Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Agnieszka Barbara Najda | + 1132 word(s) | 1132 | 2022-01-29 08:39:44 | | | |
| 2 | Nora Tang | -48 word(s) | 1084 | 2022-02-17 03:55:01 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Najda, A. Health Attributes of Trametes versicolor. Encyclopedia. Available online: https://encyclopedia.pub/entry/19543 (accessed on 07 February 2026).
Najda A. Health Attributes of Trametes versicolor. Encyclopedia. Available at: https://encyclopedia.pub/entry/19543. Accessed February 07, 2026.
Najda, Agnieszka. "Health Attributes of Trametes versicolor" Encyclopedia, https://encyclopedia.pub/entry/19543 (accessed February 07, 2026).
Najda, A. (2022, February 16). Health Attributes of Trametes versicolor. In Encyclopedia. https://encyclopedia.pub/entry/19543
Najda, Agnieszka. "Health Attributes of Trametes versicolor." Encyclopedia. Web. 16 February, 2022.
Copy Citation
Several bioactive compounds are present in the mushroom, and they attribute effective therapeutic and medicinal values to humans. Traditionally, Trametes versicolor has been consumed for its potential health attributes, and researchers have explored the health attributes of the mushroom using several in vitro, in vivo, and chemical methods [31,34]. Herein, the bioactive components responsible for the therapeutic importance are described.
mushroom
Trametes versicolor
bioactivity
health attributes
anti-inflammation
1. Role of Polysaccharopeptides as Prebiotics
Prebiotics are nondigestible ingredients of food that affect the host beneficially by promoting the growth of beneficial bacteria of the colon and improves the immunity of the host, and the intake of mushrooms as food enhances the growth of probiotic bacteria in the large intestine; therefore, it protects the human body from viral infections and inhibits the growth of disease-causing bacteria such as E. coli, Clostridium, and Salmonella (Figure 1) [1][2].
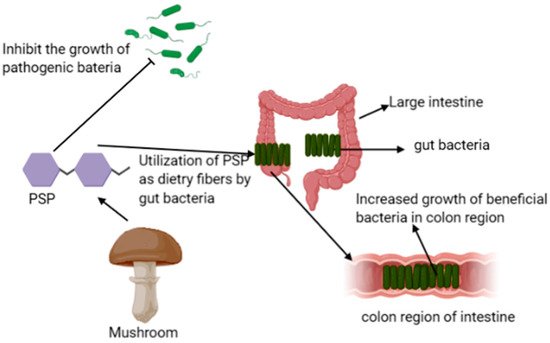
Figure 1. Possible mechanism of PSP of Trametes versicolor as prebiotics.
Edible mushrooms consist of pleuran, lentinan, schizophyllan, α and β-glucans, mannans, chitin, hemicellulose, galactans, and xylans polysaccharides that possess prebiotic effects. These polysaccharides increase the resistance to the intestinal mucosa and ulcer formation in the intestines of rats. In this regard, Yu et al. reported the effects of polysaccharopeptides (PSP) isolated from Trametes versicolor on the growth and activity of the probiotic bacteria Bifidobacteria and Lactobacilli [3]. The polysaccharides of Trametes versicolor possess different chemical constituents, including β-glucans as the principal components that are not easily digested by the intestinal enzymes. These polysaccharides, due to their non-starch and nondigestible natures, can be utilized as dietary fibers by gut probiotic bacteria in the large intestine and result in increased growth of these bacteria in the colon region, thereby providing health benefits, and are considered as potential prebiotics [2][4]. However, complete information regarding the ability of β-glycans of mushrooms to inhibit the composition of fecal microbes has not been investigated.
2. Antidiabetic and Antiobesity Properties
Type 2 diabetes results in chronic diseases, including diabetic nephropathy (disease related to the kidney), diabetic retinopathy (disease related to eyes), and diabetic neuropathy (nontraumatic lower-limb amputations). Due to continuous changes in the eating behavior and activity level of human beings, the incidence of type 2 diabetes is increasing continuously [5]. The disease results due to impaired secretion and resistance of insulin inside the body. The major risk effect for insulin resistance is obesity, and a strong relationship between these two factors has been reported in both animal and human studies [6]. Therefore, improvements in obesity result in the improvement of insulin sensitivity and the prevention of type 2 diabetes. Ternatin is a highly methylated cyclic heptapeptide.
