Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jules Tianyu ZHANG-YIN | + 2008 word(s) | 2008 | 2022-02-16 09:37:22 | | | |
| 2 | Catherine Yang | Meta information modification | 2008 | 2022-02-17 03:59:44 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Zhang-Yin, J. PET Imaging. Encyclopedia. Available online: https://encyclopedia.pub/entry/19548 (accessed on 07 February 2026).
Zhang-Yin J. PET Imaging. Encyclopedia. Available at: https://encyclopedia.pub/entry/19548. Accessed February 07, 2026.
Zhang-Yin, Jules. "PET Imaging" Encyclopedia, https://encyclopedia.pub/entry/19548 (accessed February 07, 2026).
Zhang-Yin, J. (2022, February 16). PET Imaging. In Encyclopedia. https://encyclopedia.pub/entry/19548
Zhang-Yin, Jules. "PET Imaging." Encyclopedia. Web. 16 February, 2022.
Copy Citation
PET imaging is being increasingly used to supplement MRI in the clinical management of brain tumors. The main radiotracers implemented in clinical practice include [18F]FDG, radiolabeled amino acids ([11C]MET, [18F]FDOPA, [18F]FET) and [68Ga]Ga-DOTA-SSTR, targeting glucose metabolism, L-amino-acid transport and somatostatin receptors expression, respectively.
glioma
brain metastases
1. Glioma
Amino acid PET tracers have relatively high sensitivity for gliomas as the LAT system transporters is overexpressed in most of them [1], with a good tumor-to-background contrast in the brain [2]. Thus, their use has been recommended by the Response Assessment Neuro-Oncology group (RANO) for the assessment of glioma in many situations [3].
1.1. Diagnostic and Characterization
[11C]MET is chemically equivalent to natural methionine it is incorporated into proteins and could therefore accumulate over time within tumors [4]. Nevertheless, 11C short physical half-life limits its availability and does not allow for delayed imaging. [11C]MET has good performance to diagnose brain tumors as highlighted in a meta-analysis including more than 400 patients by Zhao et al. [5]. In their study, [11C]MET had a high pooled sensitivity and specificity of 91% and 86% for neoplastic tissue, whereas those of 18F-FDG were only moderate with sensitivity and specificity of 71% and 77%, respectively.
Nowadays, the emergence of 18F radiolabeled amino acid tracers such as [18F]FDOPA and [18F]FET enabled a wide use of amino acid PET in clinical practice in many centers. In general, they have comparable diagnostic value compared to [11C]MET [6][7].
[18F]FDOPA PET has a good accuracy for the diagnosis of primary brain tumors, with a sensitivity of 96% and a specificity of 86% [8]. It is more specific than [18F]FDG [8] and performs as well as 11C-MET [9]. The visual and semi-quantitative analyses can both be used. In the visual analysis, the positivity can be defined by a lesion’s uptake greater than or equal to striatum uptake. In the semi-quantitative analysis, the only pathology-controlled thresholds to detect brain tumors is a ratio of 1.0 for tumor-to-striatum and 1.3 for tumor-to-brain [8][10] (Figure 1). It can also be used to differentiate high- and low-grade gliomas as the uptake is significantly higher in high-grade gliomas [11]. A recent meta-analysis found a pooled sensitivity of 0.88 and a pooled specificity and 0.73 for glioma grading [12], making it a valuable clinical tool [13]. The dynamic analysis also seems to be an interesting tool to integrate [14][15][16]. At diagnosis [18F]FDOPA uptake also has an independent prognostic value [17].
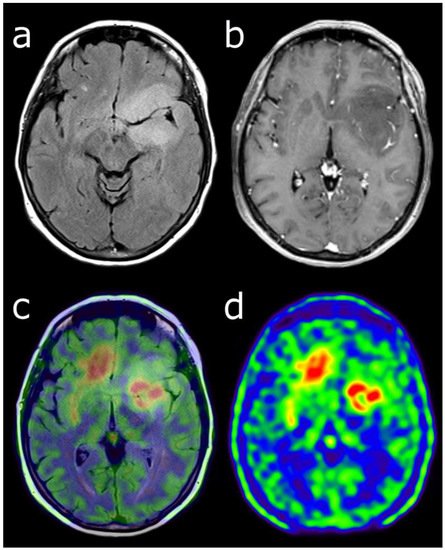
Figure 1. Characterization of a left temporo-fronto-insular brain lesion in a 60-year-old woman. This lesion was considered suggestive of grade II or III glioma according T2-FLAIR (a) and post-enhancement T1-weighted MRI (b). [18F]FDOPA PET images (fused with T2-FLAIR MRI (c) and PET only (d)) showed an uptake more intense than twice the normal cortex, predictive of high-grade tumor. Moreover, the lesion visualized with PET was much more extended than with MRI, particularly in the contralateral frontal lobe. Pathological analysis of biopsy samples revealed a WHO grade IV glioblastoma.
