longo, F.J. Fertilization; Chapman & Hall: New York, NY, USA, 1997.
- Ottolini, C.S.; Rogers, S.; Sage, K.; Summers, M.C.; Capalbo, A.; Griffin, D.K.; Sarasa, J.; Wells, D.; Handyside, A.H. Karyomapping identifies second polar body DNA persisting to the blastocyst stage: Implications for embryo biopsy. Reprod. Biomed. Online 2015, 31, 776–782.
- Battaglia, R.; Palini, S.; Vento, M.E.; La Ferlita, A.; Faro, M.J.L.; Caroppo, E.; Borzì, P.; Falzone, L.; Barbagallo, D.; Ragusa, M.; et al. Identification of extracellular vesicles and characterization of miRNA expression profiles in human blastocoel fluid. Sci. Rep. 2019, 9, 84.
- DesRochers, L.M.; Bordeleau, F.; Reinhart-King, C.A.; Cerione, R.A.; Antonyak, M.A. Microvesicles provide a mechanism for intercellular communication by embryonic stem cells during embryo implantation. Nat. Commun. 2016, 7, 11958.
- Wilding, M.; Dale, B.; Marino, M.; Di Matteo, L.; Alviggi, C.; Pisaturo, M.L.; Lombardi, L.; De Placido, G. Mitochondrial aggregation patterns and activity in human oocytes and preimplantation embryos. Hum. Reprod. 2001, 16, 909–917.
- Van Blerkom, J.; Davis, P.W.; Lee, J. ATP content of human oocytes and developmental potential and outcome after in-vitro fertilization and embryo transfer. Hum. Reprod. 1995, 10, 415–424.
- Gilula, N.B.; Epstein, M.L.; Beers, W.H. Cell-to-cell communication and ovulation. A study of the cumulus-oocyte complex. J. Cell Biol. 1978, 78, 58–75.
- Li, R.; Albertini, D.F. The road to maturation: Somatic cell interaction and self-organization of the mammalian oocyte. Nat. Rev. Mol. Cell Biol. 2013, 14, 141–152.
- Derrick, R.; Hickman, C.; Oliana, O.; Wilkinson, T.; Gwinnett, D.; Whyte, L.B.; Carby, A.; Lavery, S. Perivitelline threads associated with fragments in human cleavage stage embryos observed through time-lapse microscopy. Reprod. Biomed. Online 2017, 35, 640–645.
- Fujimoto, V.Y.; Browne, R.W.; Bloom, M.S.; Sakkas, D.; Alikani, M. Pathogenesis, developmental consequences, and clinical correlations of human embryo fragmentation. Fertil. Steril. 2011, 95, 1197–1204.
- Munné, S.; Grifo, J.; Cohen, J.; Weier, H.U. Chromosome abnormalities in human arrested preimplantation embryos: A multi-ple-probe FISH study. Am. J. Hum. Genet. 1994, 55, 150–159.
- Vanneste, E.; Voet, T.; Le Caignec, C.; Ampe, M.; Konings, P.; Melotte, C.; Debrock, S.; Amyere, M.; Vikkula, M.; Schuit, F.; et al. Chromosome instability is common in human cleavage-stage embryos. Nat. Med. 2009, 15, 577–583.
- Johnson, D.S.; Cinnioglu, C.; Ross, R.; Filby, A.; Gemelos, G.; Hill, M.; Ryan, A.; Smotrich, D.; Rabinowitz, M.; Murray, M.J. Comprehensive analysis of karyotypic mosaicism between trophectoderm and inner cell mass. Mol. Hum. Reprod. 2010, 16, 944–949.
- Chow, J.F.; Yeung, W.S.; Lau, E.Y.; Lee, V.C.; Ng, E.H.; Ho, P.-C. Array comparative genomic hybridization analyses of all blastomeres of a cohort of embryos from young IVF patients revealed significant contribution of mitotic errors to embryo mosaicism at the cleavage stage. Reprod. Biol. Endocrinol. 2014, 12, 105.
- Huang, J.; Yan, L.; Fan, W.; Zhao, N.; Zhang, Y.; Tang, F.; Xie, X.S.; Qiao, J. Validation of multiple annealing and looping-based amplification cycle sequencing for 24-chromosome aneuploidy screening of cleavage-stage embryos. Fertil. Steril. 2014, 102, 1685–1691.
- Kuo, H.-C.; Ogilvie, C.M.; Handyside, A.H. Chromosomal Mosaicism in Cleavage-Stage Human Embryos and the Accuracy of Single-Cell Genetic Analysis. J. Assist. Reprod. Genet. 1998, 15, 276–280.
