Artemisia judaica (ArJ) is a Mediterranean aromatic plant used traditionally to treat gastrointestinal ailments, skin diseases, atherosclerosis, and as an immuno-stimulant. Researches validate the curative role of ArJ in the treatment of skin wounds, which is attributed to its antioxidant and anti-inflammatory effects, as well as its high proportion of oxygenated monoterpenes and cinnamate derivatives.
1. Introduction
Artemisia is a genus of annual, perennial, and biennial herbs in the Asteraceae (Compositae) family
[1]. The plants of the genus
Artemisia are frequently used in traditional medicine as remedies for human and animal ailments. For instance,
Artemisia species have been used in traditional medicine for respiratory disorders, including coughs and phlegm, as a pain killer, worm expelling agent, diaphoretic and diuretic agent, and for the treatment of wounds, hypertension, and allergies
[2]. In addition, some of the
Artemisia plants are traditionally used to treat seizures, and the activity is confirmed through in vivo animal experiments
[3][4][5].
Artemisia species have been reported in in vitro and in vivo experiments and in clinical trials evaluating their anticancer, antimalarial, antimicrobial, and antiviral activities
[6][7].
Furthermore, several side effects and misuses have also been reported for some of the genus’ plants. For instance,
A. monosperma leaves are not recommended in pregnancy and are used to induce abortion in Jordan
[8]. However, this plant, in addition to other plants of
Artemisia, e.g.,
A. vulgaris, has been used in folklore medicine for labor induction
[8][9][10]. Besides abortion, vomiting, diarrhea, headache, pruritus, and rashes have been reported among young children and pregnant women who used
A. annua to treat malaria
[11]. The
Artemisia plants’ biological activities were attributed to the presence of essential oils, sesquiterpene lactones, flavonoids, bitter principles, coumarins, and phenolic acids
[1][2][12][13]. Several
Artemisia species grow wildly or as cultivated plants for their use as medication and as a herbal tea preparation in the Mediterranean region
[9][14][15].
Artemisia judaica L. (ArJ) is widely grown in the Mediterranean region, including Algeria, Libya, Egypt, Jordan, and Saudi Arabia
[16][17][18][19][20]. In Saudi Arabia, ArJ grows in the kingdom’s northern region, including the border area of the Hail-Qassim regions
[21]. ArJ has been reported for several traditional uses, e.g., healing external wounds and repairing snake and scorpion bites
[22]. In addition, ArJ is traditionally used to treat gastrointestinal disorders, sexual inability, hyperglycemia, heart diseases, inflammatory disorders, arthritis, cancers
[1][20], skin diseases, atherosclerosis, and enhance vision and immunity
[23][24]. The Bedouins in Egypt (Sinai) and Saudi Arabia also use the plant as a herbal tea in treating GIT disorders
[16]. Biologically, ArJ demonstrated antidiabetic, antioxidant, hepatoprotective, and anti-inflammatory activities in experimental animals
[22][25][26] due to the properties inherent in the chemical structure of the compounds it contains
[27]. The plant also exhibited weak antimicrobial activity against Gram-positive and Gram-negative bacteria
[28][29]. In vitro studies reported the plant extract’s potential antioxidant and anticancer activities
[28][29].
ArJ chemical analysis revealed the presence of flavonoids, e.g., glycosides and aglycones of apigenin, luteolin, and quercetin
[22]. Other natural classes, such as phenolics, triterpenes, bitter principles, and sesquiterpene lactones, i.e., judaicin, have also been reported from the plant
[22][30]. Additionally, ArJ is an aromatic plant. Its essential constituents have been identified from the plant species growing in different areas and climatic regions
[18][20][23][24][31]; as well known as the anthropogenic factors, environmental conditions primarily affect the composition of the plant
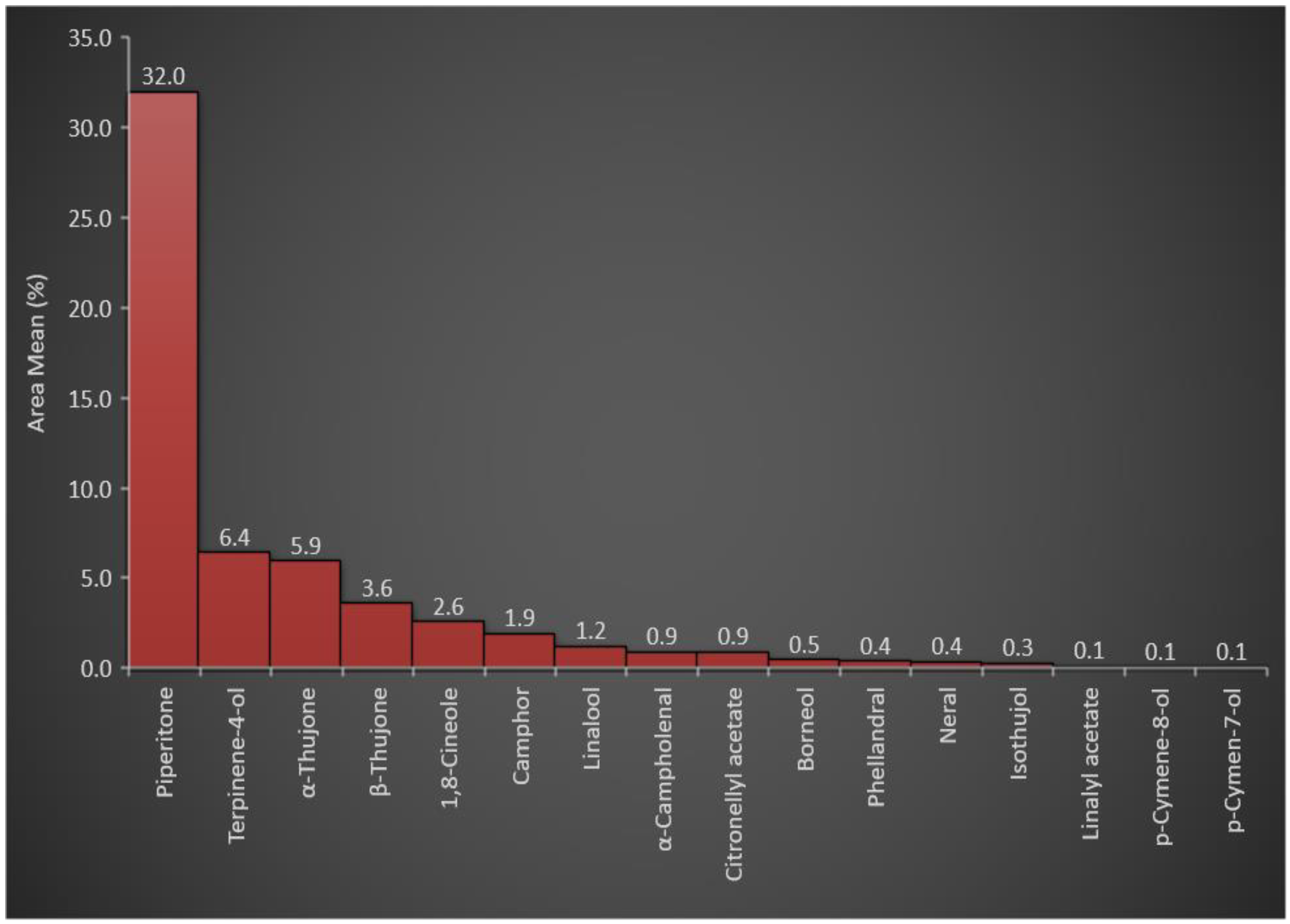
[32]. The overall analysis of the essential oil constituents of ArJ indicated that the monoterpene, i.e., piperitone, is the major chemotypic constituent in the plant from different genotypes
[1][24]. In addition, other essential constituents of the plants, such as camphor, ethyl cinnamate, and spathulenol, have also been identified in relatively high concentrations in individual plant genotypes
[24]. In addition, environmental conditions and the geographical locations of the plant growing areas have been reported to affect the major chemotypic constituents of ArJ essential oils.
