Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Garofita Mateescu | + 5139 word(s) | 5139 | 2022-02-10 10:50:28 | | | |
| 2 | Beatrix Zheng | + 320 word(s) | 5459 | 2022-02-17 04:32:36 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Mateescu, G. Cat-Scratch Disease’s Neuro-Ophthalmological Effects. Encyclopedia. Available online: https://encyclopedia.pub/entry/19544 (accessed on 03 March 2026).
Mateescu G. Cat-Scratch Disease’s Neuro-Ophthalmological Effects. Encyclopedia. Available at: https://encyclopedia.pub/entry/19544. Accessed March 03, 2026.
Mateescu, Garofita. "Cat-Scratch Disease’s Neuro-Ophthalmological Effects" Encyclopedia, https://encyclopedia.pub/entry/19544 (accessed March 03, 2026).
Mateescu, G. (2022, February 16). Cat-Scratch Disease’s Neuro-Ophthalmological Effects. In Encyclopedia. https://encyclopedia.pub/entry/19544
Mateescu, Garofita. "Cat-Scratch Disease’s Neuro-Ophthalmological Effects." Encyclopedia. Web. 16 February, 2022.
Copy Citation
Cat-scratch disease is an illness caused by Bartonella henselae that occurs as a result of contact with an infected kitten or dog, such as a bite or scratch. It is more prevalent in children and young adults, as well as immunocompromised individuals. Among the ophthalmologic disorders caused by cat-scratch disease in humans, Parinaud oculoglandular syndrome, uveitis, vitritis, retinitis, retinochoroiditis and optic neuritis are the most prevalent. The neurological disorders caused by cat-scratch disease in humans include encephalopathy, transverse myelitis, radiculitis, and cerebellar ataxia.
cat scratch disease
Bartonella henselae
neuro-ophthalmology
ocular
neurological
1. Neurologic Manifestations
Neurologic complications from B. henselae infection are infrequent, occurring in around 2–7% of infected persons. Neurological symptoms often manifest two weeks after the onset of fever and lymphadenopathy [1][2]. Recently, Bartonella infections have been related to a broader range of neurological symptoms, including hallucinations, loss of weight, muscular exhaustion, partial paralysis, and pediatric acute-onset neuropsychiatric syndrome (PANS) [3].
The findings imply that this species of bacteria may be capable of causing a spectral range of neurological symptoms, varied clinical features, and multi-organ system pathology that differs in intensity and screening difficulty across an extended time period, varying from weeks to years [4][5].
The majority of individuals with CSD who have central nervous system damage are children under the age of 18. One of the most common symptoms is encephalitis, although radiculitis, polyneuritis, myelitis, and cranial nerve involvement have all been described [6][7]. Neurologic alterations often occur about two weeks after the start of fever and significant lymphadenopathy, which are frequent in these patients. Seizures are the most frequent first symptom, but headaches, alterations in mental state, delirium, and coma are all possible [7]. Most individuals recover completely without complications in under a year [8]. Computed tomographic imaging revealed no abnormalities or localized low-density lesions; an electroencephalogram demonstrated generic, widespread slowness [7]. The pathologic characteristics of CSD in the brain are unknown, as these tissues are rarely biopsied.
However, granulomatous inflammation of the brain tissue involving meningitis has been demonstrated, and special stains and molecular testing have shown that organisms recover from such lesions [9]. Usually, the diagnosis is established based on the findings of a lymph-node biopsy or other supportive laboratory tests.
Except for the detection of B. henselae infection, the laboratory examination of infected individuals with encephalopathy usually produces inconsistent findings and is thus ineffective for diagnosis.
Electroencephalography conducted during the acute period of illness shows a widespread slowness in about 80% of patients, which resolves completely upon follow-up [10]. Approximately 19% of patients show abnormal results on a CT scan or magnetic resonance imaging (MRI) of the brain, which would include anomalies in the brain’s white matter, striatum, thalamus, and gray matter [11]. The outcome for individuals with encephalopathy is usually good, with 90% of patients recovering completely and spontaneously with no complications [2]. Just one case of fatal meningitis and encephalitis in an immunocompetent infant has been reported in the scientific literature as a consequence of B. henselae disease [12].
Examination of the cerebrospinal fluid may reveal moderate pleocytosis and increased protein content [7] but, frequently, CSF study findings are benign or suggest relatively moderate inflammation. Examination of the cerebrospinal fluid may reveal moderate pleocytosis and an increased protein content [7]. The human illness has some features in common with experimental cat-brain disease, including the temporary nature of the disruption, the existence of behavioral changes, a decline in alertness or convulsions, and the absence of major CSF irregularities. It is possible that B. henselae infects both species through comparable pathophysiologic processes.
With the development of increasingly accurate and precise screening procedures, recent microbiological studies have confirmed blood and cerebrospinal fluid (CSF) infections with one or more Bartonella species in patients with neurological, neuropsychological, and psychiatric manifestations [3]. Pediatric acute-onset neuropsychiatric syndrome (PANS) is a medical illness in children and adolescents marked by the abrupt development of neuropsychiatric symptoms such as obsessions or compulsions or dietary restriction [13]. Depression, anger, anxiousness, and academic deterioration are frequently associated with PANS manifestations. PANS may be induced by infection, electrolyte imbalances, and other inflammatory responses [13]. After failing to react to psychiatric combination therapy and an immunomodulatory operator for suspected immune-driven encephalitis, Breitschwerdt and colleagues reported a case of Bartonella henselae bacteremia in a child with schizophrenia who had symptom remission after antibiotic treatment [3].
Furthermore, individuals with bartonellosis with neurological symptoms may acquire autoantibodies, lacking normal clinical, pathological, and immunologic markers of bacteremia, and may be immunocompromised as a result of medication therapy or lengthy Bartonella spp. infection. When autoantibodies are documented in combination with negative indicators for inflammation, the clinical presumption of an infectious etiology in individuals with neuropsychiatric symptoms is reduced [14].
1.1. Encephalopathy
The most typical feature is encephalopathy, which presents in 90% of cases involving the neurological system. The neurological symptoms often manifest themselves two to three weeks following the start of lymphadenopathy. Headaches and an altered mental state are common symptoms. Seizures occur in 46–80% of individuals infected with Bartonella encephalopathy, with some individuals diagnosed with status epilepticus [2][15].
However, as many as 40% of individuals with Bartonella encephalopathy have been observed to exhibit aggressive behavior [16]. Along with an altered mental state, individuals with encephalopathy can exhibit a range of neurologic findings, such as weakness, muscle-tone changes, nuchal stiffness, extensor plantar reflexes, and hypo- or hyperreflexia [17].
Cat-scratch disease encephalopathy exhibits several features, including a prolonged time interval between infection and the progression of CNS symptoms, an accelerated initiation of significant brain impairment, and a potential and, at times, extremely rapid resolution of symptoms without residual neurologic deficits. Compartmental disorders, mental decline, and convulsions are all frequent signs of illness [10].
The link between CSD and encephalopathy remains largely unknown. Encephalopathy was initially defined as having neurological involvement in CSD 70 years ago [16] and is often associated with a favorable long-term result [10]. Numerous instances of encephalopathy have been documented in recent times. Such encephalopathies may involve the brainstem [18] or striatum [19], and may result in infarction because of a vasculitic complication [20] or status epilepticus in children [21]. Numerous hypotheses regarding the etiology of CSD encephalopathy exist, including direct penetration, neurotoxins, and vasculitis due to an immunological response [22].
1.2. Encephalitis
Encephalitis was the most frequently encountered neurologic symptom in the population studied by Nawrocki et al., which was in line with the observations of Reynolds et al., who found that the majority of children hospitalized for the neurologic complications of cat-scratch disease were diagnosed with encephalitis or encephalopathy [9]. Hence, doctors must investigate cat-scratch disease in individuals with encephalitis or newly diagnosed hepatosplenic disorders, particularly in children.
Considering the nature and infrequency of encephalitis as a result of CSD, it is fair to postulate that the disease might evoke a distinct immune response in some individuals. It is conceivable that this process could be enabled against specific receptor sectors in a way outlined in synapses in the pathogenesis of other epileptogenic encephalitis, and that, depending on the specific reactivity, this could contribute to the formation of the epileptogenic process [23].
