Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mahdieh Molanouri Shamsi | + 1276 word(s) | 1276 | 2022-02-17 03:52:08 | | | |
| 2 | Jason Zhu | + 27 word(s) | 1303 | 2022-02-18 02:57:29 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Molanouri Shamsi, M. Exercise Strategies of Athletes during COVID-19. Encyclopedia. Available online: https://encyclopedia.pub/entry/19589 (accessed on 08 February 2026).
Molanouri Shamsi M. Exercise Strategies of Athletes during COVID-19. Encyclopedia. Available at: https://encyclopedia.pub/entry/19589. Accessed February 08, 2026.
Molanouri Shamsi, Mahdieh. "Exercise Strategies of Athletes during COVID-19" Encyclopedia, https://encyclopedia.pub/entry/19589 (accessed February 08, 2026).
Molanouri Shamsi, M. (2022, February 17). Exercise Strategies of Athletes during COVID-19. In Encyclopedia. https://encyclopedia.pub/entry/19589
Molanouri Shamsi, Mahdieh. "Exercise Strategies of Athletes during COVID-19." Encyclopedia. Web. 17 February, 2022.
Copy Citation
Elite athletes use high-intensity training to maintain their fitness level. However, intense training can harm the immune system, making athletes suspectable to COVID-19 and negatively affecting their performance. Although high-intensity exercise can suppress the immune system, elite athletes should not stop training in the time of infection but use low- and moderate-intensity training. Moderate-intensity exercise can improve immune function and maintain physical fitness. In addition, it is also better for athletes not to undertake high-intensity training at the time of vaccination, but instead perform moderate to low-intensity training.
Exercise
Athletes
Immune System
1. Exercise Intensity and Immune System
1.1. Low-Intensity Exercise and Athletes’ Immune Systems
There is very little research on low-intensity training and athletes’ immune systems. Low-intensity training (e.g., below the first ventilatory threshold, at stable lactate concentrations < 2 mM or with an intensity of less than 37–45% VO2max), is also referred to as long slow distance training or zone-1 training. Steensberg et al. [1] reported increased IL-6 and IL-10 after 3 h and 26 min of low-intensity exercise. Mee-Inta et al. [2] concluded in their study that low-intensity exercise can reduce inflammation. It has also been reported that low-intensity exercise (less than 60% VO2max) for less than 60 min can reduce inflammation and improve immune function [3]. Tenorio et al. [4] examined the effect of low- versus high-intensity exercise training on inflammation and endothelial dysfunction biomarkers in adolescents with obesity in a 6-month randomized exercise intervention study. Interestingly, they found that high and low exercise intensities can improve immune function (neutrophils, monocytes, tumor necrosis factor-alpha).
Generally, low-intensity exercise has been considered to be a good strategy for elite athletes’ recovery and reducing post-competition stress [5]. Taken together, these studies indicate that low-intensity exercise can create a positive adaptation in the immune system.
1.2. Moderate-Intensity Exercise and Athletes’ Immune Systems
Moderate-intensity exercise performed between first and second lactate or ventilatory threshold (i.e., zone-2) and causes accumulated lactate levels [6]. Performing exercise with an intensity of 45 to 65% VO2max is considered moderate-intensity exercise.
MacIntosh et al. [7] reported that regular moderate-intensity training can reduce inflammation, and increase IL-10 and T-cell function. Other studies showed that if moderate-intensity exercise is performed for more than 60 min, it can increase inflammation [8]; otherwise it decreases inflammation [5]. It has been reported that IL-10, an anti-inflammatory cytokine, increases in both intense and moderate-intensity exercise [9]. However, moderate-intensity exercise has been shown to reduce cytokine storms and increase white blood cells, lymphocytes, and T cells [10].
In another study, Fashi et al. [11] concluded that four weeks of aerobic exercise reduced inflammation in the lung. Shiri et al. [12] examined the effect of six weeks of endurance training on tumor tissue IL-10 levels in breast cancer-bearing mice, and reported a significant increase in IL-10 levels.
Taken together, these studies demonstrate that moderate-intensity exercise is the best strategy in preventing suppression of the immune system.
1.3. High-Intensity Exercise and Athletes’ Immune Systems
High-intensity exercise refers to an intensity higher than 70% of VO2max [13]. High-intensity or “zone-3” training (e.g., >4 mmol lactate/L blood, >90% maximal heart rate) involves intermittent intervals exercises (short, high-intensity sprints) [14]. Some markers of the immune function change within a few days after long-term intense endurance physical exercise. Neutrophils and NK cell functions, salivary immunoglobulins A (IgA), and some types of inflammatory macrophages are shown to undergo negative changes following this kind of exercise training [15]. In addition, based on the “open window” theory, 3 to 72 h after intense exercise an infectious agent may be able to invade the host body, thus increasing the risk of opportunistic infections [16] (Figure 1).