Table 1 demonstrates the major constituents of the plant essential oils from different locations.
Table 1. Major constituents of the ArJ essential oils from plant species growing in different areas.
| Locations |
Major Constituents |
Y% |
Ref. |
| Saudi Arabia |
cis-Thujone (2.5%), thymol (3.5%), trans-sabinyl acetate (3.3%), carvacrol (3.5%), b-eudesmol (13.1%), eudesma-4 (15), 7-dien-1-b-ol (3.5%), and hexadecanoic acid (5.7%) |
0.18% (v/w) |
[24] |
| Algeria |
Piperitone (66.17%), ethyl cinnamate isomer (6.11%), spathulenol (2.34%), E-longipinane (2.55%) |
1.7% (w/w) |
[33] |
| Egypt |
Piperitone (49.1%) and camphor (34.5%), borneol (3.90%) |
|
[34] |
| Sinai, Egypt |
Camphor (31.4%), endo-borneol (5.72%), piperitone (29.9%) |
0.28% |
[18] |
| Jordan |
Artemisia ketone (9–24%), chrysanthenone (4–31%), piperitone (3–15%), camphor (0.3–16%), cinnamate (11.0%) |
0.4–0.9% (w/w) |
[20][35] |
| Libya |
cis-Chrysanthenol (9.1%), piperitone (30.2%), ethyl cinnamate (3.8%). |
0.62% (w/w) |
[23] |
The methods used for essential oil production from aromatic plants vary and mostly depend on the nature of the volatile constituents, the amount of the essential oils, and the nature of the plant samples
[36]. Thereby, distillation procedures are primarily used for the plants containing a considerable amount of the thermostable volatile constituents; however, volatile (e.g., diethyl ether) and non-volatile (e.g., lard) solvent extraction processes are used for the extraction of the highly delicate aromatic plants which contain heat-sensitive and small quantities of the essential oils
[37]. In addition, modern extraction techniques, such as CO
2, supercritical CO
2 extraction and microwave-assisted extraction techniques, are used for the industrial-scale production of the essential oils with specific advantages, e.g., time- and quantity-based efficiency and environmentally friendly properties
[38][39][40].
Burn injury traumas occur by friction, cold, heat, radiation, chemical, or electric sources, but hot liquids, solids, and fire contribute significantly towards burn injuries
[41]. In Saudi Arabia, 52% of all burns occur in young children, and males are more prone to burns than females (1.42:1). Burn wounds require immediate attention to avoid hypovolemic shock and sepsis
[42]. New approaches and drugs are being researched to facilitate faster burn wound healing
[43], thereby minimizing adverse reactions, like allergy or irritation, due to topical agents that increase the rehabilitation period
[44]. In addition to their general availability, herbal medicines have demonstrated a promising role in wound healing compared to silver sulfadiazine (SS)
[45][46][47]. Nevertheless, modern approaches and methodologies are required to validate claims for herbal compounds
[48].
2. Essential Oil Constituents of A. judaica
Several parameters have been reported as influencing factors affecting essential oil production, constituents, and quality; the parameters include the maturity stage of the plant, the oil extraction processes, and the drying methods applied to the aromatic plant samples, as well as the environmental conditions where the aromatic plant grows
[49][50][51][52]. The essential oil of wild ArJ growing in the Northern Qassim region of Saudi Arabia has been isolated by the hydro-distillation technique using a Clevenger apparatus from the shade-dried aerial parts of the plant. Three different distillation experiments have been used to calculate the essential oil production percentage of 1.71 ± 0.3%
w/
w of the dried plant aerial parts. The percentage yield was higher than the reported yields for the cultivated species of the plant growing in Saudi Arabia (0.18%
v/w)
[24], indicating the higher capacity of the wild species of ArJ to biosynthesize essential oils. In addition, the nature of the plant sample, i.e., fresh or dried, and the conditions of the drying process could be factors affecting oil production percentage. The reported oil production percentage (0.18%
v/w) has been calculated for the fresh plant samples
[24]. However, the current percentage (1.71 ± 0.3%
w/w) of essential oil production resulted from the distillation of the ten-day dried plant sample, which is consistent with the reported percentages of the essential oil production from dried samples of the aromatic plants, i.e., rosemary and sage
[53][54]. Moreover, the current essential oil recovery percentage (1.71 ± 0.3%
w/w) was nearly similar to the recorded data reported for the wild species of ArJ growing in the Southern region of Jordan (1.62%)
[20].