In the same line, [18F]FET is another very performant tracer as a meta-analysis of 13 [18F]FET PET studies, including more than 450 patients, showed pooled sensitivity and specificity both around 80% for the diagnosis of primary brain tumors [18]. More recently, a second meta-analysis, including 119 patients, reported pooled sensitivity and specificity of 0.94 and 0.88, respectively [19]. A mean tumor-to-background uptake threshold ratio of at least 1.6 and a maximum TBR of at least 2.1 seems to be the best cutoff. Nevertheless, the same authors, in another previous study, have found some contradictory results, especially a relatively low specificity of 62% for a good sensitivity of 84% [18]. The threshold is 2.1 for tumor-to-brain (TBR) max et 1.7 for TBR mean to distinguish glioma versus non-glioma [18]. It allows the biopsy guidance [20] and differentiate efficiently high- and low-grade gliomas, especially thanks to parameters derived from dynamic acquisition [21].
It is particularly important to rule out false positivity, as increased amino acid tracer uptake may also occur in nonneoplastic lesions or inflammatory processes such for [18F]FET and for [18F]FDOPA [22][23][24], but uptake intensity is generally low or moderate, below the intensity of high-grade gliomas’ uptake.
1.2. Defining Tumor Extent
Delineating glioma extent is important for further diagnostic and therapeutic management, such as biopsy, resection, or radiotherapy planning (Figure 1). To do so [18F]FDOPA and [18F]FET can complement mpMRI. Indeed, conventional mpMRI is particularly limited in its ability to identify non-enhancing glioma subregions [28], whereas [18F]FDOPA PET could discriminate glioma from benign brain lesions such as dysembryoplastic neuroepithelial tumor, and high- from low-grade gliomas, with no contrast enhancement on mpMRI [11]. In a recent biopsy-validated study, molecular information obtained from [18F]FET would reveal a more accurate glioma extent, which is critical for individualized treatment planning [29].
As there is a significant difference in evaluating tumor volume between amino acid PET and mpMRI, it suggests that the latter could substantially underestimate the metabolically active tumor volume [30][31]. Another recent evidence-based article suggested that amino acid PET could improve the delineation of high-grade gliomas compared to standard mpMRI [32]. However, till today, only one study has demonstrated that the management based on amino acid PET-guided benefits a better patient outcome [33].
1.3. Defining Tumor Heterogeneity
The extent of tumor resection is one of the most important prognostic factors in gliomas. Nevertheless, often these tumors are highly heterogeneous with a possible coexistence of high- and low-grade subregions. Thus, presurgical identification of high-grade subregions extent is of major importance. In this very recent biopsy validation study, Girard et al. found that the addition of [18F]FDOPA PET to mpMRI enlarged the delineation volumes and enhanced overall accuracy for detection of high-grade subregions. Thus, combining [18F]FDOPA PET with advanced mpMRI may improve treatment planning in newly diagnosed gliomas [34]. In another clinical trial, better defining tumor heterogeneity seems to have a high impact on radiation therapy. Indeed, [18F]FDOPA PET-guided dose-escalated radiation therapy significantly improved the overall survival in the subgroup of O6-methylguanine-DNA methyltransferase (MGMT) methylated glioblastoma patients and the progression-free survival in MGMT unmethylated glioblastoma patients [33].
1.4. Monitoring Therapy
In gliomas, frequently used systemic treatment options are alkylating chemotherapy and antiangiogenic therapy, associated with radiotherapy (protocol STUPP). The mpMRI method remains the standard tool for assessment of response but it often lacks specificity [35].
[18F]FET can provide a reliable response assessment after chemotherapy (temozolomide and nitrosourea-based) in patients with high-grade glioma at recurrence [34]. It also can predict patients’ outcomes as metabolic responders have better survival [36][37].
An early [18F]FET uptake change, seen 6 weeks after chemoradiotherapy using temozolomide, could already have prognostic information. A decrease in metabolic activity of more than 10% can be a cutoff for predicting better patients’ survival [38].
1.5. Recurrence vs. Radionecrosis
Differentiating TRC from disease progression is of critical importance for patients’ management and prognosis and it can often be challenging [41]. In some countries such as France, suspected recurrent or progressive glioma after treatment is the most common indication for [18F]FDOPA PET in brain tumors. The mpMRI method has some limitations in the case of pseudo-progression in the first 12 weeks after treatment, as in the case of radio-necrosis after 12 weeks of treatment [41][42]. These TRC can appear like a new or increasing contrast enhancement that cannot be distinguished from tumor recurrence.
The [18F]FDOPA PET has an accuracy from 82% to 96% to distinguish TRC vs. a progressive tumor [43][44]. The visual criteria, using the striatum contralateral to the tumor as the reference, or semi-quantitatively, with a tumor-to-striatum ratio of 1.4 for SUVmax and 1.2 for SUV mean, could both be effective [43] (Figure 2).