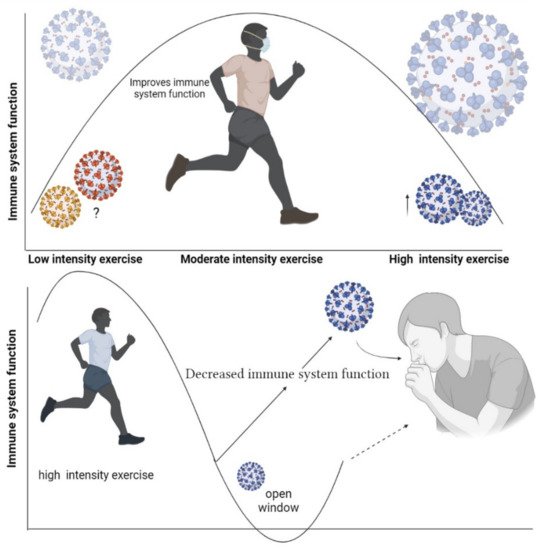
Figure 1. Open window theory after high-intensity training.
2. Respiratory Infections and Exercise
Evidence suggests that chronic exercise can increase upper respiratory tract infections in athletes [17]. The risk of athletes getting respiratory infections after intense exercise training is 7 times more than inactive individuals and 2 times more than active individuals [17]. Recurrent infections in athletes can be dangerous during a coronavirus pandemic [18].
Svenndsen et al. [19] reported that intense skiing increases the risk of infection by 3 times compared to recreational skiers. Nieman & Wentz [20] reported that intense physical activity lasting less than 60 min can suppress the immune system. In line with these results, other studies have reported that intense exercise lasting more than 60 to 90 min can suppress immune function [21][22]. It has also been found that elite athletes who perform intense exercise to prepare for professional competitions may be more susceptible to infectious diseases [23]. Thus, elite athletes are more prone to URTI during preparation for a professional competition. Being overtired may make this situation worse. Mackinnon et al. [24] reported that after 4 weeks of high-intensity training in swimmers, 33% of athletes showed symptoms of restlessness and 42% self-reported symptoms of URTI. During intense exercise, the activities of the lymphatic system are disrupted, which can negatively affect the immune system [25].
Allergic rhinitis is common among athletes due to regular high-intensity exercise [26]. To prepare for competitions, elite athletes undertake high-intensity training chronically trying to improve their physical fitness. It has been reported that, of 216 Olympic athletes, 56% had a history of conjunctivitis and rhinitis [26]. Allergic rhinitis impairs physical performance in professional athletes by affecting sleep, decreasing the ability to concentrate, or reducing physical fitness [26]. Continuous exposure to allergic rhinitis can increase the number of lymphocytes, eosinophils, neutrophils, basophils, and other leukocytes. This can cause the airways to overreact and eventually lead to fibrosis [27]. Allergens can also stimulate the airway epithelium to release IL-25, IL33, and thymic stromal lymphopoietin (TSLP). These cytokines can activate innate submucosal lymphocytes (ILC2) and release IL-4, IL-5, IL-9, and IL-13 [27], thereby causing airway wall remodeling, bronchial hyperresponsiveness, and goblet cell metaplasia [27]. These data, along with the open window theory, suggest that elite athletes are at high risk for COVID-19, highlighting the importance of vaccination and health care during infection.
3. Management during Athletes Infection
It has been found that viral infections can happen due to intense training in elite athletes, leading to a decrease in aerobic performance, especially among those affected by COVID-19 [28]. It has also been shown that athletes develop a fever during infection, and their muscle strength decreases [29]. The first step in managing athletes’ infections is to reduce exercise intensity and use nutritional strategies [30]. Infection management in athletes can be divided into two categories: (a) strategies for severe infections, and (b) strategies for athletes with minor symptoms. Athletes who develop viral infections due to intense exercise and have a severe physiological condition must have active rest (i.e., low-intensity exercise) [31]. Protein catabolism increases in this situation [32]; thus, low-intensity resistance training and protein supplementation are the best choice [33]. For the second group, aerobic and moderate-intensity training can be appropriate. It is recommended that athletes do not stop exercising when they develop URTI or COVID-19 as it makes the situation worse because the sudden cessation of exercise due to illness can further weaken the immune system.