The produced oil samples obtained from each distillation experiment were independently subjected to GC-FID analysis. The results expressed in
Table 1 show the mean relative percentage of the individual compounds plus standard deviations obtained from the three GC-FID spectroscopic runs. Kovats retention index was calculated with the C
8–C
40 series of
n-alkenes analyzed under identical extermination conditions. The reported retention indexes were also used to identify the ArJ essential constituents. The results shown in
Table 2 indicated that oxygenated monoterpenes represented ≈ 57% of the plants’ essential constituents among all essential oil classes. The higher percentage of oxygenated monoterpenes was attributed to the presence of piperitone in a high concentration (31.99% of the total essential oils in the plant). In addition, other oxygenated monoterpenes, e.g., terpinene-4-ol, α-thujone, β-thujone, 1,8-cineole, camphor, and linalool, were represented at relatively high concentrations of 6.42, 5.94, 3.61, 2.56, 1.92, and 1.21%, respectively, with a total percentage of 21.66%. The concentration of piperitone (31.99%) and the total oxygenated monoterpene concentrations (57%) among the total essential oil constituents (
Figure 1) were consistent with the reported chemotypic properties of the plant
[24] that have been found, 30–70% of piperitone in the essential oil of ArJ growing wildly in different regions of the Mediterranean countries, such as Egypt, Algeria, and Jordan
[20][31][55][56].
Figure 1. Representation of the oxygenated monoterpenes in A. judaica essential oil.
Table 2. Essential oil constituents of A. judaica growing in the Northern Qassim region of Saudi Arabia.
| RT |
Chemical Compounds |
Area Mean |
RIcal |
RIrep |
m/z |
Weight g/100 g of the Plant |
| 12.096 |
(Z)-3-Hexenol |
0.50 ± 0.08 |
845 |
845 |
|
0.0085 |
| 12.209 |
2-Methyl-ethylbutanoate |
0.4 ± 0.06 |
850 |
853 |
|
0.0068 |
| 16.403 |
Sabinene |
0.13 ± 0.11 |
953 |
954 |
59.04 (100%), 81.05 (96.37%), 96.07 (83.12%) |
0.0022 |
| 18.449 |
α-Phellandrene |
1.30 ± 0.16 |
1000 |
999 |
68.04 (100%), 79.03 (42.69%), 93.04 (93.23%) |
0.0222 |
| 19.795 |
Limonene |
0.72 ± 0.01 |
1029 |
1028 |
85.04 (100%), 55.03 (12.29%), 70.07 (7.28%) |
0.0123 |
| 20.239 |
1,8-Cineole |
2.56 ± 0.05 |
1038 |
1040 |
69.04 (100%), 110.08 (70.37%), 95.06 (46.98%) |
0.0437 |
| 21.359 |
γ-Terpinene |
3.58 ± 0.18 |
1062 |
1063 |
135.05 (100%), 91.03 (20.36%), 107.03 (11.26%) |
0.0612 |
| 23.548 |
Linalool |
1.21 ± 0.04 |
1108 |
1104 |
91.04 (100%), 92.04 (98.97%), 55.04 (47.04%) |
0.0207 |
| 23.739 |
α-Thujone |
5.94 ± 0.09 |
1112 |
1112 |
95.06 (100%), 81.04 (68.49%), 109.04 (35.48%) |
0.1016 |
| 24.24 |
β-Thujone |
3.61 ± 0.03 |
1123 |
1124 |
84.0 (100%), 55.02 (80.71%), 126.05 (47.17%) |
0.0617 |
| 24.49 |
α-Campholenal |
0.91 ± 0.02 |
1128 |
|
82.04 (100%), 110.06 (91.66%), 95.04 (43.11%) |
0.0156 |
| 24.639 |
Terpinene-4-ol |
6.42 ± 0.17 |
1132 |
1140 |
70.04 (100%), 83.03 (71.89%), 71.03 (29.17) |
0.1098 |
| 25.443 |
Isothujol |
0.27 ± 0.47 |
1149 |
1145 |
|
0.0046 |
| 25.651 |
Camphor |
1.92 ± 0.09 |
1154 |
1155 |
68.01 (100%), 81.02 (26.97%), 55.04 (18.33%) |
0.0328 |
| 26.454 |
Borneol |
0.47 ± 0.01 |
1170 |
1170 |
95.04 (100%), 110.04 (48.36%), 54.06 (25.12%) |
0.0080 |
| 27.156 |
p-Cymene-8-ol |
0.08 ± 0.14 |
1186 |
1185 |
135.06 (100%), 150.08 (40.42), 91.03 (32.05) |
0.0014 |
| 29.403 |
Neral |
0.36 ± 0.00 |
1235 |
1236 |
69.02 (100%), 68.01 (17.21%), 83.01 (11.68%) |
0.0062 |
| 30.034 |
Linalyl acetate |
0.10 ± 0.17 |
1249 |
1250 |
107.06 (100%), 95.04 (34.18), 55.03 (14.79%) |
0.0017 |
| 30.845 |
Piperitone |
31.99 ± 0.50 |
1268 |
1260 |
82.04 (100%), 110.02 (37.44%), 95.06 (19.20%) |
0.5470 |
| 31.485 |
Phellandral |
0.38 ± 0.02 |
1281 |
|
|
0.0065 |
| 31.965 |
p-Cymen-7-ol |
0.08 ± 0.13 |
1292 |
1290 |
|
0.0014 |
| 32.146 |
Thymol |
1.81 ± 0.03 |
1296 |
1297 |
135.06 (100%), 107.03 (11.26), 77.01 (10.75%) |
0.0309 |
| 33.406 |
Carvacrol |
0.10 ± 0.12 |
1325 |
1324 |
|
0.0017 |
| 33.643 |
Citronellyl acetate |
0.89 ± 0.02 |
1330 |
1334 |
107.06 (100%), 91.04 (39.11), 122.08 (15.51) |