This compound inhibits the effect of fat accumulation, which was studied using 3T3-L1 adipocytes [7]. According to Figure 2, ternatin reduced the mRNA levels of CCAAT/enhancer-binding protein-a (C/EBP-a and sterol regulatory element-binding protein-1c (SREBP-1c)) at an early stage of differentiation in preadipocytes; this results in the suppression of peroxisome proliferative-activated receptors c (PPAR-cmRNA) [8]. The suppression results in the reduction of messenger RNA levels of adipocyte fatty acid-binding protein (aP2), lipoprotein lipase, FAS (fatty acid synthase), and ACC2 (acetyl-CoA carboxylase) due to the cellular lipid accumulation becoming reduced [9]. The consumption of ternatin also reduced triglyceride synthesis, but its effectiveness is lower in the differentiation of adipocytes than observed in preadipocytes. D-Leu7 is a derivative of ternatin that is demonstrated to inhibit the accumulation of fats in 3T3-L1 cells and is eight times more effective with a 12-fold higher cytotoxic activity (IC50) than that of ternatin [10]. Kobayashi et al., in their study, reported that [D-Leu7] ternatin acts by suppressing the expression of lipogenic genes in diabetic Kuo Kondo yellow obese (KK-A(y)) mice and inhibits the accumulation of fats in cultured adipocytes [11]. They also reported that ternatin and its derivative compound can be a valuable drug for the treatment of obesity. The finding of this study proved that the compound present in mushrooms significantly (p < 0.05) lowers the glucose and triglycerides. Furthermore, Xian et al. reported the effect of an aqueous extract of mushrooms in improving the insulin resistance with the Pl3K/Akt and p38 MAPK signaling pathways, which are involved in diabetic skeletal muscles [12]. In their study, they induced insulin-resistant rat models by T2DM with HFD feeding and STZ injection for the manifestation of serious metabolic diseases that exhibit the characteristics of hyperglycemia, hypertension, and dyslipidemia. The insulin-resistant rats in their study displayed certain changes in their bodies that included an increase in venous blood glucose and physiological indexes like the body weight, intake of water, and intake of food. When these rats were treated with mushroom extract in different concentrations of the dosage, the physiological index and glucose level improved compared to the untreated T2DM. In their experiment, it was revealed for the first time that a direct demonstration of the mushroom extract could improve the level of glucose in skeletal muscles. The antiobesity effect of the polysaccharides obtained from Trametes versicolor was investigated by in vivo methods, and it was observed that the polysaccharides triggered the splenocytes of mice via the MAPK-NF-κB signaling pathway that induced an immunomodulatory effect [13].
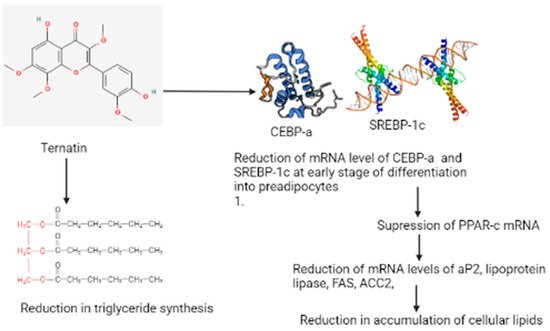
Figure 2. Possible mechanism of the antidiabetic and antiobesity properties of the ternatin compound isolated from Trametes versicolor.
3. Anti-Inflammatory Properties
Inflammation is, although a defensive phenomenon in the body with insufficient regulation and unsuitable in the disparity of self-tissue, could be the reason for severe diseases and injuries [14]. Trametes versicolor consists of bioactive compounds, including terpenoids and sterols, with anti-inflammatory properties. The effects of these compounds were evaluated primarily on the secretion of cytokines nitric oxide, TNF α, and IL-6 in RAW 264.7 cells by Jin et al. [15]. In their study, they found that LPS induced the production of tumor-associated genes cytokines TNF α and IL-6 in RAW 264.7. The cytokines TNF α and IL-6 are inflammatory mediators and are responsible for inflammation. The activity of these mediators was suppressed by the bioactive compounds obtained from Trametes versicolor. For instance, 5α,8α-epidioxy-22E-ergosta-6 suppress the production of TNF α induced by LPS, while ursolic acid, corosolic acids, 2E-7α-methoxy-5α,6α-epoxyergosta-8, ergosta-7,22-dien-3β-ol, and dihydrouridine showed an inhibitory effect of IL-6 induced by LPS (Figure 3). Furthermore, Kojic et al., in their study, treated grape pomace with Trametes versicolor for 15 days under solid-state fermentation (SSF) to enhance the anti-inflammatory properties, and they observed an increase of flavan-3-ol and the flavonol rutin within 1–4 days of treatment [16].
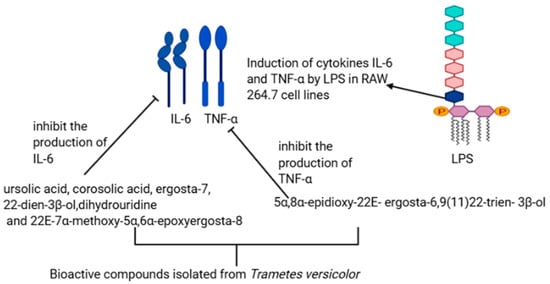
Figure 3. Possible mechanism for the anti-inflammatory activity of bioactive compounds isolated from the extract of Trametes versicolor.