When evaluating all the etiologies of juvenile viral encephalitis, focal neurologic symptoms are prevalent [24]. In a massive study by Kolski et al. [24], around 60% of juvenile encephalitis cases were preceded by partial epilepsy, and 50%, by other focused neurologic symptoms. Encephalopathy, convulsions, status epilepticus, retinitis, transverse myelitis, and cerebral vasculitis are among the presentations recorded in individuals with cat-scratch illness who have neurological signs [25][26][27]. Encephalopathy has been the most prevalent neurologic symptom of cat-scratch illness and may occur many weeks after the first contact with a cat [28].
Cathiers et al. [10] examined the prevalence of bartonellosis’ various neurological symptoms, with encephalitis being the most common. The age range of the encephalopathy patients was 10.6 years. Convulsions happened in 46%, whereas confrontational behavior occurred in 40%. Without neurologic sequelae, all the patients (n = 76) recovered within 12 months, and 78% in 1–12 weeks. However, there have been reports of neurological symptoms persisting for more than a year [20].
According to Fouch et al., a six-year-old boy arrived with a lymphatic axillar nodule, and his condition deteriorated over the following few days, with headaches, seizures, and mental state abnormalities, which culminated in a vegetative state and the patient’s death. A postmortem examination revealed widespread and severe perivascular lymphocytic infiltrates with microglial nodules across the frontal, parietal, and occipital lobes, as well as the pons. Bartonella henselae was identified as the causal agent [29].
A recent study has shown a variety of antineuronal autoantibodies in the circulation and CSF of certain patients with status epilepticus and encephalopathy of unclear cause, indicating that autoimmunity may play a role in these instances [30]. These autoantibodies may be oriented against a range of different cell-surface antigens, and several have been linked to particular seizure conditions, including limbic encephalitis. If autoantibodies are detected in cat-scratch disease encephalopathy, immunotherapy in the acute stage, such as injectable immunoglobulins or systemic steroids, may be tried.
1.3. Other Neurological Manifestations
Less-frequent neurologic consequences include meningomyeloradiculopathy, which manifests as paresthesias, paralysis, and sphincter dysfunction in the lower extremities, facial nerve palsy, Guillain–Barré Syndrome [30][31], continuous partial epilepsy [32][33], acute hemiplegia, transverse myelitis [34], and cerebral arteritis [35]. In research by Easley et al., two children were reported who first presented with status epilepticus and normal CSF fluid tests [36].
Multiple-sclerosis-related demyelinating illness [37], acute stroke-related CNS disease [22], transverse myelitis [38], psychiatric conditions [39], and persistent intellectual and locomotor impairments [3] are further types of central nervous system dysfunction.
Facial nerve invasion is quite uncommon in Bartonella infection. Walter et al. [30] described the first instance of acute facial nerve paralysis in a cat-scratch disease patient with characteristic lymphadenitis who was immunologically confirmed.
According to Nakamura et al. [40], a seven-year-old child presented with fever, cervical lymphadenopathy, and peripheral facial nerve palsy, all of which were serologically confirmed to be due to cat-scratch illness. On the left, the stapedius muscle reflex was missing, and magnetic resonance imaging of the brain showed a mass lesion in the left internal auditory meatus. Mutucumarana et al. [41] described a five-year-old girl who had a nearly three-week-long fever, along with exhaustion, headaches, a six-pound weight loss, and late-onset left-sided facial palsy.
Premachandra et al. [42] documented a case of a child who had a parotid lump and a lower motor neurone facial paralysis affecting the marginal mandibular branch of the facial nerve, suggesting a malignant parotid tumor. Additionally, Ganesan et al. [31] described a novel etiology of parotid edema, brief facial paralysis, and mild ptosis. Chiu et al. [43] documented the case of a 10-year-old child who had two weeks of left facial paralysis. The patient presented with left-eye conjunctivitis, facial edema, and weakness, and also had a severe left neck mass. Unilateral facial nerve branch paralysis was discovered during the test. Thompson et al. [44] documented an adult with peripheral facial nerve palsy caused by cat-scratch illness; the patient also had neuroretinitis.
In the report of Rocha et al. [34], a child presented with left hemiplegia and a right frontoparietal lesion on head neuroimaging due to cat-scratch illness. Another report by Cerpa et al. [45] was regarding a child who had transitory right hemiparesis during cat-scratch disease with status epilepticus, a finding consistent with Todd’s paralysis. Furthermore, two cases of cat-scratch-disease-associated vasculitis as the cause of stroke and subsequent hemiparesis have been reported [46]. Vermeulen et al. performed a retrospective analysis and identified 20 instances of cat-scratch disease-related spinal osteomyelitis, one of which had severe transitory paresis [47]. From these findings, it can be concluded that the flaccid paralysis associated with cat-scratch-illness encephalitis may be triggered by a specific contamination of the right hemisphere, postictal Todd’s paralysis, or spinal-cord invasion.
Yep et al. [48] reported on the case of a young woman presenting with meningitis and neuroretinitis who had fever, sweating, a left frontal headache, hazy vision, and painful left-eye movements for five days. A central scotoma decreased color vision, and enlargement of the optic disc and macula were seen in the left eye. The patient reported cat contact a few months prior to the beginning of the symptomatology. A thorough examination of the fundus with a tropicamide-dilated pupil revealed a severely enlarged macula in addition to optic nerve damage.
Anabu et al. described a seven-year-old boy who acquired choreoathetosis after CSD encephalopathy and showed abnormal basal ganglion alterations on an MRI scan. He also developed convulsions with a fluctuating level of consciousness; initially he was still unable to walk or talk, though after several weeks, his condition improved—but without a complete recovery [19].
2. Ocular Manifestations
B. henselae was discovered to be the causal agent of this disease in only the past decade [49]. The infection is believed to be spread through direct conjunctival inoculation. Common symptoms include a foreign-body sensation, unilateral eye redness, serous discharge, and increased tear secretion. Patients usually present necrotic granuloma with conjunctival epithelium ulceration, and also local lymphadenopathy involving the preauricular, submandibular, or cervical lymphatic system [50]. After many weeks, the granuloma often resolves without scarring.
Ocular involvement develops in 5–10% of people with CSD [10]. The eye could serve as the major infection site, resulting in Parinaud oculoglandular syndrome, which is defined by the infection of the conjunctiva and eyelids, as well as local lymphadenopathy (Figure 1). Within 2–3 weeks of the onset of systemic illness, various ocular symptoms might arise. Neuroretinitis, optic neuropathy, and a variety of other kinds of intraocular inflammation are among these symptoms. Regarding the annexal manifestation, Parinaud Syndrome would be representative. Considering vitreous changes, intermediate uveitis and vitreous hemorrhage can be present. Inner retinitis and chorioretinitis can also be included in the retinal and/or choroidal manifestations. The retinal vascular manifestations are retinal vasculitis, angiomatous-like proliferation, retinal arteriolar branch occlusion and retinal vein branch occlusion. The macular complications include serous macular detachment, macular stars (stellar maculopathy), macular edema, and macular hole. The optic-nerve manifestations are considered to be neuroretinitis, optic-disc edema, and optic-nerve head mass.