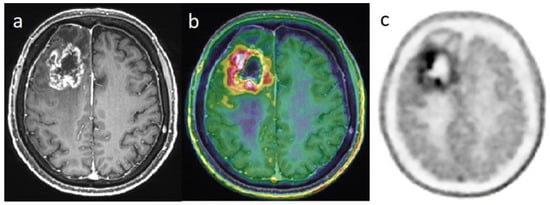
Figure 2. Differential diagnosis between recurrence versus radiation necrosis in a 70-year-old man who suffered from a right frontal glioblastoma IDH1-wt and was treated with surgery then chemoradiation with Temodal. Nine months later, there is an increase in edema in the site of the initial tumor. Post-enhancement T1-weighted MRI (a) revealed an increasing contrast enhancement which could correspond to a recurrence or a radionecrosis. [18F]FDOPA PET images (fused with T1-weighted MRI (b) and PET only (c)) showed an intense uptake of this lesion, with tumor/striata ratio = 1.5, suggesting tumor recurrence.
2. Metastases
Brain metastases (BM) are the most common malignant brain tumors, as they are part of the natural course of several types of cancer, especially breast and lung cancer, as well as melanomas. Contrast-enhanced mpMRI is the cornerstone of metastatic brain tumor evaluation. It has widespread availability and excellent spatial resolution, but its specificity can be low, especially in distinguishing TRC from progression, resulting in substantial diagnostic challenges [47]. Amino acid PET tracers can be useful because, as gliomas, metastatic brain tumors overexpress LAT, independently of the primitive tumor. However, [18F]FDG has also a role to play.
2.1. Diagnostic and Characterization
For the detection of BM, the [18F]FDG has poor accuracy, as highlighted in a recent meta-analysis including more than 900 lung cancer patients with brain metastases and comparing contrast-enhanced mpMRI to [18F]FDG PET. It was found that mpMRI has a substantially higher cumulative sensitivity (77%) than [18F]FDG PET (21%) [48].
Amino acid PET tracers are substantially more performant than [18F]FDG, especially for lesions more than 1 cm. Unterrainer et al. reported that [18F]FET were positive for approximately 90% BM, using a ratio ≥ 1.6 for tumor/brain [49]. For lesions smaller than 1 cm, the detection rate by mpMRI remains the best, nearly 100% [49]. So, mpMRI is the reference imaging modality for the detection of brain metastases.
There is limited evidence to support the use of PET to distinguish between BM and other brain tumors, especially high-grade glioma [48]. Some authors reported that [18F]FDG is generally lower in metastases than in PCNSL and amino acid PET tracers could identify aggressive tumor features and thus predict a worse prognosis [50][51].
2.2. Detecting Occult Primary Extracerebral Malignancy Revealed by Brain Metastases
In the case of newly discovered BM in patients with no history of cancer, primary lesion and other extracerebral metastases must be sought. Several studies investigating [18F]FDG PET revealed its good performance.
Roh et al. showed that the sensitivity of [18F]FDG PET (87.5%) was significantly higher than that of CT (43.7%) in the detection of the primary tumor in patients with BM [52]. The main sites of other extracerebral metastases are in lymph nodes, especially mediastinal, hilar and retroperitoneal ones [53][54], and the lung was the most frequent primary tumor in patients with brain metastases [54][55]. Moreover, [18F]FDG PET detected additional extracerebral metastatic sites in 42% to 63% of patients [53][54][55][56].
2.3. Recurrence vs. Radionecrosis
Among irradiated patients, brain radionecrosis is a common complication, mainly depending on irradiation technique [57], which occurs with an incidence up to 25% [58]. Most of the brain radionecroses are diagnosed during the year after the end of radiotherapy and in 80% within 3 years [59]. Differentiating brain metastases recurrence from radionecrosis can be challenging during mpMRI follow-up after stereotactic radiotherapy.
According to the RANO/PET working group, amino acids PET tracer should be preferred in this indication [47][60]. Reported diagnostic performance of amino acids PET is high and reproducible with sensitivity ranging between 74 and 90% and specificity between 75 and 100% [61][62][63][64][65][66][67][68].
Reported sensitivity of standard [18F]FDG ranges from 40% to 83% and specificity between 50% and 94%, respectively [59][69][70][71]. This limited diagnostic performance is mainly due to low tumor-to-brain on standard images. To overcome this issue, some authors performed additional delayed PET images 4 to 5 h after [18F]FDG injection. With such protocols, [18F]FDG PET reached sensitivity of 93–95% and specificity of 94–100% [72][73] (Figure 3).
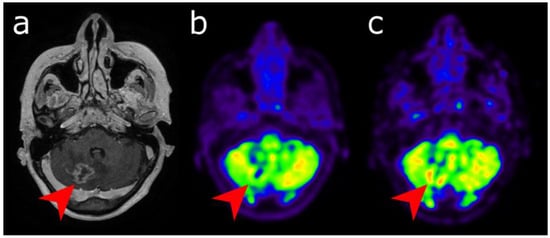
Figure 3. Differential diagnosis between recurrence versus radionecrosis in a 73-year-old woman who was treated with stereotactic radiation therapy 2 years earlier for a cerebellar metastasis (red arrow) of breast cancer. Post-enhancement T1-weighted MRI (a) revealed an increasing contrast enhancement. While standard [18F]FDG PET imaging performed 60 min (b) displayed no significant uptake, delayed images performed 4 h post-injection (c) revealed uptake higher than the background activity, suggesting tumor recurrence.