- Baart, E.; Martini, E.; Berg, I.V.D.; Macklon, N.; Galjaard, R.-J.; Fauser, B.; Van Opsta, D. Preimplantation genetic screening reveals a high incidence of aneuploidy and mosaicism in embryos from young women undergoing IVF. Hum. Reprod. 2005, 21, 223–233.
- Munné, S.; Velilla, E.; Colls, P.; Bermúdez, M.G.; Vemuri, M.C.; Steuerwald, N.; Garrisi, J.; Cohen, J. Self-correction of chromosomally abnormal embryos in culture and implications for stem cell production. Fertil. Steril. 2005, 84, 1328–1334.
- Barbash-Hazan, S.; Frumkin, T.; Malcov, M.; Yaron, Y.; Cohen, T.; Azem, F.; Amit, A.; Ben-Yosef, D. Preimplantation aneuploid embryos undergo self-correction in correlation with their developmental potential. Fertil. Steril. 2009, 92, 890–896.
- Pellestor, F. Chromothripsis: How does such a catastrophic event impact human reproduction? Hum. Reprod. 2014, 29, 388–393.
- Pellestor, F.; Girardet, A.; Andréo, B.; Arnal, F.; Humeau, C. Relationship between morphology and chromosomal constitution in human preimplantation embryo. Mol. Reprod. Dev. 1994, 39, 141–146.
- Munné, S.; Alikani, M.; Tomkin, G.; Grifo, J.; Cohen, J. Embryo morphology, developmental rates, and maternal age are correlated with chromosome abnormalities. Fertil. Steril. 1995, 64, 382–391.
- Munne, S. Chromosome abnormalities in human embryos. Hum. Reprod. Updat. 1998, 4, 842–855.
- Cohen, J. Apoptosis: Mechanisms of life and death in the immune system. J. Allergy Clin. Immunol. 1999, 103, 548–554.
- Maderna, P.; Godson, C. Phagocytosis of apoptotic cells and the resolution of inflammation. Biochim Biophys Acta. 2003, 1639, 141–151.
- Martin, S.; Reutelingsperger, C.E.M.; McGahon, A.J.; Rader, J.; Van Schie, R.C.A.A.; LaFace, D.M.; Green, D. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: Inhibition by overexpression of Bcl-2 and Abl. J. Exp. Med. 1995, 182, 1545–1556.
- Koopman, G.; Reutelingsperger, C.P.; Kuijten, G.A.; Keehnen, R.M.; Pals, S.T.; van Oers, M.H. Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood 1994, 84, 1415–1420.
- Gavrieli, Y.; Sherman, Y.; Ben-Sasson, S.A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J. Cell Biol. 1992, 119, 493–501.
- Yang, H.W.; Hwang, K.J.; Kwon, H.C.; Kim, H.S.; Choi, K.W.; Oh, K.S. Detection of reactive oxygen species (ROS) and apoptosis in human fragmented embryos. Hum. Reprod. 1998, 13, 998–1002.
- Antczak, M.; Van Blerkom, J. Temporal and spatial aspects of fragmentation in early human embryos: Possible effects on de-velopmental competence and association with the differential elimination of regulatory proteins from polarized domains. Hum. Reprod. 1999, 14, 429–447.
- Levy, R.; Benchaib, M.; Cordonier, H.; Souchier, C.; Guerin, J.F. Annexin V labelling and terminal transferase-mediated DNA end labelling (TUNEL) assay in human arrested embryos. Mol. Hum. Reprod. 1998, 4, 775–783.
- Jurisicova, A.; Varmuza, S.; Casper, R. Programmed cell death and human embryo fragmentation. Mol. Hum. Reprod. 1996, 2, 93–98.
- Jurisicova, A.; Antenos, M.; Varmuza, S.; Tilly, J.L.; Casper, R.F. Expression of apoptosis-related genes during human preimplantation embryo development: Potential roles for the Harakiri gene product and Caspase-3 in blastomere fragmentation. Mol. Hum. Reprod. 2003, 9, 133–141.
- Martinez, F.; Rienzi, L.; Iacobelli, M.; Ubaldi, F.; Mendoza, C.; Greco, E.; Tesarik, J. Caspase activity in preimplantation human embryos is not associated with apoptosis. Hum. Reprod. 2002, 17, 1584–1590.
- Hardy, K.; Spanos, S.; Becker, D.; Iannelli, P.; Winston, R.M.L.; Stark, J. From cell death to embryo arrest: Mathematical models of human preimplantation embryo development. Proc. Natl. Acad. Sci. USA 2001, 98, 1655–1660.