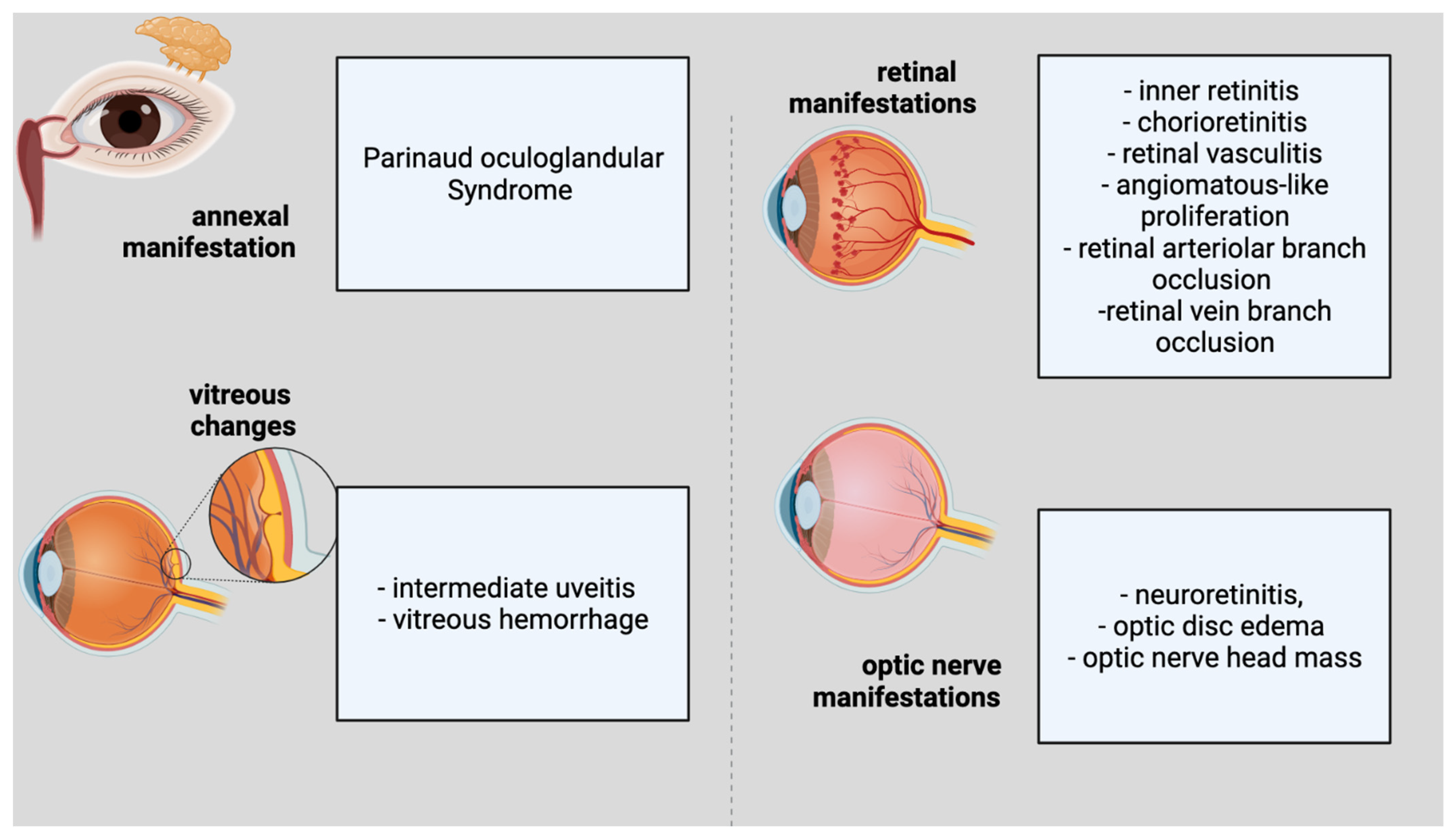 Figure 1. Ophthalmological features of cat scratch disease.
Figure 1. Ophthalmological features of cat scratch disease.The most frequent clinical observations in previous investigations have been neuroretinitis associated with macular stars [51] and/or isolated foci of retinitis or choroiditis [52]. Macular stars can become visible in a few days as vision loss begins and become increasingly apparent over the following two to three weeks. Isolated ocular neuritis was also reported before [53], highlighting the necessity of eliminating infectious agents such as Bartonella henselae prior to initiating therapy with pulse methylprednisolone, particularly in children.
The authors of a Turkish study of 13 eyes of 10 patients with ocular bartonellosis highlighted the variety of ocular symptoms, including an acute endophthalmitis case [53]. Nine patients had a history of cat interaction and systemic symptoms for up to three months before presenting with ocular symptoms, and three had them earlier, but they had initially been misinterpreted as noninfectious intraocular inflammation [53]. The lack of vitreous cells helped to distinguish these lesions from the retinitis/retinal infiltrates observed in Behcet disease or toxoplasmosis, the investigators reported. They advised the careful monitoring of retinal infiltrates simulating cotton-wool patches, since these may progress to a branch retinal artery occlusion [53].
Ocular CSD may potentially manifest as occlusion of the retinal artery, retinal infiltrates resembling cotton-wool exudates, or endophthalmitis [54]. The pathogenesis of retinal infiltrates is most likely related to ischemia caused by the occlusion of the retinal arterioles [55]. It is essential for the ophthalmologist to distinguish the superficial retinal infiltrates in ocular CSD from retinitis or retinal infiltrates seen in individuals with sarcoidosis, Adamantiades-Behcet’s disease, toxoplasmosis or rickettsia infection [53].
Previously, Eiger-Moscovich et al. [56] described the clinical course of BRAO (Branch Retinal Artery Occlusion) in six individuals (seven eyes). One suffered numerous arterial occlusions. BRAO was found in three eyes. In one patient with unilateral BRAO, the other eye had neuroretinitis and, in two cases, both appeared in the ipsilateral eye. BRAO developed focal retinal infiltrates in both eyes or in one eye. A central scotoma left just one eye with low visual acuity (1/60). However, BRAO caused persistent visual field reduction in both eyes [56]. The authors recommended careful history-taking and serologic testing for Bartonella infection, particularly in children with BRAO.
Numerous case reports and series on ophthalmic vascular occlusions as a result of CSD have been published [56][57][58][59]. Surprisingly, the changes and distortions in visual acuity are location-dependent. In some instances of secondary epiretinal membranes, the epiretinal membrane (ERM) spontaneously releases. The therapy, it has been claimed, may promote this occurrence [60][61][62]. The release of the ERM (epiretinal membrane) results in a reduction or elimination of the macula’s tractional pressures, thus allowing the macular hole to close.
When a persistent tractional epiretinal membrane develops (as a consequence of uveitis), even without the formation of a macular hole, pars plana vitrectomy and ERM peeling are the recommended treatment options. In immunocompromised or immunosuppressed individuals, CSD is anticipated to present a more severe systemic effect. It has been observed that, in HIV-positive individuals, it causes bacillary angiomatosis [63].
Best et al. [64] reported a case in which a patient came to the emergency department with a unilateral central scotoma and no prodromal symptoms, a rare presentation for this illness. The patient was afebrile and had no visible skin lesions associated with a cat scratch or bite, nor did she have lymphadenopathy. An ophthalmologic examination revealed findings suggestive of retinal edema.
Suhler et al. [65] discovered that B. henselae infection was the most frequently observed etiology of neuroretinitis in their clinic in the northwestern USA. The degree to which these results apply to certain other areas is unclear.
Tey et al. [66] found that 10 out of 13 patients (76.9%) exhibited posterior segment symptoms, whereas three (23.1%) had Parinaud’s oculoglandular syndrome, lacking the participation of the posterior segment. Eighty-two percent of the eyes had small foci of retinal white lesions. Subretinal fluid (SRF) beneath the fovea was visible on SD-OCT in eight eyes (34.8%), and Parinaud’s oculoglandular syndrome, in four eyes (17.4%).
In the case described by Annoura et al. [67], Parinaud oculoglandular syndrome, anterior uveitis, neuroretinitis, a serous retinal detachment, and a flame-like retinal hemorrhage suggesting retinal vein blockage were all identified. Additionally, the frequency of concurrent eye disorders in the anterior and posterior chambers is notable. Additionally, the present instance was noteworthy in that ocular involvement occurred in the absence of systemic diseases, and neither lymphatic nor systemic disease emerged.
Neuroretinitis
Neuroretinitis is characterized by unilateral vision loss, optic-disc edema, and macular star formation. Given the appearance of the macular star, it was first believed to be a macular disease and was labeled as such [68]. Soon afterwards, according to Gass et al., it was revealed that the macular star was caused by leakage resulting from the optic-disc edema, not by a retinal process in itself, so the optic-nerve edema preceded the macular star [69]. The causes of neuroretinitis are diverse, and the typical components of the triad do not usually manifest simultaneously, especially in the early stages of the illness.