References
- Papin-Michault, C.; Bonnetaud, C.; Dufour, M.; Almairac, F.; Coutts, M.; Patouraux, S.; Virolle, T.; Darcourt, J.; Burel-Vandenbos, F. Study of LAT1 Expression in Brain Metastases: Towards a Better Understanding of the Results of Positron Emission Tomography Using Amino Acid Tracers. PLoS ONE 2016, 11, e0157139.
- Wester, H.; Herz, M.; Weber, W.; Heiss, P.; Senekowitsch-Schmidtke, R.; Schwaiger, M.; Stöcklin, G. Synthesis and radiopharmacology of O-(2-fluoroethyl)-L-tyrosine for tumor imaging. J. Nucl. Med. 1999, 40, 205–212.
- Albert, N.; Weller, M.; Suchorska, B.; Galldiks, N.; Soffietti, R.; Kim, M.; la Fougère, C.; Pope, W.; Law, I.; Arbizu, J.; et al. Response Assessment in Neuro-Oncology working group and European Association for Neuro-Oncology recommendations for the clinical use of PET imaging in gliomas. Neuro Oncol. 2016, 18, 1199–1208.
- Borbély, K.; Nyáry, I.; Tóth, M.; Ericson, K.; Gulyás, B. Optimization of semi-quantification in metabolic PET studies with 18F-fluorodeoxyglucose and 11C-methionine in the determination of malignancy of gliomas. J. Neurol. Sci. 2006, 246, 85–94.
- Zhao, C.; Zhang, Y.; Wang, J. A meta-analysis on the diagnostic performance of (18)F-FDG and (11)C-methionine PET for differentiating brain tumors. Am. J. Neuroradiol. 2013, 35, 1058–1065.
- Grosu, A.; Astner, S.; Riedel, E.; Nieder, C.; Wiedenmann, N.; Heinemann, F.; Schwaiger, M.; Molls, M.; Wester, H.; Weber, W. An interindividual comparison of O-(2-fluoroethyl)-L-tyrosine (FET)- and L-methionine (MET)-PET in patients with brain gliomas and metastases. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 1049–1058.
- Janvier, L.; Olivier, P.; Blonski, M.; Morel, O.; Vignaud, J.; Karcher, G.; Taillandier, L.; Verger, A. Correlation of SUV-Derived Indices with Tumoral Aggressiveness of Gliomas in Static 18F-FDOPA PET: Use in Clinical Practice. Clin. Nucl. Med. 2015, 40, e429–e435.
- Chen, W.; Silverman, D.H.; Delaloye, S.; Czernin, J.; Kamdar, N.; Pope, W.; Satyamurthy, N.; Schiepers, C.; Cloughesy, T. 18F-FDOPA PET imaging of brain tumors: Comparison study with 18F-FDG PET and evaluation of diagnostic accuracy. J. Nucl. Med. 2006, 47, 904–911.
- Becherer, A.; Karanikas, G.; Szabó, M.; Zettinig, G.; Asenbaum, S.; Marosi, C.; Henk, C.; Wunderbaldinger, P.; Czech, T.; Wadsak, W.; et al. Brain tumour imaging with PET: A comparison between fluorodopa and methionine. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 1561–1567.
- Lizarraga, K.; Allen-Auerbach, M.; Czernin, J.; DeSalles, A.; Yong, W.; Phelps, M.; Chen, W. (18)F-FDOPA PET for differentiating recurrent or progressive brain metastatic tumors from late or delayed radiation injury after radiation treatment. J. Nucl. Med. 2013, 55, 30–36.
- Bund, C.; Heimburger, C.; Imperiale, A.; Lhermitte, B.; Chenard, M.; Lefebvre, F.; Kremer, S.; Proust, F.; Namer, I. FDOPA PET-CT of Nonenhancing Brain Tumors. Clin. Nucl. Med. 2017, 42, 250–257.
- Xiao, J.; Jin, Y.; Nie, J.; Chen, F.; Ma, X. Diagnostic and grading accuracy of 18F-FDOPA PET and PET/CT in patients with gliomas: A systematic review and meta-analysis. BMC Cancer 2019, 19, 767.
- Verger, A.; Arbizu, J.; Law, I. Role of amino-acid PET in high-grade gliomas: Limitations and perspectives. Q. J. Nucl. Med. Mol. Imaging 2018, 62, 254–266.
- Verger, A.; Kas, A.; Darcourt, J.; Chinot, O.; Taillandier, L.; Hoang Xuan, K.; Guedj, E.; Bouvet, C.; Bund, C.; Guedj, E.; et al. Joint SFMN/ANOCEF focus on 18F-FDOPA PET imaging in glioma: Current applications and perspectives. Méd. Nucl. 2020, 3, 164–171.
- Girard, A.; Saint-Jalmes, H.; Chaboub, N.; Le Reste, P.J.; Metais, A.; Devillers, A.; Le Jeune, F.; Palard-Novello, X. Optimization of time frame binning for FDOPA uptake quantification in glioma. PLoS ONE 2020, 15, e0232141.
