| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Qiongyu Hao | + 906 word(s) | 906 | 2022-02-17 08:41:33 |
Video Upload Options
Exosomes are a class of small membrane-bound extracellular vesicles released by almost all cell types and present in all body fluids. Based on the studies of exosome content and their interactions with recipient cells, exosomes are now thought to mediate “targeted” information transfer. Tumor-derived exosomes (TEX) carry a cargo of molecules different from that of normal cells-derived exosomes. TEX functions to mediate distinct biological effects such as receptor discharge and intercellular cross-talk. The immune system defenses, which may initially restrict tumor progression, are progressively blunted by the broad array of TEX molecules that activate suppressive pathways in different immune cells. Herein, we provide a review of the latest research progress on TEX in the context of tumor-mediated immune suppression, and discuss the potential as well as challenges of TEX as a target of immunotherapy.
Biogenesis of Exosomes
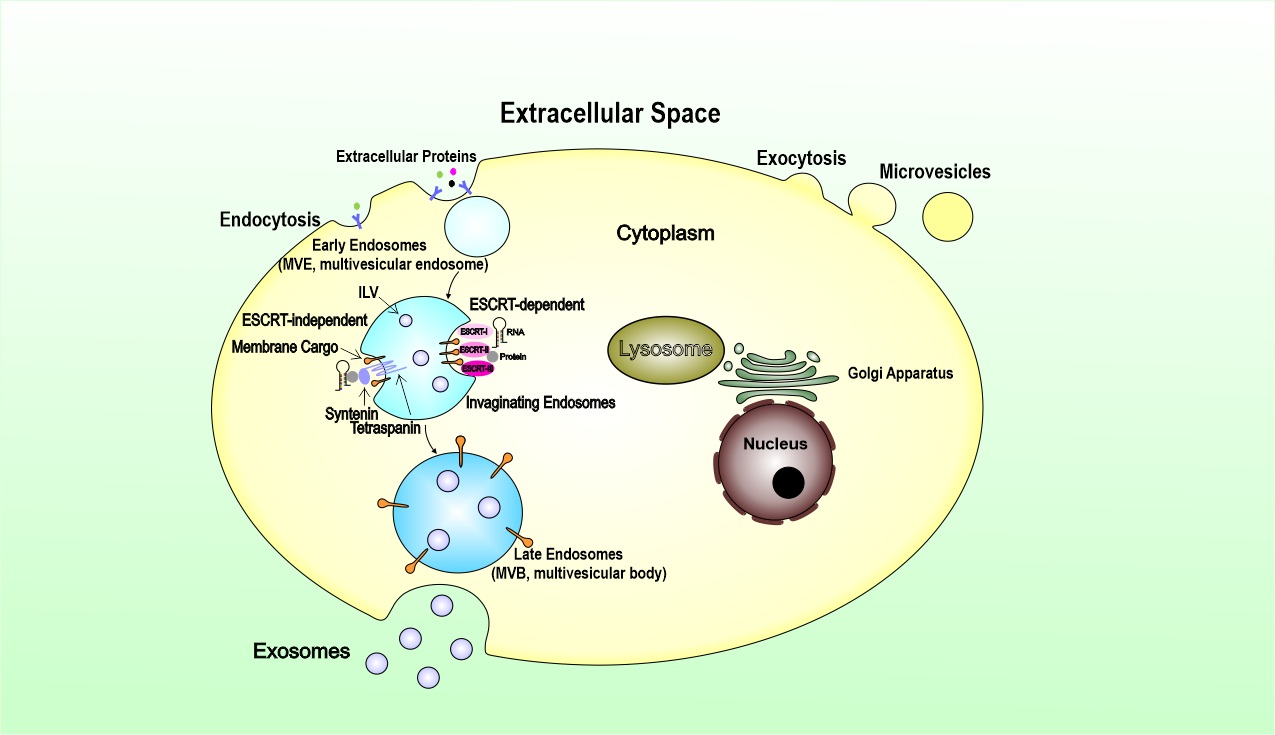
Although microvesicles and exosomes have different modes of biogenesis, both entities involve membrane trafficking processes. Briefly, microvesicles originate by an outward budding at the plasma membrane. In contrast, exosomes are generated within the endosomal system as intraluminal vesicles (ILVs) and secreted during the fusion of multivesicular endosomes (MVEs) with the cell surface. For microvesicles, cargoes are enriched in the forming vesicles by a stepwise mechanism of clustering and budding, then followed by fission and vesicle release for secretion within extracellular vesicles. The process of exosomes biogenesis begins with the invagination of the plasma membrane to form endosomes. Exosomes are generated as ILVs within the lumen of endosomes during their maturation into MVEs, a process that involves particular sorting machinery. Therefore, exosomes are derived from the endocytic pathway of donor cells.