Neuroretinitis is described as inflammation of the optic nerve and peripapillary retina, marked by edema of the optic disc and the eventual development of a macular star. Neuroretinitis is often unilateral and might be bilateral in immunocompetent as well as immunocompromised people [70]. Ocular symptoms often manifest 2–3 weeks following the onset of systemic symptoms. The most prevalent ocular symptom is eyesight loss [71]. A subjective afferent pupillary deficiency, a visual field defect, and dyschromatopsia are often present [71]. On occasion, cells and flares are visible in the anterior chamber, and moderate vitritis is prevalent. The fundoscopic observations often include edema of the optic disc and lipid exudation in the macula in the form of a whole or partial star [72]. A half star shape is most often found in the nasal macula.
In neuroretinitis, the optic disc is the major site of inflammation [69]. At first glance, the macroscopic star could be missing. It typically resolves 1–12 weeks following the beginning of optic-disc edema. The disc edema starts to subside after two weeks and often resolves completely after 8–12 weeks. The key role of nicotinamide adenine dinucleotide (NAD+) in retinal cells’ metabolism might influence the recovery potential of the retina, as already revealed by research regarding degenerative retinal conditions [73]. The macular star disappears after four weeks but could remain for almost a year [74]. The extreme sensitivity of macular cells has to be considered as an important factor in the recovery of the macula [75][76].
Neuroretinitis usually follows a feverish illness with lymphadenopathy, rashes, arthralgia, and headache. The ophthalmologic findings include reduced visual acuity, dyschromatopsia, a relative afferent pupillary deficit, and visual field abnormalities including a cecocentral/central scotoma. However, 2–6 weeks after the onset of symptoms, stellate macula exudates (the ‘macular star’) form. Due to the slow development of this finding, the maculopathy is frequently missed at first presentation [74]. Weeks later, individuals may develop multifocal deep-white retinal lesions and vitreous irritation. Anterior chamber response is uncommon [77]. The macular star may last for months and impede the recovery of visual acuity.
In two previous studies, retinal or choroidal white lesions predominated. According to Solley et al. [52], they were found in the superficial retina (30%), deep retina (49%), full-thickness retina (14%), and choroid (7%). Neovessels were discovered during an OCT angiography of a Bartonella focal chorioretinitis. It may assist in differentiating Bartonella lesions from other noninfectious or infectious fundus lesions because Bartonella spp. have a unique vascular proliferative characteristic [78].
In neuroretinitis, optic-disc edema causes serous retinal detachment, resulting in immediate unilateral vision loss, followed by macular exudates organized in a partial or full star pattern around the fovea [79][80]. OCT showed subretinal fluid in all eyes with Bartonella neuroretinitis, while ophthalmoscopy or fluorescein angiography did not. According to Parikh et al. [81], OCT may be helpful in detecting intraretinal edema associated with optic-disc inflammation preceding serous retinal detachment and subsequent macular-star formation. In 53 patients (62 eyes) with Bartonella optic neuropathy, the authors found that the frequency of optic neuropathy surpassed that of neuroretinitis, and that the lack of a macular star did not rule out optic neuropathy [82]. The majority of patients had unilateral involvement, 58% had prodromal systemic symptoms, and 26% had a history of cat encounters (53%). An exposure history was not required to diagnose ocular bartonellosis, even in areas abounding with cats.
Optic-nerve involvement such as a peripapillary angioma or disc granuloma is uncommon. Ocular granuloma with pineal-gland enlargement, indicating pineoblastoma, has recently been described by Aziz et al. [83]. In this case, enucleation of the globe revealed suppurative granulomatous inflammation surrounded by vascular growth, indicative of Bartonella granuloma [83]. The authors summarized 15 reported instances of optic-disc granulomas associated with CSD [83]. Moreover, Freitas-Neto et al. [84] showed that multimodal fundus imaging assisted in diagnosing CSD in a patient with a peripapillary angiomatous lesion.
3. Therapy
Fundus imaging, optical coherence tomography (OCT), and fluorescence angiography can aid in the diagnosis and treatment of CSD [85]. Late leakage from the optic disc is the most frequently observed finding in FA. Peripapillary angiomatosis and peripapillary serous retinal detachment are additional FA observations [50][86]. Although neuroretinitis is a well-described complication of B. henselae infection, angiomatous lesions remain uncommon.
Habot-Wilner et al. [87][88] have shown the use of retinal OCT in evaluating macular alterations in CSD. The research discovered a lowering of the foveal contour, hypertrophy of the neurosensory retina, and production of subretinal fluid in each of the tested eyes. In the outer plexiform layer, the retinal exudates looked hyperreflective. Follow-up examinations revealed that the macula was normal. The authors also recommended the use of OCT in conjunction with other imaging modalities to monitor patients with CSD neuroretinitis [87][88].
B. henselae-induced ocular inflammation is often benign and self-limiting, with good visual outcomes. However, significant, irreversible visual loss is possible. The indication for therapy varies according to the severity of the condition (Table 1). In vitro, Bartonella henselae is sensitive to a variety of antibiotics [89]. There is no conclusive randomized clinical research demonstrating the effectiveness of antibiotic or corticosteroid therapy for B. henselae infection, but an uncontrolled study indicated the effectiveness of antibiotic therapy [89].
While B. henselae seems to be the most frequently observed infectious cause of neuroretinitis, treatment persists due to the disease’s self-limiting nature. Thus, according to two specialists, the rarity of Bartonella neuroretinitis complicates treatment [90]. Lee et al. believe that antimicrobial treatment reduces the duration of the disease symptoms and that, therefore, it should be treated with antibiotics until the laboratory testing is completed [91].
On the other hand, Bhatti asserts that therapy has no effect on the cure rate [90]. Given the rarity of Bartonella neuroretinitis, recruiting participants for a randomized clinical study would be very challenging and would require multicenter research [92]. Reed et al. [93] found that antibiotics resulted in a faster visual recovery and a shorter duration of illness. Chiu et al. [43] found no difference in visual results between treated and untreated patients (antibiotics, CS, or both). Rostad et al. cured an eight-year-old child of B. henselae with oral doxycycline (60 mg) every 12 h [94].
Chiu et al. found no link between systemic corticosteroid usage and visual results [43]. Meanwhile, Habot-Wilner et al. [87][88] found, in a multicenter retrospective cohort analysis, that a combination antibacterial and systemic corticosteroid regimen was related to a better visual result than an antibiotic-only regimen in patients with BCVA worse than 6/9 at presentation. Kodoma et al. described a case study of 14 patients in Japan, 13 of whom received systemic steroids and two of whom received 1000 mg of methylprednisolone pulse treatment [95].
In Garcia’s case report, oral doxycycline and rifampicin were effectively used to treat an elderly patient with a parotid abscess and aseptic meningitis [96]. The majority of the complications resolve spontaneously, while recovery from central nervous system symptoms might take up to a year [97]. There is insufficient evidence to support the advantages of particular antibiotic treatment in immunocompetent individuals with uncommon CSD manifestations [98]. Numerous antibiotic treatments, particularly gentamicin, trimethoprim/sulfamethoxazole, azithromycin, ciprofloxacin, erythromycin, doxycycline, and rifampin, were utilized in atypical CSD, alone or in various combinations.
Bejarano et al. reported a case in which a child presenting fever and tonic–clonic seizures was effectively treated with an approximately four-month course of azithromycin and rifampin [99].
Taking into consideration the available research, status epilepticus caused by this illness is often resistant to anticonvulsant medication. Even with the prompt initiation of treatment and the usage of various anticonvulsants, seizure management might not be achieved for several hours [100].
Due to their deep penetration of the central nervous system, the majority of researchers and doctors strongly advise a protocol of doxycycline and rifampin for 10–14 days [21], with a treatment course of 2–4 weeks in immunocompetent individuals and four months in immunosuppressed patients, but the antibiotic treatments used may vary in terms of the drug category and length of time. Antiepileptics usually cure the majority of patients within three days to two years [99]. Bogue et al. revealed that clinical signs of systemic CSD rapidly resolved in three critically sick patients following intravenous gentamicin-sulfate therapy [101].
The course of therapy would be determined by the responsiveness and the awareness that, in the normal course of the illness, recuperation from CSDE is typically quick (4–14 days). Collipp [102] noted an outstanding recovery due to trimethoprim–sulfamethoxazole in 11 CSD cases. Along with seizure management and respiratory assistance, the most critical part of treating individuals with encephalopathy is establishing a diagnosis as soon as possible. This may minimize lengthy and unsatisfying investigations that may cause the patient distress. During a coma, supporting measures such as adequate nursing care and constant monitoring of the patient are suggested.