- Girard, A.; Le Reste, P.J.; Metais, A.; Chaboub, N.; Devillers, A.; Saint-Jalmes, H.; Jeune, F.L.; Palard-Novello, X. Additive Value of Dynamic FDOPA PET/CT for Glioma Grading. Front. Med. 2021, 8, 705996.
- Patel, C.; Fazzari, E.; Chakhoyan, A.; Yao, J.; Raymond, C.; Nguyen, H.; Manoukian, J.; Nguyen, N.; Pope, W.; Cloughesy, T.; et al. 18F-FDOPA PET and MRI characteristics correlate with degree of malignancy and predict survival in treatment-naïve gliomas: A cross-sectional study. J. Neurooncol. 2018, 139, 399–409.
- Dunet, V.; Rossier, C.; Buck, A.; Stupp, R.; Prior, J. Performance of 18F-fluoro-ethyl-tyrosine (18F-FET) PET for the differential diagnosis of primary brain tumor: A systematic review and Metaanalysis. J. Nucl. Med. 2012, 53, 207–214.
- Dunet, V.; Pomoni, A.; Hottinger, A.; Nicod-Lalonde, M.; Prior, J. Performance of 18F-FET versus 18F-FDG-PET for the diagnosis and grading of brain tumors: Systematic review and meta-analysis. Neuro Oncol. 2015, 18, 426–434.
- Pauleit, D.; Floeth, F.; Hamacher, K.; Riemenschneider, M.J.; Reifenberger, G.; Müller, H.W.; Zilles, K.; Coenen, H.H.; Langen, K.J. O-(2-fluoroethyl)-L-tyrosine PET combined with MRI improves the diagnostic assessment of cerebral gliomas. Brain 2005, 128, 678–687.
- Pöpperl, G.; Kreth, F.; Mehrkens, J.; Herms, J.; Seelos, K.; Koch, W.; Gildehaus, F.; Kretzschmar, H.; Tonn, J.; Tatsch, K. FET PET for the evaluation of untreated gliomas: Correlation of FET uptake and uptake kinetics with tumour grading. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 1933–1942.
- Pichler, R.; Dunzinger, A.; Wurm, G.; Pichler, J.; Weis, S.; Nußbaumer, K.; Topakian, R.; Aigner, R. Is there a place for FET PET in the initial evaluation of brain lesions with unknown significance? Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1521–1528.
- Calabria, F.F.; Chiaravalloti, A.; Jaffrain-Rea, M.L.; Zinzi, M.; Sannino, P.; Minniti, G.; Rubello, D.; Schillaci, O. 18F-DOPA PET/CT Physiological Distribution and Pitfalls: Experience in 215 Patients. Clin. Nucl. Med. 2016, 41, 753–760.
- Sala, Q.; Metellus, P.; Taieb, D.; Kaphan, E.; Figarella-Branger, D.; Guedj, E. 18F-DOPA, a clinically available PET tracer to study brain inflammation? Clin. Nucl. Med. 2014, 39, e283–e285.
- Hutterer, M.; Nowosielski, M.; Putzer, D.; Jansen, N.; Seiz, M.; Schocke, M.; McCoy, M.; Göbel, G.; la Fougère, C.; Virgolini, I.; et al. -fluoro-ethyl-l-tyrosine PET: A valuable diagnostic tool in neuro-oncology, but not all that glitters is glioma. Neuro Oncol. 2013, 15, 341–351.
- Jansen, N.; Graute, V.; Armbruster, L.; Suchorska, B.; Lutz, J.; Eigenbrod, S.; Cumming, P.; Bartenstein, P.; Tonn, J.; Kreth, F.; et al. MRI-Suspected low-grade glioma: Is there a need to perform dynamic FET PET? Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 1021–1029.
- Suchorska, B.; Giese, A.; Biczok, A.; Unterrainer, M.; Weller, M.; Drexler, M.; Bartenstein, P.; Schüller, U.; Tonn, J.; Albert, N. Identification of time-to-peak on dynamic 18F-FET-PET as a prognostic marker specifically in IDH1/2 mutant diffuse astrocytoma. Neuro Oncol. 2017, 20, 279–288.
- Langen, K.J.; Galldiks, N.; Hattingen, E.; Shah, N.J. Advances in neuro-oncology imaging. Nat. Rev. Neurol. 2017, 13, 279–289.
- Song, S.; Cheng, Y.; Ma, J.; Wang, L.; Dong, C.; Wei, Y.; Xu, G.; An, Y.; Qi, Z.; Lin, Q.; et al. Simultaneous FET-PET and contrast-enhanced MRI based on hybrid PET/MR improves delineation of tumor spatial biodistribution in gliomas: A biopsy validation study. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1458–1467.
- Lohmann, P.; Werner, J.; Shah, N.; Fink, G.; Langen, K.; Galldiks, N. Combined Amino Acid Positron Emission Tomography and Advanced Magnetic Resonance Imaging in Glioma Patients. Cancers 2019, 11, 153.