The endosomal sorting complex required for transport (ESCRT) is the most well-established driver of early endosomes (ILVs), which maturate and differentiate into late endosomes within the multivesicular bodies (MVBs). The presence of ESCRT subunits in exosomes and their machinery in ILV biogenesis opens up a new way of perceiving and understanding the formation of exosomes through manipulation of the ESCRT components (Fig. 1). Exosomes can also be generated in an ESCRT independent manner, which was revealed by studies showing that MVEs, featuring ILVs loaded with CD63, were still formed upon depletion of the four ESCRT complexes. It has been suggested that lysosomes can regulate exosome biogenesis by altering the fate of MVBs. In summary, exosome biogenesis is undoubtedly complex. It seems that both ESCRT-dependent and ESCRT-independent mechanisms operate in exosome biogenesis, and their contributions may vary depending on the cargoes and cell type and can be influenced by other signals and pathological stimuli that the cell can receive. TEX acquires its cargo from the parent tumor cell via the complex process of biogenesis. ILVs formed in MVBs contain receptors/transmembrane proteins and signal molecules derived from the parent cell surface membrane and the cytosol. The sorting process of these parent cell components is cell-specific. TEX, carrying information from the parent tumor cell to recipient cells, is released into the extracellular space when MVBs enclosing pools of future exosomes fuse with the cell membrane. The cargo delivery