Balakrishnan et al. [20] reported on an 11-year-old girl who suffered from a variety of neurological symptoms, which included migraines, multisensory hallucinations, nervousness, visual impairment affecting both eyes, episodes of generalized paralysis, VII-th nerve palsy, severe sleeping problems, convulsions, extreme fatigue, cognitive deficits, and memory problems. Physiopathologically, originally, B. henselae generated vasculitis, which resulted in subsequent cerebral ischemia, tissue destruction, and surgical excision.
Rosas et al. [103] described a case of cat-scratch-illness encephalitis associated with left-arm flaccid paralysis. The patient manifested an altered mental state and a probable seizure during the acute phase. Initially, hemiparesis was thought to be a sign of Todd’s paralysis. Clinically, the patient recovered on the fifth day of hospitalization, regaining use of her left arm, and recovered completely a week afterwards with doxycycline and levetiracetam treatment.
Raihan et al. reported four instances in which patients had a background of interaction with cats, had a fever prior to developing ocular symptoms, had optic-disc enlargement and macular edema, and tested positive for B. henselae [104].
Zakhour et al. [105] described a 10-year-old girl who had transverse myelitis and Guillain–Barré syndrome as a consequence of cat-scratch illness. Lower-extremity weakness and sensory loss, as well as lower-extremity discomfort, were all associated with the symptomatology. The treatment consisted of doxycycline for 14 days and five days of rifampin and intravenous immunoglobulin, and the case evolution was positive; at the end of the treatment, the patient only presented mild sensory deficits.
In the study conducted by Bilavsky et al. [106], over a period of 11 years, of cat-scratch disease in eight pregnant women, it was found that five of the eight had classic cat-scratch illness, including regional lymphadenitis; two experienced local lymphadenitis accompanied by arthralgia, myalgia, and erythema nodosum; and one had neuroretinitis.
Table 1. Treatment of cat-scratch disease with neuro-ophthalmologic features.
| Author | Site of Lesion | Treatment | Dosage | Time | |
|---|---|---|---|---|---|
| Antibiotics | Corticoids | ||||
| Lee et al. [91] | Nodule of the upper lid | Topical gentamicin and systemic erythromycin | - | N/A | N/A |
| Kodoma et al. [95] | Neuroretinitis | 14 patients: Antibiotics: ciprofloxacin, doxycycline, sulfamethoxazole, erythromycin or cephems |
14 patients: prednisolone, betamethasone, methylprednisolone | N/A | N/A |
| Garcia Garcia et al. [96] | Parotid gland abscess and aseptic meningitis | Doxycycline and rifampicin | - | N/A | Two weeks |
| Canneti et al. [98] | Thirty-nine CSD patients | 31 patients (8 patients with neurologic manifestations of CSD) | 2 patients with neurologic manifestations of CSD | N/A | N/A |
| Bejarano et al. [99] | encephalopathy | Clarithromycin (5 days), cefotaxime (3 days), Meropenem (2 days), Vancomycin (2 days), Piperacillin-tazobactam (5 days), Azithromycin (134 days), rifampin (134 days) | - | Clarithromycin 15 mg/kg/day, cefotaxime 90 mg/kg/4 h, meropenem 40 mg/kg/8 h, vancomycin 10 mg/kg/6 h, piperacillin-tazobactam 80 mg–10 mg/kg/6 h, azithromycin 10 mg/kg/day | NA |
| Armengol et al. [15] | encephalopathy | Erythromycin | - | NA | 5 days |
| Fouch et al. [29] | encephalitis | Cephalexin | - | NA | 7 days |
| Balakrishnan et al. [20] | Vasculitis, cerebral infarction | Azithromycin Ceftriaxone | Azithromycin 500 mg Ceftriaxone 2 g |
Azithromycin 8 weeks Ceftriaxone 8 weeks |
|
| Cerpa et al. [45] | Encephalitis with convulsive status | Ciprofloxacin Cotrimoxazole Rifampicin Azithromycin |
Ciprofloxacin 300 mg × 3/day Cotrimoxazole 110 mg × 3/day Rifampicin 450 mg Azithromycin 350 mg |
Ciprofloxacin two weeks Cotrimoxazole two weeks Rifampicin 4 weeks Azithromycin 4 weeks |
|
| Schuster et al. [21] | Neurologic CSD with hyperactivity | Doxycycline Rifampin | NA | 2 weeks | |
| Rosas et al. [103] | encephalitis associated with left arm flaccid paralysis | Doxycycline | 100 mg × 2/zi | 2 weeks | |
| Bilawsky et al. [106] | Neuroretinitis in pregnant woman | None | |||
| Celiker et al. [70] | Neuroretinitis in three patients | Doxycycline | NA | NA | |
| Raihan et al. [104] | Neuroretinitis in four patients | Azithromycin (3 cases) Doxycycline (1 case) |
Azithromycin 250 mg Doxycycline 200 mg |
Azithromycin 4–6 weeks Doxycycline 4 weeks |
|
| Mutucumarana et al. [41] | VII-th nerve palsy | Azithromycin and rifampin | NA | 2 weeks | |
| Zakhour et al. [105] | Transverse myelitis and Guillain-Barré syndrome | Ceftriaxone, vancomycin, doxycycline | Ceftriaxone and vancomycin a few days; Doxycycline 2 weeks |
||
| Fouch et al. [29] | Disseminated Bartonella henselae | Cephalexin | NA | ||
| Farooque et al. [27] | Persistent focal seizures and encephalopathy | NA | 4 weeks | ||
| Pinto et al. [107] | aseptic meningitis and neuroretinitis | Azythromicin, Doxycicline, Rifampin | Azythromicin 500 mg; Doxycicline 100 mg; Rifampin 300 mg |
Azythromicin a few days; Doxycicline and Rifampin a month | |
This disease is especially concerning when it occurs in pregnancy because the course of cat-scratch disease is longer and frequently febrile, lasting weeks or months. B. henselae DNA was found in postmortem cells of a neonate delivered by a mother who had been diagnosed with B. henselae infection; the baby died nine days after birth. These findings indicate that B. henselae infection could be detrimental to the fetus, explicitly or implicitly, and might even present a danger of horizontal transmission [3].
4. Conclusions
By and large, medical information concerning neurobartonellosis is restricted to case reports and case series, and a clear molecular mechanism behind the emergence of symptoms and pathogenesis of the lesions has not been discovered.
Neurological and ophthalmological symptoms and syndromes can develop at the same time. The period between the beginning of CSD and the commencement of encephalopathy is between days and two months. Encephalopathy is often signaled by disorientation, which might be followed by a decreased state of awareness, varying from sleepiness to a comatose state. Around 50% of individuals have seizures, which vary from localized, self-limited clonic convulsions to status epilepticus. Aphasia, hemiparesis, cranial nerve palsy, and ataxia are all examples of focal neurological symptoms that occur in a minority of individuals. Despite Bartonella henselae infrequently generating encephalopathy and meningitis, a favorable prognosis following antibiotic therapy requires clinicians to consider them in routine practice.
Infected individuals may have a variety of visual symptoms of Bartonella infections. Neuroretinitis, optic neuritis, focal retinitis, choroiditis, chorioretinitis, exudative maculopathy, serous retinal detachment, and vitritis have all been described in the literature. Other ocular issues, such as branch retinal artery blockage, macular holes, and peripapillary angiomatosis, have also been reported. There have also been reports of conjunctival symptoms such as Parinaud’s oculoglandular syndrome and nonspecific follicular conjunctivitis.
B. henselae is the most often identified cause of neuroretinitis, with about two-thirds of patients exhibiting serologic evidence of prior B. henselae infection. Patients with ocular bartonellosis may also present with a variety of posterior segment symptoms, such as posterior uveitis, neuroretinitis, optic neuritis/papillitis, optic disc granuloma, retinal vasculitis, retinal venous or arteriolar occlusions, angiomatosis, acute multifocal inner retinitis or retinal white-dot syndrome and/or retinal infiltrates.
Whenever a patient presents with injected conjunctivae and granulomatous follicular conjunctivitis, the possibility of Parinaud’s oculoglandular syndrome must be considered, especially if the patient has indicated interaction with cats or kittens.