- Dale, B.; Gualtieri, R.; Talevi, R.; Tosti, E.; Santella, L.; Elder, K. Intercellular communication in the early human embryo. Mol. Reprod. Dev. 1991, 29, 22–28.
- Hardy, K.; Warner, A.; Winston, R.M.; Becker, D.L. Expression of intercellular junctions during preimplantation development of the human embryo. Mol. Hum. Reprod. 1996, 2, 621–632.
- Mantikou, E.; Wong, K.M.; Repping, S.; Mastenbroek, S. Molecular origin of mitotic aneuploidies in preimplantation embryos. Biochim. Biophys. Acta 2012, 1822, 1921–1930.
- Santos, M.A.; Teklenburg, G.; Macklon, N.S.; Van Opstal, D.; Schuring-Blom, G.H.; Krijtenburg, P.-J.; de Vreeden-Elbertse, J.; Fauser, B.C.; Baart, E.B. The fate of the mosaic embryo: Chromosomal constitution and development of Day 4, 5 and 8 human embryos. Hum. Reprod. 2010, 25, 1916–1926.
- Athavale, D.M.; Barré, A.; Kranyak, A.C.; Lal, A.; Blalock, J.L.; Zimmerman, S.; Chang, T.A.; Robinson, R.D.; Wininger, J.D.; Roudebush, W.E.; et al. Pro-apoptotic gene expression in blastocoel fluid from euploid day-5 embryos is associated with negative pregnancy outcomes. Fertil. Steril. 2019, 112, e261.
- Rule, K.; Chosed, R.; Chang, T.A.; Wininger, J.D.; Roudebush, W.E. Relationship between blastocoel cell-free DNA and day-5 blastocyst morphology. J. Assist. Reprod. Genet. 2018, 35, 1497–1501.
- Goto, Y.; Noda, Y.; Mori, T.; Nakano, M. Increased generation of reactive oxygen species in embryos cultured in vitro. Free. Radic. Biol. Med. 1993, 15, 69–75.
- Agarwal, A.; Saleh, R.; Bedaiwy, M. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil. Steril. 2003, 79, 829–843.
- Guérin, P.; El Mouatassim, S.; Ménézo, Y. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Hum. Reprod. Update 2001, 7, 175–189.
- Bedaiwy, M.A.; Falcone, T.; Mohamed, M.S.; Aleem, A.A.N.; Sharma, R.K.; Worley, S.E.; Thornton, J.; Agarwal, A. Differential growth of human embryos in vitro: Role of reactive oxygen species. Fertil. Steril. 2004, 82, 593–600.
- Bedaiwy, M.A.; Mahfouz, R.Z.; Goldberg, J.M.; Sharma, R.; Falcone, T.; Hafez, M.F.A.; Agarwal, A. Relationship of reactive oxygen species levels in day 3 culture media to the outcome of in vitro fertilization/intracytoplasmic sperm injection cycles. Fertil. Steril. 2010, 94, 2037–2042.
- Lee, T.-H.; Lee, M.-S.; Liu, C.-H.; Tsao, H.-M.; Huang, C.-C.; Yang, Y.-S. The Association Between Microenvironmental Reactive Oxygen Species and Embryo Development in Assisted Reproduction Technology Cycles. Reprod. Sci. 2012, 19, 725–732.
- Lan, K.-C.; Lin, Y.-C.; Chang, Y.-C.; Lin, H.-J.; Tsai, Y.-R.; Kang, H.-Y. Limited relationships between reactive oxygen species levels in culture media and zygote and embryo development. J. Assist. Reprod. Genet. 2019, 36, 325–334.
- Daughtry, B.L.; Rosenkrantz, J.L.; Lazar, N.H.; Fei, S.S.; Redmayne, N.; Torkenczy, K.A.; Adey, A.; Yan, M.; Gao, L.; Park, B.; et al. Single-cell sequencing of primate preimplantation embryos reveals chromosome elimination via cellular fragmentation and blastomere exclusion. Genome Res. 2019, 29, 367–382.
- Lemmen, J.; Agerholm, I.; Ziebe, S. Kinetic markers of human embryo quality using time-lapse recordings of IVF/ICSI-fertilized oocytes. Reprod. Biomed. Online 2008, 17, 385–391.
- Stensen, M.H.; Tanbo, T.G.; Storeng, R.; Åbyholm, T.; Fedorcsak, P. Fragmentation of human cleavage-stage embryos is related to the progression through meiotic and mitotic cell cycles. Fertil. Steril. 2015, 103, 374–381.