4. How Can Athletes Get Vaccinated While They Continue Training?
In the previous sections, the importance of vaccination in elite athletes was highlighted. One concern with athlete vaccination is that the first dose may have short-term side effects. These side effects vary depending on the type of vaccine [34]. It is recommended that athletes reduce exercise intensity when receiving the first dose of the vaccine [35]. Athletes performing moderate- to low-intensity exercise training do not need to reduce exercise intensity [35]. It has been found that nearly 94% of athletes experience arm pain, general fatigue, and fever after receiving the vaccine for 2 days [36]. Therefore, it can be suggested that, during this period, the intensity of training should be reduced in athletes who undertake high-intensity training. After the symptoms disappear, the intensity can gradually increase intensity. High-intensity training can continue until a day before the second dose, when training intensity should be reduced again. Elite athletes have been shown to experience headaches, chills, fever, and muscle aches for 1 to 3 days after receiving the second dose [36][37]. Thus, the exercise intensity should kept low until the fourth day after receiving the second dose and then increase gradually [34][38].
References
- Steensberg, A.; Fischer, C.P.; Keller, C.; Møller, K.; Pedersen, B.K. IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E433–E437.
- Mee-Inta, O.; Zhao, Z.-W.; Kuo, Y.-M. Physical Exercise Inhibits Inflammation and Microglial Activation. Cells 2019, 8, 691.
- Rose, G.L.; Skinner, T.L.; Mielke, G.I.; Schaumberg, M.A. The effect of exercise intensity on chronic inflammation: A systematic review and meta-analysis. J. Sci. Med. Sport 2021, 24, 345–351.
- Tenório, T.R.S.; Balagopal, P.B.; Andersen, L.B.; Ritti-Dias, R.M.; Hill, J.O.; Lofrano-Prado, M.C.; Prado, W.L. Effect of Low- Versus High-Intensity Exercise Training on Biomarkers of Inflammation and Endothelial Dysfunction in Adolescents with Obesity: A 6-Month Randomized Exercise Intervention Study. Pediatr. Exerc. Sci. 2018, 30, 96–105.
- Stöggl, T.L.; Sperlich, B. Editorial: Training Intensity, Volume and Recovery Distribution Among Elite and Recreational Endurance Athletes. Front. Physiol. 2019, 10, 10.
- Goodwin, M.L.; Harris, J.E.; Hernández, A.; Gladden, L.B. Blood lactate measurements and analysis during exercise: A guide for clinicians. J. Diabetes Sci. Technol. 2007, 1, 558–569.
- MacIntosh, B.R.; Murias, J.M.; Keir, D.A.; Weir, J.M. What Is Moderate to Vigorous Exercise Intensity? Front. Physiol. 2021, 12, 682233.
- Cerqueira, É.; Marinho, D.A.; Neiva, H.P.; Lourenço, O. Inflammatory Effects of High and Moderate Intensity Exercise—A Systematic Review. Front. Physiol. 2019, 10, 1550.
- Suzuki, K.; Nakaji, S.; Yamada, M.; Totsuka, M.; Sato, K.; Sugawara, K. Systemic inflammatory response to exhaustive exercise. Cytokine kinetics. Exerc. Immunol. Rev. 2002, 8, 6–48.
- Suzuki, K.; Hayashida, H. Effect of exercise intensity on cell-mediated immunity. Sports 2021, 9, 8.
- Fashi, M.; Agha-Alinejad, H.; Asilian Mahabadi, H.; Rezaee Seraji, B.; Pak Rad, B. The Effect of Aerobic Exercise in Carbon Black Particulates Air Pollution on TLR4 and TNF-α Gene Expression in Lung Tissue of Male Rats. J. Sport Biosci. 2015, 7, 605–618.
- Shiri, Y.; Agha-Alinejad, H.; Gharakhanlou, R.; Amani Shalamzari, S.; Saei, M.A. Effect of six weeks endurance training on tumor tissue IL-10 cytokine levels in breast cancer bearing mice. Iran. J. Endocrinol. Metab. 2014, 16, 205–210.
- Laursen, P.B.; Jenkins, D.G. The scientific basis for high-intensity interval training. Sports Med. 2002, 32, 53–73.
- Esteve-Lanao, J.; Foster, C.; Seiler, S.; Lucia, A. Impact of training intensity distribution on performance in endurance athletes. J. Strength Cond. Res. 2007, 21, 943–949.
- Campbell, J.P.; Turner, J.E. Debunking the Myth of Exercise-Induced Immune Suppression: Redefining the Impact of Exercise on Immunological Health Across the Lifespan. Front. Immunol. 2018, 9, 648.
- Pedersen, B.K.; Bruunsgaard, H. How physical exercise influences the establishment of infections. Sports Med. 1995, 19, 393–400.
- Gałązka-Franta, A.; Jura-Szołtys, E.; Smółka, W.; Gawlik, R. Upper respiratory tract diseases in athletes in different sports disciplines. J. Hum. Kinet. 2016, 53, 99.