References
- Opavsky, M.A. Cat scratch disease: The story continues. Can. J. Infect. Dis. 1997, 8, 43–49.
- Seals, J.E.; Oken, H.A. Cat Scratch Encephalopathy. Md Med. J. 1999, 48, 176–178.
- Breitschwerdt, E.B.; Mascarelli, P.E.; Schweickert, L.A.; Maggi, R.G.; Hegarty, B.C.; Bradley, J.M.; Woods, C.W. Hallucinations, sensory neuropathy, and peripheral visual deficits in a young woman infected with Bartonella koehlerae. J. Clin. Microbiol. 2011, 49, 3415–3417.
- Vayssier-Taussat, M.; Le Rhun, D.; Deng, H.K.; Biville, F.; Cescau, S.; Danchin, A.; Marignac, G.; Lenaour, E.; Boulouis, H.J.; Mavris, M.; et al. The Trw type IV secretion system of Bartonella mediates host-specific adhesion to erythrocytes. PLoS Pathog. 2010, 6, e1000946.
- Pulliainen, A.T.; Dehio, C. Bartonella henselae: Subversion of vascular endothelial cell functions by translocated bacterial effector proteins. Int. J. Biochem. Cell Biol. 2009, 41, 507–510.
- Klein, J.D. Cat scratch disease. Pediatr. Rev. 1994, 15, 348–353.
- Lyon, L.W. Neurologic manifestations of cat-scratch disease. Report of a case and review of the literature. Arch. Neurol. 1971, 25, 23–27.
- Shinall, E.A. Cat-scratch disease: A review of the literature. Pediatr. Dermatol. 1990, 7, 11–18.
- Reynolds, M.G.; Holman, R.C.; Curns, A.T.; O’Reilly, M.; McQuiston, J.H.; Steiner, C.A. Epidemiology of cat-scratch disease hospitalizations among children in the United States. Pediatr. Infect. Dis. J. 2005, 24, 700–704.
- Carithers, H.A. Cat-scratch disease: An overview based on a study of 1200 patients. Am. J. Dis. Child. 1985, 139, 1124.
- Seah, A.B.H.; Azran, M.S.; Rucker, J.C.; Biousse, V.; Martin, D.F.; Newman, N.J. Magnetic resonance imaging abnormalities in cat-scratch disease encephalopathy. J. Neuroophthalmol. 2003, 23, 16–21.
- Gerber, J.E.; Johnson, J.E.; Scott, M.A.; Madhusudhan, K.T. Fatal meningitis and encephalitis due to Bartonella henselae bacteria. J. Forensic Sci. 2002, 47, 640–644.
- Swedo, S.E.; Seidlitz, J.; Kovacevic, M.; Latimer, M.E.; Hommer, R.; Lougee, L.; Grant, P. Clinical presentation of pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections in research and community settings. J. Child Adolesc. Psychopharmacol. 2015, 25, 26–30.
- Harms, A.; Dehio, C. Intruders below the radar: Molecular pathogenesis of Bartonella spp. Clin. Microbiol. Rev. 2012, 25, 42–78.
- Armengol, C.E.; Hendley, J.O. Cat-scratch disease encephalopathy: A cause of status epilepticus in school-aged children. J. Pediatr. 1999, 134, 635–638.
- Stevens, H. Cat-scratch fever encephalitis. AMA Am. J. Dis. Child. 1952, 84, 218–222.
- Wheeler, S.W.; Wolf, S.M.; Steinberg, E.A. Cat-scratch encephalopathy. Neurology 1997, 49, 876–878.
- Genizi, J.; Kasis, I.; Schif, A.; Shahar, E. Effect of high-dose methyl-prednisolone on brainstem encephalopathy and basal ganglia impairment complicating cat scratch disease. Brain Dev. 2007, 29, 377–379.
- Anbu, A.T.; Foulerton, M.; McMaster, P.; Bakalinova, D. Basal ganglia involvement in a child with cat-scratch disease. Pediatr. Infect. Dis. J. 2003, 22, 931–932.
- Balakrishnan, N.; Ericson, M.; Maggi, R.; Breitschwerdt, E.B. Vasculitis, cerebral infarction and persistent Bartonella henselae infection in a child. Parasit. Vectors 2016, 9, 254.
- Schuster, A.L.; Honeycutt, T.C.B.; Hamrick, H.J. Status epilepticus due to cat scratch disease: Recognition, diagnosis, and thoughts on pathogenesis. Pediatr. Emerg. Care 2016, 32, 789–791.
- Marienfeld, C.B.; Dicapua, D.B.; Sze, G.K.; Goldstein, J.M. Expressive aphasia as a presentation of encephalitis with Bartonella henselae infection in an immunocompetent adult. Yale J. Biol. Med. 2010, 83, 67–71.
- Andrews, P.I.; McNamara, J.O. Rasmussen’s encephalitis: An autoimmune disorder? Curr. Opin. Neurol. 1996, 9, 141–145.
- Kolski, H.; Ford-Jones, E.L.; Richardson, S.; Petric, M.; Nelson, S.; Jamieson, F.; Blaser, S.; Gold, R.; Otsubo, H.; Heurter, H.; et al. Etiology of acute childhood encephalitis at The Hospital for Sick Children, Toronto, 1994–1995. Clin. Infect. Dis. 1998, 26, 398–409.
- Marra, M. Neurologic complications of Bartonella henselae infection Christina. Curr. Opin. Neurol. 1995, 8, 164–169.
- Baylor, P.; Garoufi, A.; Karpathios, T.; Lutz, J.; Mogelof, J.; Moseley, D. Transverse myelitis in 2 patients with Bartonella henselae infection (cat scratch disease). Clin. Infect. Dis. 2007, 45, e42–e45.
- Farooque, P.; Khurana, D.S.; Melvin, J.J. Persistent focal seizures after cat scratch encephalopathy. Pediatr. Neurol. 2010, 42, 215–218.
- Cherinet, Y.; Tomlinson, R. Cat scratch disease presenting as acute encephalopathy. Emerg. Med. J. 2008, 25, 703–704.
- Fouch, B.; Coventry, S. A case of fatal disseminated Bartonella henselae infection (cat-scratch disease) with encephalitis. Arch. Pathol. Lab. Med. 2007, 131, 1591–1594.
- Walter, R.S.; Eppes, S.C. Cat scratch disease presenting with peripheral facial nerve paralysis. Pediatrics 1998, 101, E13.
- Ganesan, K.; Mizen, K. Cat scratch disease: An unusual cause of facial palsy and partial ptosis: Case report. J. Oral Maxillofac. Surg. 2005, 63, 869–872.
- Nowakowski, G.S.; Katz, A. Epilepsia partialis continua as an atypical presentation of cat scratch disease in a young adult. Neurology 2002, 59, 1815–1816.
- Puligheddu, M.; Giagheddu, A.; Genugu, F.; Giagheddu, M.; Marrosu, F. Epilepsia partialis continua in cat scratch disease. Seizure 2004, 13, 191–195.
- Rocha, J.L.; Pellegrino, L.N.; Riella, L.V.; Martins, L.T. Acute hemiplegia associated with cat-scratch disease. Braz. J. Infect. Dis. 2004, 8, 263–266.
- Selby, G.; Walker, G.L. Cerebral arteritis in cat-scratch disease. Neurology 1979, 29, 1413–1418.
- Easley, R.B.; Cooperstock, M.S.; Tobias, J.D. Cat-scratch disease causing status epilepticus in children. South. Med. J. 1999, 92, 73–76.
- Brinar, V.V.; Habek, M. Rare infections mimicking MS. Clin. Neurol. Neurosurg. 2010, 112, 625–628.
- Salgado, C.D.; Weisse, M.E. Transverse myelitis associated with probable cat-scratch disease in a previously healthy pediatric patient. Clin. Infect. Dis. 2000, 31, 609–611.
- Schaller, J.L.; Burkland, G.A.; Langhoff, P.J. Do bartonella infections cause agitation, panic disorder, and treatment-resistant depression? MedGenMed 2007, 9, 54.