0.0152 |
| 34.649 |
(E)-Methyl cinnamate |
0.35 ± 0.02 |
1354 |
1355 |
131.02 (100%), 103.03 (61.07), 162.04 (49.31%) |
0.0060 |
| 35.894 |
Cis-Ethyl cinnamate |
4.02 ± 0.06 |
1383 |
1376 |
131.04 (100%), 103.04 (48.29%), 77.03 (30.40%) |
0.0687 |
| 36.303 |
Jasmone |
0.71 ± 0.02 |
1392 |
1396 |
91.03 (100%), 95.03 (66.88%), 79.03 (60.65%) |
0.0121 |
| 36.658 |
β-Bourbounene |
4.06 ± 0.17 |
1401 |
1401 |
111.02 (100%), 137.07 (41.99%), 180.09 (21.39%) |
0.0694 |
| 37.754 |
β-Caryophyllene |
0.43 ± 0.08 |
1427 |
1429 |
161.12 (100%), 105.04 (57.31%), 93.05 (27.09%) |
0.0075 |
| 39.75 |
Trans-Ethyl cinnamate |
13.67 ± 0.55 |
1477 |
1455 |
131.04 (100%), 103.04 (48.29%), 77.03 (30.40%) |
0.2337 |
| 40.542 |
Valencene |
3.24 ± 0.09 |
1497 |
1497 |
161.12 (100%), 105.04 (57.31%), 91.04 (53.35%) |
0.0554 |
| 41.138 |
γ-Cadinene |
0.79 ± 0.14 |
1511 |
1513 |
161.11 (100%), 133.07 (30.58%), 120.07 (27.73%) |
0.0135 |
| 41.968 |
σ-Cadinene |
2.37 ± 0.09 |
1532 |
1526 |
91.04 (100%), 205.11 (86.11%), 77.02 (46.25%) |
0.04052476 |
| 44.321 |
Spathulenol |
3.33 ± 0.07 |
1593 |
1575 |
91.04 (100%), 93.05 (73.39), 77.02 (46.25%) |
0.0569 |
| 47.198 |
β-Eudesmol |
0.22 ± 0.19 |
1671 |
1672 |
59.04 (100%), 149.11 (67.04%), 146.14 (33.10%) |
0.0038 |
| 48.344 |
a-Caryophylene acetate |
1.11 ± 0.31 |
1702 |
1696 |
67.04 (100%), 95.06 (62.38%), 96.07 (41.92%) |
0.0190 |
| Total |
100 |
1.71 |
| Monoterpene hydrocarbons |
5.74 |
| Oxygenated monoterpenes |
57.20 |
| Sesquiterpene hydrocarbons |
10.88 |
| Oxygenated sesquiterpenes |
4.66 |
| Phenolics |
1.87 |
| Cinnamic acid derivatives |
18.03 |
Besides the monoterpenes, GC-FID analysis of ArJ also showed a comparatively high percentage of cinnamic acid derivatives (18.03%), represented by the presence of three essential constituents, i.e., (
E)-methyl cinnamate (0.35%),
cis-ethyl cinnamate (4.02%), and
trans-ethyl cinnamate (13.67%). Notably, ethyl cinnamate has been reported as one of the major chemotypes of the plant
[24]. Monoterpene hydrocarbons, sesquiterpene hydrocarbons, oxygenated sesquiterpenes, and phenolic essential oils were also represented in the essential oil to a lesser extent, with 5.74, 10.88, 4.66, and 1.87%, respectively (
Table 2).
3. In Vitro Antioxidant Activity of the ArJ Essential Oil
Antioxidants are promising therapeutic agents in wound healing
[57]. Most of the reported plants with wound healing activity possess noticeable antioxidant potency, which has been examined by different in vitro and in vivo assays
[58]. The essential oils obtained from several plants of the genus
Artemisia, e.g.,
A. diffusa and
A. herba-alba, have exhibited potential free radical scavenging, reducing, and metal-chelating properties
[59][60]. The measurements, i.e., TAC, DPPH-SA, FRAP, and MCA, were conducted for the essential oil of ArJ quantitatively. The plant’s essential oil reduced the molybdate ions (VI) to molybdenum (V) in the TAC assay at a level of 59.32 mg of Trolox equivalents per gram of the plant essential oil.
Moreover, the ArJ essential oil exhibited notable reducing characteristics towards the ferric ion measured by the FRAP assay (22.34 mg of Trolox equivalent per gram of the essential oil). This ArJ essential oil-reducing characteristic in the TAC and FRAP contributes to the overall activity of this oil as an antioxidant agent
[61]. The noticeable reducing characteristic of the ArJ essential oil could be attributed to the presence of camphor (1.92%), ethyl cinnamate (4.02%), and piperitone (31.99%) in relatively high concentrations
[62][63][64]. The results also revealed that the essential oil of ArJ can chelate iron by 26.99 mg of EDTA equivalents per gram of essential oil, which was consistent with the reported ferrous ion-chelating activity of ArJ
[35]. As iron has a primary role in the Fenton reaction involving the conversion of the oxidizing agent hydrogen peroxide (H2O2) into the more reactive hydroxyl radical (HO·), the iron-chelating agents, such as the ArJ essential oil, interfere with the progression and exaggeration of the oxidative stress
[65].