leads to markedly biological effects, from the cellular transcriptome and proteome to cellular functions in recipient cells. The transmission of exosomes is tissue- and organ-specific in the body. Different integrins expressed on TEX are proved to dictate exosome adhesion to specific cell types and extracellular matrix molecules in particular organs. However, it remains unclear mainly what are the components in the exosomes determine their organ specific location or cell-type specificity. Collectively, the knowledge of vesicle biogenesis, secretion, and uptake is not complete and deserves further exploration.
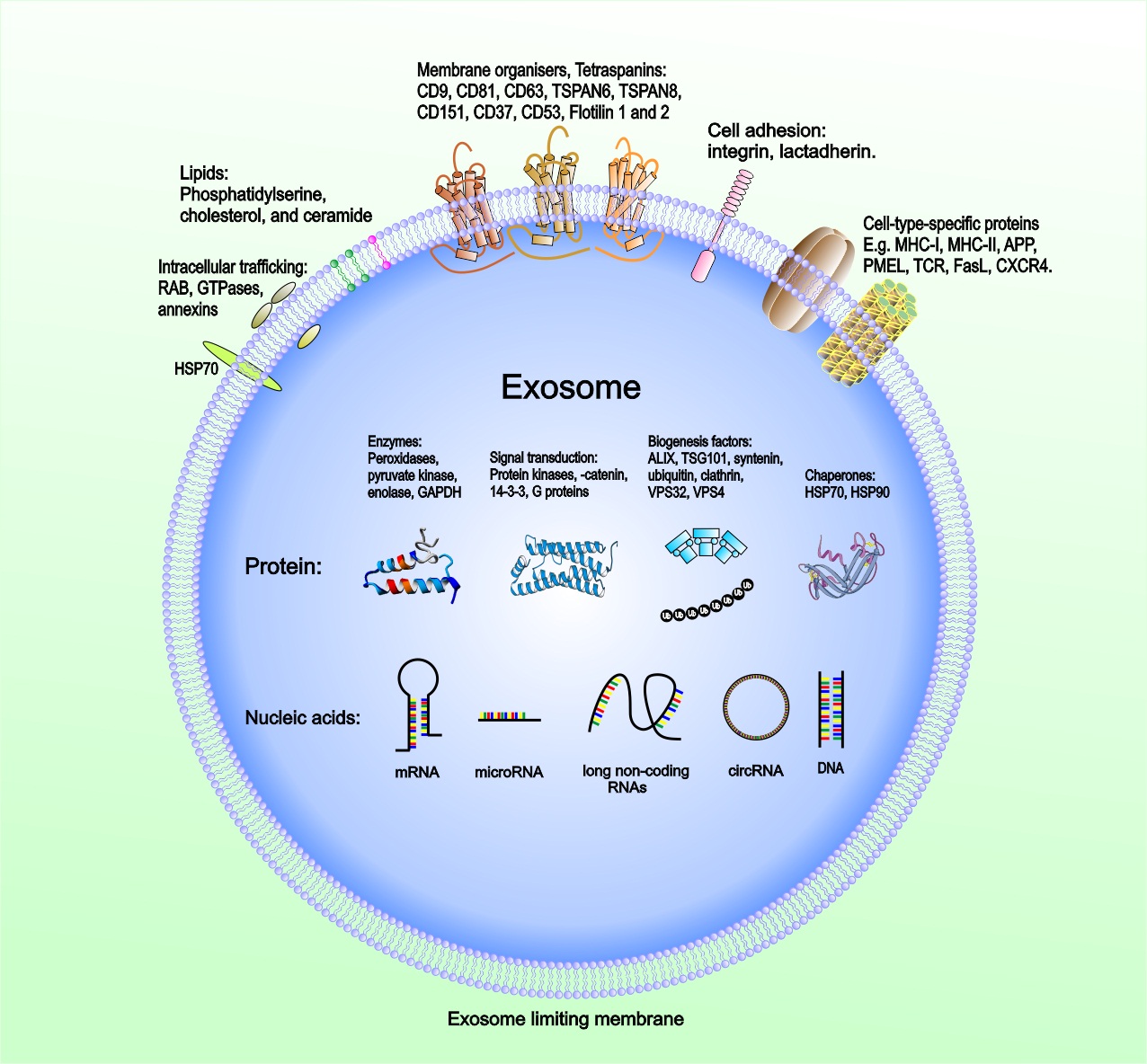
The molecular composition of TEX
Exosomes have emerged as crucial regulators of intercellular communication in cancer. Exosomes released into TME and body fluids could be taken up by recipient cells through direct fusion of their membrane in different manners such as lipid raft, calveolae, and clathrin-dependent endocytosis, macropinocytosis, and phagocytosis (41-44). The intravesicular cargo of exosomes is made up of proteins, lipids, DNAs (mtDNA, ssDNA, dsDNA), and RNAs (mRNA, miRNA, long non-coding RNA, circRNA), which are all functional when transferred into recipient cells (45, 46). Extensive reports of exosome composition have illustrated that exosomes derived from tumors and carrying various cargoes are markedly involved in regulating the biological activities of their recipient cells via the transfer of their oncogenic content that can vary widely between cells and conditions (Fig. 2).