- Nakamura, C.; Inaba, Y.; Tsukahara, K.; Mochizuki, M.; Sawanobori, E.; Nakazawa, Y.; Aoyama, K. A pediatric case with peripheral facial nerve palsy caused by a granulomatous lesion associated with cat scratch disease. Brain Dev. 2018, 40, 159–162.
- Mutucumarana, C.P.; Kumar, R.D.; Foradori, D.M.; Ramgopal, V.; Demmler, G.J. Facial nerve palsy in a child with fever of unknown origin. Clin. Pediatr. 2020, 59, 516–518.
- Premachandra, D.J.; Milton, C.M. Cat scratch disease in the parotid gland presenting with facial paralysis. Br. J. Oral Maxillofac. Surg. 1990, 28, 413–415.
- Chiu, A.G.; Hecht, D.A.; Prendiville, S.A.; Mesick, C.; Mikula, S.; Deeb, Z.E. Atypical presentations of cat scratch disease in the head and neck. Otolaryngol. Head Neck Surg. 2001, 125, 414–416.
- Thompson, P.K.; Vaphiades, M.S.; Saccente, M. Cat-scratch disease presenting as neuroretinitis and peripheral facial palsy. J. Neuroophthalmol. 1999, 19, 240–241.
- Cerpa Polar, R.; Orellana, G.; Silva Caso, W.; Sánchez Carbonel, J.; Santisteban, J.; Del Valle Mendoza, J.; Santisteban, J. Encephalitis with convulsive status in an immunocompetent pediatric patient caused by Bartonella henselae. Asian Pac. J. Trop. Med. 2016, 9, 610–613.
- Lewis, D.W.; Tucker, S.H. Central nervous system involvement in cat scratch disease. Pediatrics 1986, 77, 714–721.
- Vermeulen, M.J.; Rutten, G.J.; Verhagen, I.; Peeters, M.F.; van Dijken, P.J. Transient paresis associated with cat-scratch disease: Case report and literature review of vertebral osteomyelitis caused by Bartonella henselae: Case report and literature review of vertebral osteomyelitis caused by Bartonella henselae. Pediatr. Infect. Dis. J. 2006, 25, 1177–1181.
- Yap, S.M.; Saeed, M.; Logan, P.; Healy, D.G. Bartonella neuroretinitis (cat-scratch disease). Pract. Neurol. 2020, 20, 505–506.
- Grando, D.; Sullivan, L.J.; Flexman, J.P.; Watson, M.W.; Andrew, J.H. Bartonella henselae associated with Parinaud’s oculoglandular syndrome. Clin. Infect. Dis. 1999, 28, 1156–1158.
- Cunningham, E.T.; Koehler, J.E. Ocular bartonellosis. Am. J. Ophthalmol. 2000, 130, 340–349.
- Ormerod, L.D.; Dailey, J.P. Ocular manifestations of cat-scratch disease. Curr. Opin. Ophthalmol. 1999, 10, 209–216.
- Solley, W.A.; Martin, D.F.; Newman, N.J.; King, R.; Callanan, D.G.; Zacchei, T.; Wallace, R.T.; Parks, D.J.; Bridges, W.; Sternberg, P., Jr. Cat scratch disease: Posterior segment manifestations. Ophthalmology 1999, 106, 1546–1553.
- Oray, M.; Önal, S.; Koç Akbay, A.; Tuğal Tutkun, İ. Diverse clinical signs of ocular involvement in cat scratch disease. Turk. J. Ophthalmol. 2017, 47, 9–17.
- Amer, R.; Tugal-Tutkun, I. Ophthalmic manifestations of bartonella infection. Curr. Opin. Ophthalmol. 2017, 28, 607–612.
- Chomel, B.B.; Kasten, R.W.; Floyd-Hawkins, K.; Chi, B.; Yamamoto, K.; Roberts-Wilson, J.; Gurfield, A.N.; Abbott, R.C.; Pedersen, N.C.; Koehler, J.E. Experimental transmission of Bartonella henselae by the cat flea. J. Clin. Microbiol. 1996, 34, 1952–1956.
- Eiger-Moscovich, M.; Amer, R.; Oray, M.; Tabbara, K.F.; Tugal-Tutkun, I.; Kramer, M. Retinal artery occlusion due to Bartonella henselae infection: A case series. Acta Ophthalmol. 2016, 94, e367–e370.
- Cohen, S.M.; Davis, J.L.; Gass, D.M. Branch retinal arterial occlusions in multifocal retinitis with optic nerve edema. Arch. Ophthalmol. 1995, 113, 1271–1276.
- Batsos, G.; Kabanarou, S.A.; Fotiou, P.; Rouvas, A.; Xirou, T. Retinal arterial occlusive disease in a young patient with cat scratch disease. Case Rep. Ophthalmol. 2013, 4, 87–92.
- Gray, A.V.; Michels, K.S.; Lauer, A.K.; Samples, J.R. Bartonella henselae infection associated with neuroretinitis, central retinal artery and vein occlusion, neovascular glaucoma, and severe vision loss. Am. J. Ophthalmol. 2004, 137, 187–189.
- Schadlu, R.; Apte, R.S. Spontaneous resolution of an inflammation-associated epiretinal membrane with previously documented posterior vitreous detachment. Br. J. Ophthalmol. 2007, 91, 1252–1253.
- Andreev, A.N.; Bushuev, A.V.; Svetozarskiy, S.N. A case of secondary epiretinal membrane spontaneous release. Case Rep. Ophthalmol. Med. 2016, 2016, 4925763.
- Ozgonul, C.; Besirli, C.G. Macular hole closure following spontaneous release of vitreomacular traction. BMJ Case Rep. 2017, 2017, bcr2016218547.
- Warren, K.; Goldstein, E.; Hung, V.S.; Koehler, J.E.; Richardson, W. Use of retinal biopsy to diagnose Bartonella (formerly Rochalimaea) henselae retinitis in an HIV-infected patient. Arch. Ophthalmol. 1998, 116, 937–940.
- Best, J.A.; Price, B. Central scotoma without prodromal illness caused by Bartonella henselae neuroretinitis. Am. J. Emerg. Med. 2013, 31, 887.e1–887.e2.
- Suhler, E.B.; Lauer, A.K.; Rosenbaum, J.T. Prevalence of serologic evidence of cat scratch disease in patients with neuroretinitis11The authors have no proprietary interest in the products mentioned herein. Ophthalmology 2000, 107, 871–876.
- Tey, M.S.-Y.; Govindasamy, G.; Vendargon, F.M. The clinical spectrum of ocular bartonellosis: A retrospective study at a tertiary centre in Malaysia. J. Ophthalmic Inflamm. Infect. 2020, 10, 31.
- Annoura, K.; Sano, I.; Makino, S.; Kawashima, H. Multiple ocular manifestations in a case of cat scratch disease without systemic signs. GMS Ophthalmol. Cases 2020, 10, Doc45.
- Rodríguez-Castelblanco, Á.; Cordero-Coma, M. Leber’s idiopathic stellate neuroretinitis: Diagnostic and therapeutic conflicts. Arch. Soc. Esp. Oftalmol. (Engl. Ed.) 2019, 94, 413–416.
- Gass, J.D. Diseases of the optic nerve that may simulate macular disease. Trans. Sect. Ophthalmol. Am. Acad. Ophthalmol. Otolaryngol. 1977, 83, 763–770.
- Celiker, H.; Kazokoglu, H.; Eraslan, M.; Cerman, E.; Karabas, L. Bartonella henselae Neuroretinitis in Patients without Cat Scratch. Jpn. J. Infect. Dis. 2018, 71, 397–401.
- Rabina, G.; Goldstein, M. Cat scratch disease, ocular manifestations. In Encyclopedia of Ophthalmology; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–3. ISBN 9783642359514.
- Williams, D.M.; Schachter, J. Role of cell-mediated immunity in chlamydial infection: Implications for ocular immunity. Rev. Infect. Dis. 1985, 7, 754–759.
- Pîrvu, A.S.; Andrei, A.M.; Stănciulescu, E.C.; Baniță, I.M.; Pisoschi, C.G.; Jurja, S.; Ciuluvica, R. NAD+ metabolism and retinal degeneration (Review). Exp. Ther. Med. 2021, 22, 670.