Furthermore, scavenging activity of ArJ essential oil has been reported
[35][56]. In this entry, the essential oil of the ArJ also exhibited scavenging activity, measured as 10.70 mg of Trolox equivalent per gram of the essential oil against the stable free radical DPPH. The results of the antioxidant activity of the plant essential oil seems also to be attributable to the presence of considerable percentages of oxygenated monoterpenes, cinnamate derivatives, and phenolics in the essential oils of the plants, all of which are known for their antioxidant activity
[66][67][68]. The overall results obtained from quantitative in vitro antioxidant assays confirmed the antioxidant activity of the ArJ essential oil and supported the association between the wound healing potential of the plant and its antioxidant activity.
4. Antimicrobial Profile of ArJ Essential Oil
4.1. Preliminary Antimicrobial Activity
The results of preliminary antibacterial activity demonstrated that all the tested organisms, including Gram-positive and Gram-negative bacteria, are susceptible to ArJ essential oil, except Pseudomonas aerugenosa ATCC 9027, which showed resistance at the given concentration of ArJ essential oil, i.e., 20 μL/disc (Figure 2 and Table 3). The results further demonstrated that Bacillus cereus is a highly susceptible test organism, with an inhibition zone of 12.9 ± 0.10 mm at the given concentration of ArJ essential oil. In contrast, the lowest antibacterial activity was observed against Klebsiella pneumoniae and Shigella flexneri, with inhibition zones of 6.2 ± 0.10 mm and 6.2 ± 0.10 mm in diameter, respectively (Figure 2 and Table 3). Additionally, the findings indicated that the range for the mean zone of inhibition for Gram-positive bacteria is 7.2–12.9 mm, while for Gram-negative bacteria it is 6.2–10.0 mm, indicating that Gram-positive bacteria are more susceptible than Gram-negative bacteria to a given dose of ArJ essential oil.
Figure 2. Preliminary antimicrobial activity of ArJ essential oil. (a) Staphylococcus aureus (S. aureus) ATCC 29213; (b) Staphylococcus saptophyticus (S. saptophyticus) ATCC 43867; (c) Streptococcus pyogenes (S. pyogenes)-A ATCC 27736; (d) Streptococcus pneumoniae (S. pneumoniae) ATCC 49619; (e) Enterococcus faecalis (E. faecalis) ATCC 29212; (f) Bacillus cereus (B. cereus) ATCC 10876; (g) Escherichia coli (E. coli) ATCC 25922; (h) Klebsiella pneumonie (K. pneumoniae) ATCC 27736; (i) Salmonella typhimurium (S. typhimurium) ATCC 13311; (j) Shigella flexneri (S. flexneri) ATCC 12022; (k) Proteus vulgaris (P. vulgaris) ATCC 6380; (l) Proteus mirabilis (P. mirabilis) ATCC 29906; (m) Candida albicans (C. albicans) ATCC 10231; (n) Aspergillus niger (A. niger) ATCC 6275.
Table 3. Preliminary antimicrobial activity of ArJ essential oil.
| Microorganisms |
Zone of Inhibition (mm) |
| ArJ Essential Oil |
Control Drugs |
| S. aureus ATCC 29213 |
7.7 ± 0.20 |
14.2 ± 0.20 |
| S. saprophyticus ATCC 43867 |
8.8 ± 0.20 |
12.8 ± 0.20 |
| S. pyogenes (A) ATCC 27736 |
7.4 ± 0.30 |
11.7 ± 0.10 |
| S. pneumoniae ATCC 49619 |
7.2 ± 0.17 |
11.7 ± 0.20 |
| E. faecalis ATCC 29212 |
8.7 ± 0.17 |
11.9 ± 0.10 |
| B. cereus ATCC 10876 |
12.9 ± 0.10 |
19.6 ± 0.35 |
| E. coli ATCC 25922 |
6.4 ± 0.10 |
23.1 ± 0.20 |
| K. pneumoniae ATCC 27736 |
6.2 ± 0.10 |
21.1 ± 0.10 |
| S. typhimurium ATCC 13311 |
10.0 ± 0.20 |
16.3 ± 0.30 |
| S. flexneri ATCC 12022 |
6.2 ± 0.10 |
17.9 ± 0.17 |
| P. vulgaris ATCC 6380 |
8.1 ± 0.17 |
16.2 ± 0.35 |
| P. mirabilis ATCC 29906 |
7.7 ± 0.20 |
18.7 ± 0.20 |
| C. albicans ATCC 10231 |
25.2 ± 0.20 |
25.0 ± 0.20 |
| A. niger ATCC 6275 |
15.0 ± 0.20 |
13.1 ± 0.35 |
The results for preliminary antifungal activity indicate that both the tested fungal strains are highly susceptible to ArJ essential oil. The results also indicated that the highest antifungal activity was observed against Candida albicans with an inhibition zone of 25.2 ± 0.20 mm, while Aspergillus niger had an inhibition zone of 15.0 ± 0.20 mm at the given concentration of ArJ essential oil. The control antibiotics inhibited the growth of all the tested organisms at the given concentrations, i.e., 5 μg/disc for levofloxacin and 50 μg/disc for clotrimazole, respectively (Figure 2 and Table 3).
4.2. Minimum Inhibitory Concentration (MIC), Minimum Biocidal Concentration (MBC), Minimum Biofilm Inhibitory Concentration (MBIC), and Minimum Biofilm Eradication Concentration (MBEC)
The MIC and MBC results for the tested bacteria revealed that the MIC values ranged from 6.25 to 100 µL/mL, while MBC values ranged from 12.5 to >100 µL/mL (Table 4). The MIC and MBC results for the tested fungi demonstrated that Candida albicans had MIC and MBC values of 3.125 µL/mL and 6.25 µL/mL, respectively, whereas Aspergillus niger had values of 6.25 µL/mL and 12.5 µL/mL, respectively. The MBIC and MBEC results revealed that the MBIC values for the tested bacteria ranged from 6.25 to 100 µL/mL, whereas the MBEC values ranged from 12.5 to 200 µL/mL (Table 4).