- Kunz, M.; Thon, N.; Eigenbrod, S.; Hartmann, C.; Egensperger, R.; Herms, J.; Geisler, J.; la Fougere, C.; Lutz, J.; Linn, J.; et al. Hot spots in dynamic (18)FET-PET delineate malignant tumor parts within suspected WHO grade II gliomas. Neuro Oncol. 2011, 13, 307–316.
- Verburg, N.; Hoefnagels, F.; Barkhof, F.; Boellaard, R.; Goldman, S.; Guo, J.; Heimans, J.; Hoekstra, O.; Jain, R.; Kinoshita, M.; et al. Diagnostic Accuracy of Neuroimaging to Delineate Diffuse Gliomas within the Brain: A Meta-Analysis. Am. J. Neuroradiol. 2017, 38, 1884–1891.
- Laack, N.; Pafundi, D.; Anderson, S.; Kaufmann, T.; Lowe, V.; Hunt, C.; Vogen, D.; Yan, E.; Sarkaria, J.; Brown, P.; et al. Initial Results of a Phase 2 Trial of 18F-DOPA PET-Guided Dose-Escalated Radiation Therapy for Glioblastoma. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 1383–1395.
- Galldiks, N.; Kracht, L.; Burghaus, L.; Ullrich, R.; Backes, H.; Brunn, A.; Heiss, W.; Jacobs, A. Patient-Tailored, imaging-guided, long-term temozolomide chemotherapy in patients with glioblastoma. Mol. Imaging 2010, 9, 40–46.
- Dhermain, F.G.; Hau, P.; Lanfermann, H.; Jacobs, A.H.; van den Bent, M.J. Advanced MRI and PET imaging for assessment of treatment response in patients with gliomas. Lancet Neurol. 2010, 9, 906–920.
- Roelcke, U.; Wyss, M.; Nowosielski, M.; Rudà, R.; Roth, P.; Hofer, S.; Galldiks, N.; Crippa, F.; Weller, M.; Soffietti, R. Amino acid positron emission tomography to monitor chemotherapy response and predict seizure control and progression-free survival in WHO grade II gliomas. Neuro Oncol. 2015, 18, 744–751.
- Suchorska, B.; Unterrainer, M.; Biczok, A.; Sosnova, M.; Forbrig, R.; Bartenstein, P.; Tonn, J.; Albert, N.; Kreth, F. 18F-FET-PET as a biomarker for therapy response in non-contrast enhancing glioma following chemotherapy. J. Neurooncol. 2018, 139, 721–730.
- Galldiks, N.; Langen, K.; Holy, R.; Pinkawa, M.; Stoffels, G.; Nolte, K.; Kaiser, H.; Filss, C.; Fink, G.; Coenen, H.; et al. Assessment of treatment response in patients with glioblastoma using O-(2-18F-fluoroethyl)-L-tyrosine PET in comparison to MRI. J. Nucl. Med. 2012, 53, 1048–1057.
- Harris, R.; Cloughesy, T.; Pope, W.; Nghiemphu, P.; Lai, A.; Zaw, T.; Czernin, J.; Phelps, M.; Chen, W.; Ellingson, B. 18F-FDOPA and 18F-FLT positron emission tomography parametric response maps predict response in recurrent malignant gliomas treated with bevacizumab. Neuro Oncol. 2012, 14, 1079–1089.
- Schwarzenberg, J.; Czernin, J.; Cloughesy, T.; Ellingson, B.; Pope, W.; Grogan, T.; Elashoff, D.; Geist, C.; Silverman, D.; Phelps, M.; et al. Treatment response evaluation using 18F-FDOPA PET in patients with recurrent malignant glioma on bevacizumab therapy. Clin. Cancer Res. 2014, 20, 3550–3559.
- Galldiks, N.; Stoffels, G.; Filss, C.; Rapp, M.; Blau, T.; Tscherpel, C.; Ceccon, G.; Dunkl, V.; Weinzierl, M.; Stoffel, M.; et al. The use of dynamic O-(2-18F-fluoroethyl)-l-tyrosine PET in the diagnosis of patients with progressive and recurrent glioma. Neuro Oncol. 2015, 17, 1293–1300.
- Shah, A.; Snelling, B.; Bregy, A.; Patel, P.; Tememe, D.; Bhatia, R.; Sklar, E.; Komotar, R. Discriminating radiation necrosis from tumor progression in gliomas: A systematic review what is the best imaging modality? J. Neurooncol. 2013, 112, 141–152.
- Herrmann, K.; Czernin, J.; Cloughesy, T.; Lai, A.; Pomykala, K.; Benz, M.; Buck, A.; Phelps, M.; Chen, W. Comparison of visual and semiquantitative analysis of 18F-FDOPA-PET/CT for recurrence detection in glioblastoma patients. Neuro Oncol. 2013, 16, 603–609.
- Karunanithi, S.; Sharma, P.; Kumar, A.; Khangembam, B.; Bandopadhyaya, G.; Kumar, R.; Gupta, D.; Malhotra, A.; Bal, C. 18F-FDOPA PET/CT for detection of recurrence in patients with glioma: Prospective comparison with 18F-FDG PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 1025–1035.