- Ray, S.; Gragoudas, E. Neuroretinitis. Int. Ophthalmol. Clin. 2001, 41, 83–102.
- Jurja, S.; Hîncu, M.; Dobrescu, M.A.; Golu, A.E.; Bălăşoiu, A.T.; Coman, M. Ocular cells and light: Harmony or conflict? Rom. J. Morphol. Embryol. 2014, 55, 257–261.
- Jurja, S.; Coman, M.; Hîncu, M.C. The ultraviolet influence upon soft eye tissues. Rom. J. Morphol. Embryol. 2017, 58, 45–52.
- Maitland, C.G.; Miller, N.R. Neuroretinitis. Arch. Ophthalmol. 1984, 102, 1146–1150.
- Dehio, C. Bartonella-host-cell interactions and vascular tumour formation. Nat. Rev. Microbiol. 2005, 3, 621–631.
- Purvin, V.; Sundaram, S.; Kawasaki, A. Neuroretinitis: Review of the literature and new observations. J. Neuroophthalmol. 2011, 31, 58–68.
- Kahloun, R.; Khairallah-Ksiaa, I.; Abroug, N.; Mahmoud, A.; Ben Yahia, S.; Zaouali, S.; Jelliti, B.; Khairallah, M. Final diagnosis in patients referred with a diagnosis of neuroretinitis. Neuroophthalmology 2015, 39, 266–270.
- Parikh, P.C.; Brucker, A.J.; Tamhankar, M.A. An atypical presentation of Bartonella neuroretinitis. Retina 2016, 36, e18–e19.
- Chi, S.L.; Stinnett, S.; Eggenberger, E.; Foroozan, R.; Golnik, K.; Lee, M.S.; Bhatti, M.T. Clinical characteristics in 53 patients with cat scratch optic neuropathy. Ophthalmology 2012, 119, 183–187.
- Aziz, H.A.; Plesec, T.P.; Sabella, C.; Udayasankar, U.K.; Singh, A.D. Cat scratch disease: Expanded spectrum. Ocul. Oncol. Pathol. 2016, 2, 246–250.
- Freitas-Neto, C.A.; Oréfice, F.; Costa, R.A.; Oréfice, J.L.; Dhanireddy, S.; Maghsoudlou, A.; Foster, C.S. Multimodal imaging assisting the early diagnosis of cat-scratch neuroretinitis. Semin. Ophthalmol. 2016, 31, 495–498.
- McClintic, J.; Srivastava, S.K. Imaging in the diagnosis and management of ocular cat scratch disease. Int. Ophthalmol. Clin. 2012, 52, 155–161.
- Fish, R.H.; Hogan, R.N.; Nightingale, S.D.; Anand, R. Peripapillary angiomatosis associated with cat-scratch neuroretinitis. Arch. Ophthalmol. 1992, 110, 323.
- Habot-Wilner, Z.; Tiosano, L.; Sanchez, J.M.; Shulman, S.; Barequet, D.; Rahat, O.; Amarilyo, G.; Amer, R. Demographic and clinical features of pediatric uveitis in Israel. Ocul. Immunol. Inflamm. 2020, 28, 43–53.
- Habot-Wilner, Z.; Zur, D.; Goldstein, M.; Goldenberg, D.; Shulman, S.; Kesler, A.; Giladi, M.; Neudorfer, M. Macular findings on optical coherence tomography in cat-scratch disease neuroretinitis. Eye 2011, 25, 1064–1068.
- Conrad, D.A. Treatment of cat-scratch disease. Curr. Opin. Pediatr. 2001, 13, 56–59.
- Bhatti, M.T.; Lee, M.S. Should patients with bartonella neuroretinitis receive treatment? J. Neuroophthalmol. 2014, 34, 412–416.
- Lee, W.R.; Chawla, J.C.; Reid, R. Bacillary angiomatosis of the conjunctiva. Am. J. Ophthalmol. 1994, 118, 152–157.
- Prutsky, G.; Domecq, J.P.; Mori, L.; Bebko, S.; Matzumura, M.; Sabouni, A.; Shahrour, A.; Erwin, P.J.; Boyce, T.G.; Montori, V.M.; et al. Treatment outcomes of human bartonellosis: A systematic review and meta-analysis. Int. J. Infect. Dis. 2013, 17, e811–e819.
- Reed, J.B.; Scales, D.K.; Wong, M.T.; Lattuada, C.P., Jr.; Dolan, M.J.; Schwab, I.R. Bartonella henselae neuroretinitis in cat scratch disease. Diagnosis, management, and sequelae. Ophthalmology 1998, 105, 459–466.
- Rostad, C.A.; McElroy, A.K.; Hilinski, J.A.; Thompson, M.P.; Drew, C.P.; Denison, A.M.; Zaki, S.R.; Mahle, W.T.; Rogers, J.; Abramowsky, C.R.; et al. Bartonella henselae-mediated disease in solid organ transplant recipients: Two pediatric cases and a literature review. Transpl. Infect. Dis. 2012, 14, E71–E81.
- Kodama, T.; Masuda, H.; Ohira, A. Neuroretinitis associated with cat-scratch disease in Japanese patients: Acta Ophthalmologica Scandinavica 2003. Acta Ophthalmol. Scand. 2003, 81, 653–657.
- García, J.C.; Núñez, M.J.; Castro, B.; Fernández, J.M.; Portillo, A.; Oteo, J.A. Hepatosplenic cat scratch disease in immunocompetent adults: Report of 3 cases and review of the literature. Medicine 2014, 93, 267–279.
- Florin, T.A.; Zaoutis, T.E.; Zaoutis, L.B. Beyond cat scratch disease: Widening spectrum of Bartonella henselae infection. Pediatrics 2008, 121, e1413–e1425.
- Canneti, B.; Cabo-López, I.; Puy-Núñez, A.; García García, J.C.; Cores, F.J.; Trigo, M.; Suárez-Gil, A.P.; Rodriguez-Regal, A. Neurological presentations of Bartonella henselae infection. Neurol. Sci. 2019, 40, 261–268.
- Peláez Bejarano, A.; Sánchez Del Moral, R.; Guisado-Gil, A.B. Bartonella henselae encephalopathy in a paediatric patient: A case report and treatment review. J. Clin. Pharm. Ther. 2020, 45, 840–844.
- Atıcı, S.; Kadayıfcı, E.K.; Karaaslan, A.; Toper, M.H.; Celikel, C.A.; Soysal, A.; Bakır, M. Atypical presentation of cat-scratch disease in an immunocompetent child with serological and pathological evidence. Case Rep. Pediatr. 2014, 2014, 397437.
- Bogue, C.W. Antibiotic therapy for cat-scratch disease? Pediatr. Infect. Dis. J. 1990, 9, 69.
- Collipp, P.J. Cat-scratch disease therapy. Arch. Pediatr. Adolesc. Med. 1989, 143, 1261.
- Rosas, L.; Rao, K.; McGough, C.; Becker, A. A rare case of Bartonella encephalitis with hemiplegia. Child Neurol. Open 2019, 6.
- Raihan, A.-R.; Zunaina, E.; Wan-Hazabbah, W.-H.; Adil, H.; Lakana-Kumar, T. Neuroretinitis in ocular bartonellosis: A case series. Clin. Ophthalmol. 2014, 8, 1459–1466.
- Zakhour, R.; Mancias, P.; Heresi, G.; Pérez, N. Transverse myelitis and Guillain-Barré syndrome associated with cat-scratch disease, Texas, USA, 2011. Emerg. Infect. Dis. 2018, 24, 1754–1755.
- Bilavsky, E.; Amit, S.; Avidor, B.; Ephros, M.; Giladi, M. Cat scratch disease during pregnancy. Obstet. Gynecol. 2012, 119, 640–644.
- Pinto, V.L., Jr.; Curi, A.L.; Pinto, A.D.S.; Nunes, E.P.; Teixeira, M.D.L.B.; Rozental, T.; Favacho, A.R.; de Lemos, E.R.S.; Bóia, M.N. Cat scratch disease complicated with aseptic meningitis and neuroretinitis. Braz. J. Infect. Dis. 2008, 12, 158–160.
More
Information
Subjects:
Ophthalmology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
4.0K
Revisions:
2 times
(View History)
Update Date:
21 Feb 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