Table 4. Results of MIC, MBC, MBIC, and MBEC of ArJ essential oil.
| Microorganisms |
MIC |
MBC |
MBIC |
MBEC |
| S. aureus ATCC 29213 |
50 |
100 |
50 |
100 |
| S. saprophyticus ATCC 43867 |
50 |
100 |
50 |
100 |
| S. pyogenes (A) ATCC 27736 |
100 |
>100 |
100 |
200 |
| S. pneumoniae ATCC 49619 |
100 |
>100 |
100 |
200 |
| E. faecalis ATCC 29212 |
100 |
>100 |
100 |
200 |
| B. cereus ATCC 10876 |
6.25 |
12.5 |
6.25 |
12.5 |
| E. coli ATCC 25922 |
50 |
100 |
50 |
100 |
| K. pneumoniae ATCC 27736 |
25 |
50 |
25 |
50 |
| S. typhimurium ATCC 13311 |
12.5 |
25 |
12.5 |
25 |
| S. flexneri ATCC 12022 |
12.5 |
25 |
12.5 |
25 |
| P. vulgaris ATCC 6380 |
25 |
50 |
25 |
50 |
| P. mirabilis ATCC 29906 |
100 |
>100 |
100 |
200 |
| C. albicans ATCC 10231 |
6.25 |
12.5 |
NT |
NT |
| A. niger ATCC 6275 |
3.125 |
6.25 |
NT |
NT |
Researchers' findings for ArJ essential oil antimicrobial activity are consistent with previously published data
[17][24][69][70][71][72]. Benmansour et al. demonstrated that ArJ essential oil had an excellent inhibitory effect against tested MRSA (methicillin-resistant
Staphylococcus aureus),
S. aureus, and
B. subtilis [17], which is consistent with researchers' results. Benderradji et al. showed that petroleum ether and ethyl acetate extracts of
A. sahariensis leaves had the highest inhibitory activity against most tested strains. The most reported significant inhibition zone was obtained with chloroform extract of the plant against
Pseudomonas [69]. These findings partially corroborate researchers' results, since ArJ essential oil could not kill
Pseudomonas, which might be a consequence of the essential oil and extract’s differing phytochemical contents, as well as species variations. Elazzouzia et al. demonstrated that the essential oil of
A. ifranensis had highly potent antibacterial activity against the tested
S. aureus [73], which is, again, consistent with researchers' results. Kazemi et al. demonstrated that the essential oil of the aerial parts of
A. kermanensis had highly potent antibacterial activity against
B. subtilis, P. aeruginosa, and
S. aureus, which is partially consistent with researchers' results
[72]. Al-Wahaibi et al. demonstrated that essential oils derived from
A. judaica and
A. herba-alba had potent antimicrobial potential against the tested organisms, including
Aspergillus fumigatus, Syncephalastrum racemosum, Geotricum candidum Candida albicans, Streptococcus pneumoniae, Bacillus subtilis, and
Escherichia coli, except
Pseudomonas aeruginosa; these results are consistent with researchers' results
[24]. The results of researchers' experiment indicated that ArJ essential oil has highly potent antimicrobial activity, demonstrating that ArJ essential oil could be a promising antimicrobial drug candidate and can cure various human infections, e.g., wound infections, boils, acne, etc., caused by various life-threatening pathogens, including bacteria and fungi. These results encouraged us to conduct wound healing testing on an animal model to verify the antimicrobial properties of ArJ essential oil in-vivo.
5. In Vivo Skin Burn Wound Healing
In this entry, the second-degree burn was induced on female rats based on the recent publication that observed significant wound healing in second and third-degree wounds
[74]. The choice of three-month-old female rats was due to their quicker wound healing and greater wound contraction ability as compared to males
[75].
5.1. Morphological Appearance and Histological Analysis of the Wounds
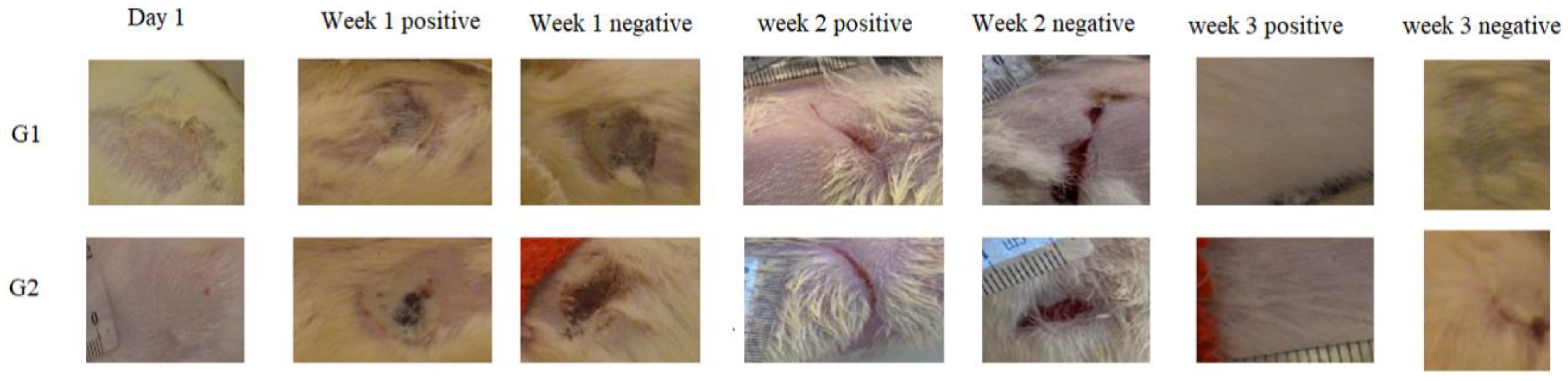
The observations of wounds over three weeks of treatment revealed the significant progression in the healing process among the treated groups. At the time of burn induction, the skin burns produced were whitish in color and round in shape. After one week of the treatment, a crust developed on the wound along with the disappearance of edema in the treated groups (ArJ and SS groups). The wound area started decreasing by the second week of the treatment; however, edema formation was still prominent in the untreated burn area. At the end of 3 weeks, the treatment wound area for both the ArJ and SS groups demonstrated recovery, while the untreated zone did not recover completely (Figure 3).