- Hull, J.H.; Loosemore, M.; Schwellnus, M. Respiratory health in athletes: Facing the COVID-19 challenge. Lancet Respir. Med. 2020, 8, 557–558.
- Svendsen, I.S.; Taylor, I.M.; Tønnessen, E.; Bahr, R.; Gleeson, M. Training-related and competition-related risk factors for respiratory tract and gastrointestinal infections in elite cross-country skiers. Br. J. Sports Med. 2016, 50, 809–815.
- Nieman, D.C. Exercise, upper respiratory tract infection, and the immune system. Med. Sci. Sports Exerc. 1994, 26, 128–139.
- Khoramipour, K.; Basereh, A.; Hekmatikar, A.A.; Castell, L.; Ruhee, R.T.; Suzuki, K. Physical activity and nutrition guidelines to help with the fight against COVID-19. J. Sports Sci. 2021, 39, 101–107.
- Hekmatikar, A.; Shalamzari, S.; Shamsi, M. Hygiene protocols during the coronavirus pandemic for athletes: Biref report. Tehran Univ. Med. J. 2021, 79, 314–318.
- Monks, L.; Seo, M.-W.; Kim, H.-B.; Jung, H.C.; Song, J.K. High-intensity interval training and athletic performance in taekwondo athletes. J. Sports Med. Phys. Fit. 2017, 57, 1252–1260.
- Mackinnon, L.T.; Hooper, S.L. Plasma glutamine and upper respiratory tract infection during intensified training in swimmers. Med. Sci. Sports. Exerc. 1996, 28, 285–290.
- Born, D.-P.; Zinner, C.; Sperlich, B. The Mucosal Immune Function Is Not Compromised during a Period of High-Intensity Interval Training. Is It Time to Reconsider an Old Assumption? Front. Physiol. 2017, 8, 485.
- Katelaris, C.H.; Carrozzi, F.M.; Burke, T.V.; Byth, K. A springtime Olympics demands special consideration for allergic athletes. J. Allergy Clin. Immunol. 2000, 106, 260–266.
- Kiboneka, A.; Kibuule, D. The Immunology of Asthma and Allergic Rhinitis. In Rhinosinusitis; IntechOpen: London, UK, 2019.
- Grimby, G. Exercise in man during pyrogen-induced fever. Scand. J. Clin. Lab. Investig. 1962, 14, 1–112.
- Friman, G.; Wesslén, L. Infections and exercise in high-performance athletes. Immunol. Cell Biol. 2000, 78, 510–522.
- Gleeson, M.; Nieman, D.C.; Pedersen, B.K. Exercise, nutrition and immune function. J. Sports Sci. 2004, 22, 115–125.
- Suzuki, M.; Umeda, T.; Nakaji, S.; Shimoyama, T.; Mashiko, T.; Sugawara, K. Effect of incorporating low intensity exercise into the recovery period after a rugby match. Br. J. Sports Med. 2004, 38, 436–440.
- Beisel, W.R.; Sawyer, W.D.; Ryll, E.D.; Crozier, D. Metabolic effects of intracellular infections in man. Ann. Intern. Med. 1967, 67, 744–779.
- Gade, J.; Beck, A.M.; Andersen, H.E.; Christensen, B.; Rønholt, F.; Klausen, T.W.; Vinther, A.; Astrup, A. Protein supplementation combined with low-intensity resistance training in geriatric medical patients during and after hospitalisation: A randomised, double-blind, multicentre trial. Br. J. Nutr. 2019, 122, 1006–1020.
- Hull, J.H.; Schwellnus, M.P.; Pyne, D.B.; Shah, A. COVID-19 vaccination in athletes: Ready, set, go…. Lancet Respir. Med. 2021, 9, 455–456.
- Gärtner, B.C.; Meyer, T. Vaccination in elite athletes. Sports Med. 2014, 44, 1361–1376.
- Hull, J.H.; Wootten, M.; Ranson, C. Tolerability and impact of SARS-CoV-2 vaccination in elite athletes. Lancet Respir. Med. 2022, 10, e5–e6.
- Hekmatikar, A.H.A.; Shamsi, M.M.; Ashkazari, Z.S.Z.; Suzuki, K. Exercise in an Overweight Patient with COVID-19: A Case Study. Int. J. Environ. Res. Public Health 2021, 18, 5882.
- Andreato, L.V.; Coimbra, D.R.; Andrade, A. Challenges to athletes during the home confinement caused by the COVID-19 pandemic. Strength Cond. J. 2020, 42, 1–5.
More
Information
Subjects:
Acoustics
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.7K
Revisions:
2 times
(View History)
Update Date:
18 Feb 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