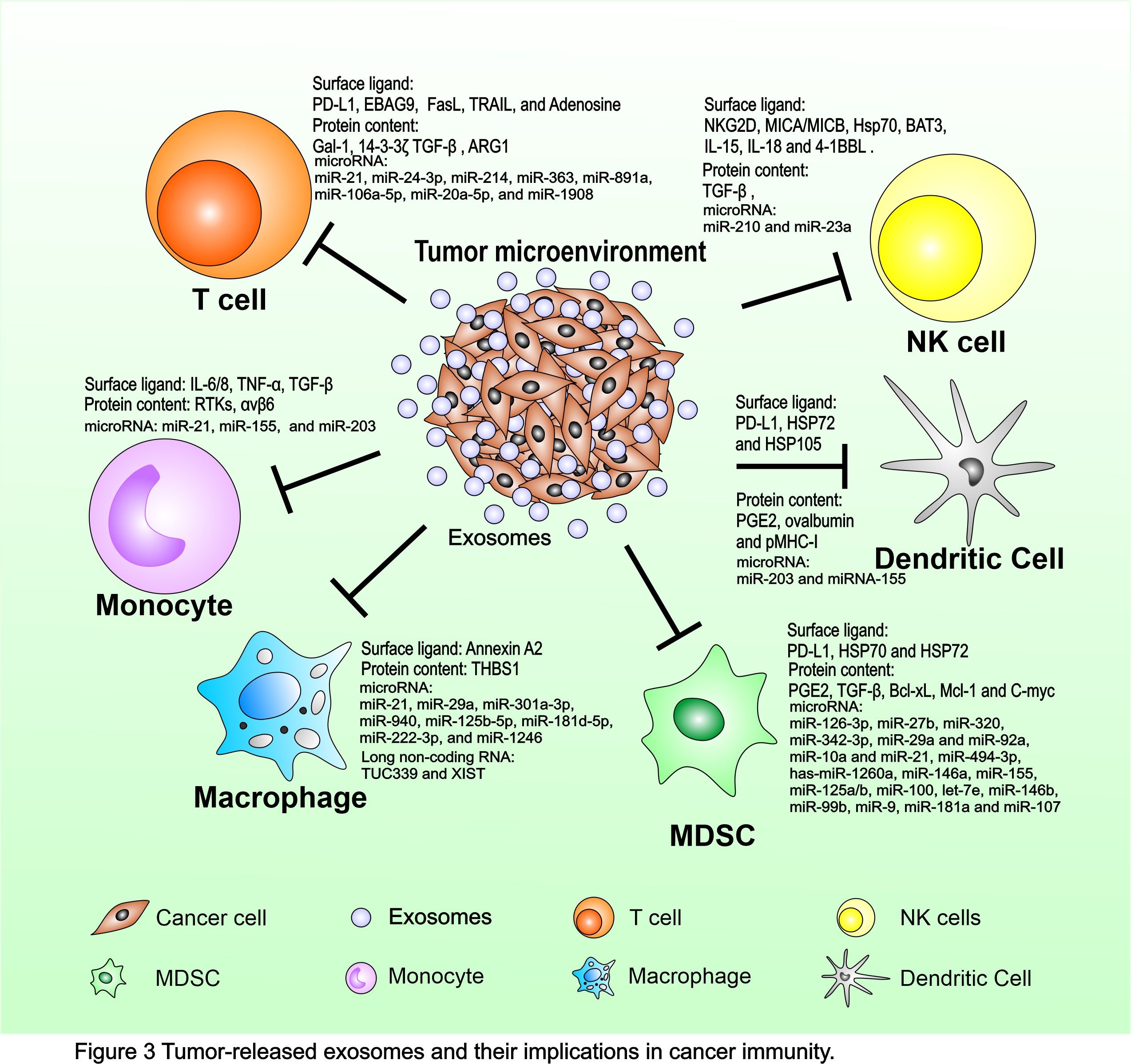
TEX-mediated Immune Suppression
Cancer immunosurveillance is a process of spontaneous cancer immunity and an attempt of the host immune system to restrain cancer growth in the early phases of development. However, the equilibrium usually fails with disease progression through escape mechanisms adopted by tumor cells to silence their immunogenic profile and survive by activating immunosuppressive/deviating pathways. Cancer cells are thought to mold microenvironment components and affect immune system function mainly by pathways involved in cell-to-cell contact and the release of soluble suppressive factors, which influence myeloid differentiation. However, the secretion of cytokines and growth factors is not responsible for the totality of the multiple and generalized immune defects in patients with cancer due to rapid degradation by serum proteases in the blood circulation. An alternative novel mechanism is now emerging involving the active release by tumor cells of immune-suppressive microvesicles, such as TEX. TEX offers an efficient vehicle for mediating tumoral immunosuppression. TEX could provide a relatively resistant transporter of bioactive molecules to promote a more effective propagation of tolerogenic signals from the tumor site to distant compartments (Fig. 3).