Figure 3. Morphological appearances of Artemisia judaica (G1)- and silver sulfadiazine (SS) (G2)-treated wounds at various time points.
Wound areas were measured using freely available Image J software. No significant differences were observed among the groups after the induction of skin burn. Two weeks after the treatment, the ArJ group’s wound area decreased significantly (p = 0.04), while the SS group’s wound size remained insignificant compared to the negative group.
Wound healing is a complex restorative process of injured tissue to its original state
[76], and it involves hemostasis, inflammation, proliferation, and remodeling
[77] to prevent complex metabolic alteration affecting body organ systems. During the process of hemostasis, blood coagulation occurs, while the inflammation process ensures safety from invasive pathogens
[77], thereby facilitating the proliferation step
[78] towards remodeling the tissue maturation process
[78][79]. During skin burn, cells and tissues are damaged substantially, thereby involving a complicated healing network compared to wound incision
[76]. Based on the deepness of the burn wounds, they are categorized as first-, second-, and third-degree burns. The first-degree burn is generally red or gray without any blisters and normal capillary network, while in a second-degree burn, blisters and partial-thickness damage to the dermis are observed. Second- and third-degree burns are treated similarly
[80]. In this entry, second-degree skin burn wounds were induced, which healed over 3 weeks for the ArJ and SS groups.
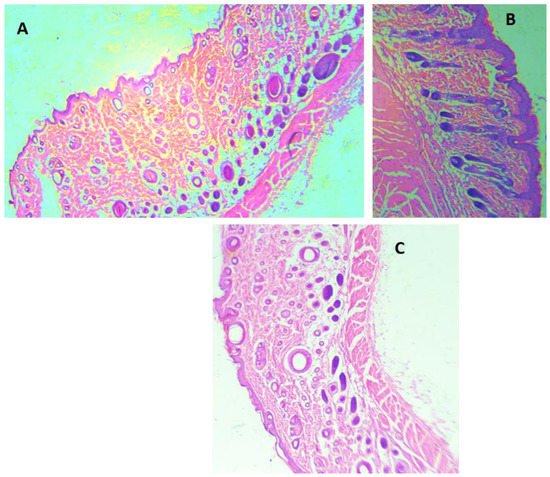
H&E staining was performed for all the animal groups (Figure 4). The H&E staining demonstrated epidermis integrity and the degree of neutrophilic infiltration in the dermis and capillaries of the ArJ and SS groups compared to the untreated wound zone. Wound healing visual appearance for ArJ was not enlarged, most probably due to the balm effect of paraffin (Figure 4B). Tissue sections were analyzed qualitatively for the amount of fibroblast, collagen, inflammation, and neovascularization in SS and ArJ groups. The data demonstrated increased collagen, fibroblast, and neovascularization, with decreased inflammation in the SS and ArJ groups compared with the negative control group.
Figure 4. Histological analysis using hematoxylin and eosin staining of control (A), Artemisia judaica (B), and SS (C). Arrows (black) indicate neutrophil infiltration; magnification 40×.
5.2. Role of Antioxidants and Oxidative Stress Markers in Wound Healing
The ArJ ointment group demonstrated significantly increased antioxidant SOD (
p = 0.03) and CAT (
p < 0.01) enzymatic activities compared to the negative group. The SOD and CAT activities were comparable in the intact and negative control. The SS treated group demonstrated a significant difference in CAT activity compared to the negative group (
p = 0.01) and was comparable to the ArJ group, while the differences were insignificant for SOD activity. The antioxidant activity contributing towards wound healing is in accordance with previous studies reporting enhanced wound healing due to potent antioxidant activities
[81]. LP significantly increased in the negative control group (
p < 0.0001) compared to the intact group, which accords with previously recorded data
[82]. No significant differences in LP were observed in treatment groups with either ArJ or SS groups compared to the negative control, while the ArJ and SS treated groups exhibited significant increases (
p < 0.0001) compared to the intact group (
Table 5).
Table 5. Effect of Artemisia judaica ointment on antioxidant and oxidant levels in skin burn rat model.
| Groups |
CAT |
SOD |
LP |
| ng/g |
| I. Intact control |
1.35 ± 0.05 A,B |
0.04 ± 0.00 A |
836.9 ± 37.75 A |
| II. Negative control (skin burn without treatment) |
1.11 ± 0.06 A |
0.04 ± 0.01 A |
1214 ± 51.46 B |
| III. Silver sulfadiazine |
1.79 ± 0.204 B |
0.19 ± 0.06 A,B |
1197 ± 30.30 B |
| IV. Artemisia judaica |
1.82 ± 0.17 B |
0.37 ± 0.13 B |
1291 ± 18.85 B |
5.3. Role of Pro- and Anti-Inflammatory Markers in Wound Healing
The pro-inflammatory markers IL-1b and IL-6 values were comparable among all the studied groups, similar to the previously published articles
[83][84]. At the same time, tumor necrosis factor α (TNF-α) significantly increased in the negative control group compared to the intact group (
p < 0.0001). TNF-α values decreased significantly after treatment with SS (
p < 0.02) and ArJ (
p < 0.002) compared to the negative control group. TNF-α, the inflammatory cytokine activated during acute inflammation by macrophages/monocytes, plays a vital role in cell signaling, leading to necrosis or apoptosis. TNF-α participates in vasodilatation and edema formation and leukocyte adhesion to the epithelium through the expression of adhesion molecules. Furthermore, TNF-α regulates blood coagulation, contributes to oxidative stress at sites of inflammation, and indirectly induces fever. The data conforms with Gushiken and Periera, where TNF-α decreased after two weeks of treatment in skin wound tissue compared to the negative control
[83][84].