- Hassold, T.; Hunt, P. To err (meiotically) is human: The genesis of human aneuploidy. Nat. Rev. Genet. 2001, 2, 280–291.
- Magli, M.C.; Capoti, A.; Resta, S.; Stanghellini, I.; Ferraretti, A.P.; Gianaroli, L. Prolonged absence of meiotic spindles by birefringence imaging negatively affects normal fertilization and embryo development. Reprod. Biomed. Online 2011, 23, 747–754.
- Magli, M.C.; Ferraretti, A.P.; Crippa, A.; Lappi, M.; Feliciani, E.; Gianaroli, L. First meiosis errors in immature oocytes generated by stimulated cycles. Fertil. Steril. 2006, 86, 629–635.
- Minciacchi, V.R.; Freeman, M.R.; Di Vizio, D. Extracellular Vesicles in Cancer: Exosomes, Microvesicles and the Emerging Role of Large Oncosomes. Semin. Cell Dev. Biol. 2015, 40, 41–51.
- Teng, F.; Fussenegger, M. Shedding Light on Extracellular Vesicle Biogenesis and Bioengineering. Adv. Sci. 2021, 8, 202003505.
- Trajkovic, K.; Hsu, C.; Chiantia, S.; Rajendran, L.; Wenzel, D.; Wieland, F.; Schwille, P.; Brügger, B.; Simons, M. Ceramide Triggers Budding of Exosome Vesicles into Multivesicular Endosomes. Science 2008, 319, 1244–1247.
- Murphy, D.E.; De Jong, O.G.; Brouwer, M.; Wood, M.J.; Lavieu, G.; Schiffelers, R.M.; Vader, P. Extracellular vesicle-based therapeutics: Natural versus engineered targeting and trafficking. Exp. Mol. Med. 2019, 51, 1–12.
- Muralidharan-Chari, V.; Clancy, J.; Plou, C.; Romao, M.; Chavrier, P.; Raposo, G.; D’Souza-Schorey, C. ARF6-Regulated Shedding of Tumor Cell-Derived Plasma Membrane Microvesicles. Curr. Biol. 2009, 19, 1875–1885.
- Giacomini, E.; Makieva, S.; Murdica, V.; Vago, R.; Viganó, P. Extracellular vesicles as a potential diagnostic tool in assisted reproduction. Curr. Opin. Obstet. Gynecol. 2020, 32, 179–184.
- Aleksejeva, E.; Zarovni, N.; Dissanayake, K.; Godakumara, K.; Vigano, P.; Fazeli, A.; Jaakma, Ü.; Salumets, A. Extracellular vesicle research in reproductive science—Paving the way for clinical achievements. Biol. Reprod. 2022.
- Giacomini, E.; Scotti, G.M.; Vanni, V.S.; Lazarevic, D.; Makieva, S.; Privitera, L.; Signorelli, S.; Cantone, L.; Bollati, V.; Murdica, V.; et al. Global transcriptomic changes occur in uterine fluid-derived extracellular vesicles during the endometrial window for embryo implantation. Hum. Reprod. 2021, 36, 2249–2274.
- Abu-Halima, M.; Häusler, S.; Backes, C.; Fehlmann, T.; Staib, C.; Nestel, S.; Nazarenko, I.; Meese, E.; Keller, A. Micro-ribonucleic acids and extracellular vesicles repertoire in the spent culture media is altered in women undergoing In Vitro Fertilization. Sci. Rep. 2017, 7, 13525.
- Kovács Árpád, F.; Fekete, N.; Turiák, L.; Ács, A.; Kőhidai, L.; Buzás, E.I.; Pállinger, É. Unravelling the Role of Trophoblastic-Derived Extracellular Vesicles in Regulatory T Cell Differentiation. Int. J. Mol. Sci. 2019, 20, 3457.
- Dissanayake, K.; Nõmm, M.; Lättekivi, F.; Ressaissi, Y.; Godakumara, K.; Lavrits, A.; Midekessa, G.; Viil, J.; Bæk, R.; Jørgensen, M.M.; et al. Individually cultured bovine embryos produce extracellular vesicles that have the potential to be used as non-invasive embryo quality markers. Theriogenology 2020, 149, 104–116.
- Mellisho, E.; Briones, M.A.; Velasquez, A.E.; Cabezas, J.; Castro, F.O.; Rodriguez-Alvarez, L. Extracellular vesicles secreted during blastulation show viability of bovine embryos. Reproduction 2019, 158, 477–492.
More
Information
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Revisions:
2 times
(View History)
Update Date:
18 Feb 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