The SSF of grape pomace with mushroom increased the inhibition of enzymes 5-lipoxygenase and hyaluronidase that are involved in the inflammatory process resulting in the lowest IC50 values of 37 and 444 μg/mL, respectively, on the second and third days of fermentation.
References
- El Enshasy, H.A.; Hatti-Kaul, R. Mushroom immunomodulators: Unique molecules with unlimited applications. Trends Biotechnol. 2013, 31, 668–677.
- Singdevsachan, S.K.; Auroshree, P.; Mishra, J.; Baliyarsingh, B.; Tayung, K.; Thatoi, H. Mushroom polysaccharides as potential prebiotics with their antitumor and immunomodulating properties: A review. Bioact. Carbohydr. Diet. Fibre 2016, 7, 1–14.
- Yu, Z.T.; Liu, B.; Mukherjee, P.; Newburg, D.S. Trametes versicolor extract modifies human fecal microbiota composition in vitro. Plant Foods Hum. Nutr. 2013, 68, 107–112.
- Pallav, K.; Dowd, S.E.; Villafuerte, J.; Yang, X.; Kabbani, T.; Hansen, J.; Dennis, M.; Leffler, D.A.; Newburg, D.S.; Kelly, C.P. Effects of polysaccharopeptide from Trametes versicolor and amoxicillin on the gut microbiome of healthy volunteers: A randomized clinical trial. Gut Microbes 2014, 5, 458–467.
- Ramberg, J.E.; Nelson, E.D.; Sinnott, R.A. Immunomodulatory dietary polysaccharides: A systematic review of the literature. Nutr. J. 2010, 9, 54.
- Louis, P.; Hold, G.L.; Flint, H.J. The gut microbiota, bacterial metabolites and colorectal cancer. Nat. Rev. Microbiol. 2014, 12, 661–672.
- Beverly, E.A.; Miller, C.K.; Wray, L.A. Spousal support and food-related behavior change in middle-aged and older adults living with type 2 diabetes. Health Educ. Behav. 2008, 35, 707–720.
- Guilherme, A.; Virbasius, J.V.; Puri, V.; Czech, M.P. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat. Rev. Mol. Cell Biol. 2008, 9, 367–377.
- Shimokawa, K.; Miwa, R.; Yamada, K.; Uemura, D. Importance of the backbone conformation of (−)-ternatin in its fat-accumulation inhibitory activity against 3T3-L1 adipocytes. Org. Biomol. Chem. 2009, 7, 777–784.
- Shimokawa, K.; Iwase, Y.; Yamada, K.; Uemura, D. Synthesis and inhibitory effect on fat accumulation of (−)-ternatin derivatives modified in the β-OH-D-Leu7 moiety. Org. Biomol. Chem. 2008, 6, 58–60.
- Kobayashi, M.; Kawashima, H.; Takemori, K.; Ito, H.; Murai, A.; Masuda, S.; Yamada, K.; Uemura, D.; Horio, F. Ternatin, a cyclic peptide isolated from mushroom, and its derivative suppress hyperglycemia and hepatic fatty acid synthesis in spontaneously diabetic KK-A(y) mice. Biochem. Biophys. Res. Commun. 2012, 427, 299–304.
- Xian, H.M.; Che, H.; Qin, Y.; Yang, F.; Meng, S.Y.; Li, X.G.; Bai, Y.L.; Wang, L.H. Coriolus versicolor aqueous extract ameliorates insulin resistance with PI3K/Akt and p38 MAPK signaling pathways involved in diabetic skeletal muscle. Phytother. Res. PTR 2018, 32, 551–560.
- Mao, G.H.; Ren, Y.; Feng, W.W.; Li, Q.; Wu, H.Y.; Jin, D.; Zhao, T.; Xu, C.Q.; Yang, L.Q.; Wu, X.Y. Antitumor and immunomodulatory activity of a water-soluble polysaccharide from Grifola frondosa. Carbohydr. Polym. 2015, 134, 406–412.
- Chung, J.-H.; Kong, J.-N.; Choi, H.-E.; Kong, K.-H. Antioxidant, anti-inflammatory, and anti-allergic activities of the sweet-tasting protein brazzein. Food Chem. 2018, 267, 163–169.
- Jin, M.; Zhou, W.; Jin, C.; Jiang, Z.; Diao, S.; Jin, Z.; Li, G. Anti-inflammatory activities of the chemical constituents isolated from Trametes versicolor. Nat. Prod. Res. 2019, 33, 2422–2425.
- Bucić-Kojić, A.; Fernandes, F.; Silva, T.; Planinić, M.; Tišma, M.; Šelo, G.; Šibalić, D.; Pereira, D.M.; Andrade, P.B. Enhancement of the anti-inflammatory properties of grape pomace treated by Trametes versicolor. Food Funct. 2020, 11, 680–688.
More
Information
Subjects:
Medicine, Research & Experimental
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Revisions:
2 times
(View History)
Update Date:
19 Apr 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