The anti-inflammatory or the pro-angiogenic markers IL-10 and transforming growth factor beta 1 (TGF-b1) increased significantly in both ArJ (
p < 0.0001 and
p < 0.0001) and SS (
p < 0.0001 and
p < 0.0001) groups compared to the negative and intact control groups. However, differences in IL-10 and TGF-b1 levels in the negative control group were insignificant compared to the intact group (
Table 6). The data confirm previous studies where IL-10 increased significantly in skin wound healing tissue after two weeks of treatment compared to the negative control group
[83][84].
Table 6. Effect of Artemisia judaica ointment on inflammatory and pro-angiogenic markers in skin burn rat model.
| Groups |
IL-1b |
IL-6 |
TNF-α |
TGF-b1 |
IL-10 |
| ng/g |
| I. Intact control |
20.77 ±1.95 A |
806.1 ±10.20 A |
9.57 ±0.55 A |
4.19 ±0.24 A |
3.54 ±0.19 A |
| II. Negative control (skin burn without treatment) |
19.37 ± 2.33 A |
776.2 ±32.77 A |
15.54 ± 0.92 B |
3.87 ± 0.09 A |
2.99 ± 0.25 A |
| III. Sulfadiazine |
23.55± 0.88 A |
789.4 ±18.02 A |
12.85± 0.26 C |
19.28± 0.30 B |
12.68± 0.15 B |
| IV. Artemisia judaica |
19.55 ± 1.34 A |
869.2 ±51.91 A |
11.96 ± 0.34 C |
19.18 ± 0.33 B |
13.39 ± 0.35 B |
Various plant and herbal products are economically cheap to procure and demonstrate modest therapeutic potency with minimum toxicity relative to synthetic drugs
[45][46][47][85]. Eupolin ointment, derived from an aqueous extract of
C. odorata leaves, is the first Vietnam FDA-approved product
[86]. The wound healing mechanism, even though it remains unclear, nevertheless enhanced blood flow, decreased inflammatory response, and reduced infection rates, all of which are contributing factors to angiogenesis. Rats are loose-skinned, in contrast to the tight human skin, with quicker constriction of the wound than the epithelization process; as such, rat wound healing, even though it is resemblant, is not entirely similar to wound healing in human skin
[87][88]. However, rats are widely used animal models due to their genetic and behavioral similarity, ease of handling, and being economically viable. Thus, the rat skin burn model serves as a vital knowledge resource.
Mortality has been one of the major concerns in patients with deep burns due to infections, and researchers have attempted to minimize wound infection risk and accelerate the healing process
[89]. Topical antimicrobial ointments are commonly employed for such purposes as SS 1% with low toxicity and have a potent antibacterial effect in burn wound therapy management
[90][91][92].
Increased antioxidant levels have demonstrated wound healing potency
[93] by protecting tissue from oxidative stress
[94][95]. In this entry, ArJ demonstrated antioxidant (augmented SOD and CAT) enzymatic activities, validating the role of antioxidant enzymes, in addition to potentiating wound healing through increased anti-inflammatory levels. TGF-b1 is involved in wound healing, angiogenesis, immune regulation, and cancer. On the contrary, TGF-b1, along with inflammatory marker IL-6, helps in T helper 17 differentiation (Th17), aggravating inflammation
[96]. In researchers' study, the levels of TGF-b1 increased significantly while no statistical difference was observed for IL-1b and IL-6 in the ArJ group compared to the control group.
Moreover, IL-10 is a key regulator of the immune system by limiting the inflammatory response, which could otherwise cause tissue damage. In one study, IL-10 knockout mice were found prone to colitis
[97][98], and blocking of IL-10 resulted in severe pathology. On the contrary, increased IL-10 levels cause chronic infection, and IL-10 blocking paved the way for pathogen clearance
[99]. Mucosal secretion of IL-10 and TNF-α were augmented during wound healing, demonstrating the protective effect of IL-10 against inflammation. On the contrary, treatment with
P. pinnata increased the serum IL-10 concentration while downregulating TNF-α and IL-6
[45]. Researchers' experiment showed a significant increase in IL-10 concomitant with a significant decrease in TNF-α in ArJ compared to the negative control group, thus confirming the potential role of IL-10 in promoting wound healing.
The current findings for the in vivo and in vitro antioxidant activity and the anti-inflammatory effect of ArJ could be attributed to the presence of higher percentages of oxygenated monoterpenes (57.2%) in the plant essential oils
[100]. Oxygenated monoterpenes have been found as major constituents in the plants used traditionally to accelerate wound healing, e.g., the plant essential oil of
Helichrysum italicum [101][102] has exhibited a primary role in potential antimicrobial activity and anti-inflammatory effects
[102]. Furthermore, some of the major ArJ essential oils reported as antioxidant and anti-inflammatory agents, e.g., thujone (both α and β-thujone, 9.55%), 1,8-cineole (2.56%), camphor (1.92%), and borneol (0.47%), have chiefly contributed to the rosemary anti-inflammatory effect
[103]. Furthermore, 1,8-cineole antioxidant and anti-inflammatory effects have been reported, and the compound effect as an inhibitor for the inflammatory markers, TNF-α, IL-6, IL-8, LTB 4, PGE 2, and IL-1β, as well as down-regulation of 5-lipoxygenase (LOX) and cyclooxygenase (COX) pathways, are well documented
[104][105]. Moreover, methyl cinnamate, a major constituent in ArJ essential oil (4.02%), has demonstrated potent anti-inflammatory activity
[106]. All these compounds participated in the antioxidant and anti-inflammatory effects of the ArJ essential oil as well as in the wound healing activity. However, other identified constituents in this entry could also be playing a role in the demonstrated plant activities.