- Nihashi, T.; Dahabreh, I.J.; Terasawa, T. Diagnostic accuracy of PET for recurrent glioma diagnosis: A meta-analysis. Am. J. Neuroradiol. 2013, 34, 944–950.
- Salber, D.; Stoffels, G.; Pauleit, D.; Oros-Peusquens, A.; Shah, N.; Klauth, P.; Hamacher, K.; Coenen, H.; Langen, K. Differential uptake of O-(2-18F-fluoroethyl)-L-tyrosine, L-3H-methionine, and 3H-deoxyglucose in brain abscesses. J. Nucl. Med. 2007, 48, 2056–2062.
- Galldiks, N.; Langen, K.; Albert, N.; Chamberlain, M.; Soffietti, R.; Kim, M.; Law, I.; Le Rhun, E.; Chang, S.; Schwarting, J.; et al. PET imaging in patients with brain metastasis-report of the RANO/PET group. Neuro Oncol. 2019, 21, 585–595.
- Li, Y.; Jin, G.; Su, D. Comparison of gadolinium-enhanced MRI and 18FDG PET/PET-CT for the diagnosis of brain metastases in lung cancer patients: A meta-analysis of 5 prospective studies. Oncotarget 2017, 8, 35743–35749.
- Unterrainer, M.; Galldiks, N.; Suchorska, B.; Kowalew, L.; Wenter, V.; Schmid-Tannwald, C.; Niyazi, M.; Bartenstein, P.; Langen, K.; Albert, N. 18F-FET PET Uptake Characteristics in Patients with Newly Diagnosed and Untreated Brain Metastasis. J. Nucl. Med. 2016, 58, 584–589.
- Kaira, K.; Oriuchi, N.; Imai, H.; Shimizu, K.; Yanagitani, N.; Sunaga, N.; Hisada, T.; Tanaka, S.; Ishizuka, T.; Kanai, Y.; et al. Prognostic significance of L-type amino acid transporter 1 expression in resectable stage I-III nonsmall cell lung cancer. Br. J. Cancer 2008, 98, 742–748.
- Yanagisawa, N.; Ichinoe, M.; Mikami, T.; Nakada, N.; Hana, K.; Koizumi, W.; Endou, H.; Okayasu, I. High expression of L-type amino acid transporter 1 (LAT1) predicts poor prognosis in pancreatic ductal adenocarcinomas. J. Clin. Pathol. 2012, 65, 1019–1023.
- Roh, J.; Kim, J.; Lee, J.; Cho, K.; Choi, S.; Nam, S.; Kim, S. Utility of combined (18)F-fluorodeoxyglucose-positron emission tomography and computed tomography in patients with cervical metastases from unknown primary tumors. Oral Oncol. 2009, 45, 218–224.
- Wolpert, F.; Weller, M.; Berghoff, A.; Rushing, E.; Füreder, L.; Petyt, G.; Leske, H.; Andratschke, N.; Regli, L.; Neidert, M.; et al. Diagnostic value of 18F-fluordesoxyglucose positron emission tomography for patients with brain metastasis from unknown primary site. Eur. J. Cancer 2018, 96, 64–72.
- Koç, Z.P.; Kara, P.Ö.; Dağtekin, A. Detection of unknown primary tumor in patients presented with brain metastasis by F-18 fluorodeoxyglucose positron emission tomography/computed tomography. CNS Oncol. 2018, 7, 12.
- Cengiz, A.; Göksel, S.; Yürekli, Y. Diagnostic Value of 18F-FDG PET/CT in Patients with Carcinoma of Unknown Primary. Mol. Imaging Radionucl. Ther. 2018, 27, 126–132.
- Mohamed, D.M.; Kamel, H.A. Diagnostic efficiency of PET/CT in patients with cancer of unknown primary with brain metastasis as initial manifestation and its impact on overall survival. Egypt J. Radiol. Nucl. Med. 2021, 52, 65–70.
- Ruben, J.; Dally, M.; Bailey, M.; Smith, R.; McLean, C.; Fedele, P. Cerebral radiation necrosis: Incidence, outcomes, and risk factors with emphasis on radiation parameters and chemotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 499–508.
- Vellayappan, B.; Tan, C.; Yong, C.; Khor, L.; Koh, W.; Yeo, T.; Detsky, J.; Lo, S.; Sahgal, A. Diagnosis and Management of Radiation Necrosis in Patients with Brain Metastases. Front. Oncol. 2018, 28, 395.
- Walker, A.; Ruzevick, J.; Malayeri, A.; Rigamonti, D.; Lim, M.; Redmond, K.; Kleinberg, L. Postradiation imaging changes in the CNS: How can we differentiate between treatment effect and disease progression? Future Oncol. 2014, 10, 1277–1297.
- Galldiks, N.; Kocher, M.; Ceccon, G.; Werner, J.; Brunn, A.; Deckert, M.; Pope, W.; Soffietti, R.; Le Rhun, E.; Weller, M.; et al. Imaging challenges of immunotherapy and targeted therapy in patients with brain metastases: Response, progression, and pseudoprogression. Neuro Oncol. 2019, 22, 17–30.
- Tomura, N.; Kokubun, M.; Saginoya, T.; Mizuno, Y.; Kikuchi, Y. Differentiation between Treatment-Induced Necrosis and Recurrent Tumors in Patients with Metastatic Brain Tumors: Comparison among 11C-Methionine-PET, FDG-PET, MR Permeability Imaging, and MRI-ADC-Preliminary Results. Am. J. Neuroradiol. 2017, 38, 1520–1527.
- Yomo, S.; Oguchi, K. Prospective study of 11C-methionine PET for distinguishing between recurrent brain metastases and radiation necrosis: Limitations of diagnostic accuracy and long-term results of salvage treatment. BMC Cancer 2017, 17, 713.
- Tsuyuguchi, N.; Sunada, I.; Iwai, Y.; Yamanaka, K.; Tanaka, K.; Takami, T.; Otsuka, Y.; Sakamoto, S.; Ohata, K.; Goto, T.; et al. Methionine positron emission tomography of recurrent metastatic brain tumor and radiation necrosis after stereotactic radiosurgery: Is a differential diagnosis possible? J. Neurosurg. 2003, 98, 1056–1064.
- Cicone, F.; Minniti, G.; Romano, A.; Papa, A.; Scaringi, C.; Tavanti, F.; Bozzao, A.; Maurizi Enrici, R.; Scopinaro, F. Accuracy of F-DOPA PET and perfusion-MRI for differentiating radionecrotic from progressive brain metastases after radiosurgery. Eur. J. Nucl. Med. Mol. Imaging 2014, 42, 103–111.
- Cicone, F.; Carideo, L.; Scaringi, C.; Romano, A.; Mamede, M.; Papa, A.; Tofani, A.; Cascini, G.; Bozzao, A.; Scopinaro, F.; et al. Long-term metabolic evolution of brain metastases with suspected radiation necrosis following stereotactic radiosurgery: Longitudinal assessment by F-DOPA PET. Neuro Oncol. 2020, 23, 1024–1034.
- Galldiks, N.; Stoffels, G.; Filss, C.; Piroth, M.; Sabel, M.; Ruge, M.; Herzog, H.; Shah, N.; Fink, G.; Coenen, H.; et al. Role of O-(2-(18)F-fluoroethyl)-L-tyrosine PET for differentiation of local recurrent brain metastasis from radiation necrosis. J. Nucl. Med. 2012, 53, 1367–1374.
- Ceccon, G.; Lohmann, P.; Stoffels, G.; Judov, N.; Filss, C.; Rapp, M.; Bauer, E.; Hamisch, C.; Ruge, M.; Kocher, M.; et al. Dynamic O-(2-18F-fluoroethyl)-L-tyrosine positron emission tomography differentiates brain metastasis recurrence from radiation injury after radiotherapy. Neuro Oncol. 2017, 19, 281–288.
- Romagna, A.; Unterrainer, M.; Schmid-Tannwald, C.; Brendel, M.; Tonn, J.; Nachbichler, S.; Muacevic, A.; Bartenstein, P.; Kreth, F.; Albert, N. Suspected recurrence of brain metastases after focused high dose radiotherapy: Can FET- PET overcome diagnostic uncertainties? Radiat. Oncol. 2016, 11, 139.
- Galldiks, N.; Lohmann, P.; Albert, N.; Tonn, J.; Langen, K. Current status of PET imaging in neuro-oncology. Neurooncol. Adv. 2019, 1, 1–10.
- Bělohlávek, O.; Šimonová, G.; Kantorová, I.; Novotný, J.; Liščák, R. Brain metastases after stereotactic radiosurgery using the Leksell gamma knife: Can FDG PET help to differentiate radionecrosis from tumour progression? Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 96–100.
- Lai, G.; Mahadevan, A.; Hackney, D.; Warnke, P.; Nigim, F.; Kasper, E.; Wong, E.; Carter, B.; Chen, C. Diagnostic Accuracy of PET, SPECT, and Arterial Spin-Labeling in Differentiating Tumor Recurrence from Necrosis in Cerebral Metastasis after Stereotactic Radiosurgery. Am. J. Neuroradiol. 2015, 36, 2250–2255.
- Horky, L.; Hsiao, E.; Weiss, S.; Drappatz, J.; Gerbaudo, V. Dual phase FDG-PET imaging of brain metastases provides superior assessment of recurrence versus post-treatment necrosis. J. Neurooncol. 2010, 103, 137–146.
- Matuszak, J.; Waissi, W.; Clavier, J.B.; Noël, G.; Namer, I.J. Métastases cérébrales: Apport de l’acquisition tardive en TEP/TDM au 18F-FDG pour le diagnostic différentiel entre récurrence tumorale et radionécrose. Méd. Nucl. 2016, 40, 129–141.
More
Information
Subjects:
Neuroimaging
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
2 times
(View History)
Update Date:
17 Feb 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




