Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Dr. Nargis Sahib | + 1982 word(s) | 1982 | 2022-02-16 06:55:24 | | | |
| 2 | Rita Xu | Meta information modification | 1982 | 2022-02-16 09:47:07 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Sahib, D.N. Juniperus turbinata Guss.. Encyclopedia. Available online: https://encyclopedia.pub/entry/19494 (accessed on 07 February 2026).
Sahib DN. Juniperus turbinata Guss.. Encyclopedia. Available at: https://encyclopedia.pub/entry/19494. Accessed February 07, 2026.
Sahib, Dr. Nargis. "Juniperus turbinata Guss." Encyclopedia, https://encyclopedia.pub/entry/19494 (accessed February 07, 2026).
Sahib, D.N. (2022, February 16). Juniperus turbinata Guss.. In Encyclopedia. https://encyclopedia.pub/entry/19494
Sahib, Dr. Nargis. "Juniperus turbinata Guss.." Encyclopedia. Web. 16 February, 2022.
Copy Citation
Juniperus turbinata Guss. is a native species of Morocco; however, an exhaustive taxonomic description based on phenotypical characterization of north-eastern Moroccan population species is lacking, which might lead to taxonomic confusion.
Juniperus turbinata
phenotypic characters
population
diversity
1. Introduction
The complex Juniperus phoenicea is widely distributed in the Mediterranean area. It exhibits a significant degree of phenotypic variability in relation to its biogeographical distribution pattern [1]. The western region of the Mediterranean circum and Macaronesian region are assumed to include the highest rate of phenotypical variation of the complex. Three species were identified based on morphological, biochemical, and genetic analyses: Juniperus phoenicea L. sensu stricto (s.s), J. turbinata Guss., and J. canariensis Guyot & Mathou [2].
Juniperus turbinata is a native species in Morocco, with few specific ecological demands; it may occur at low precipitation levels of 200–400 mm/year and can reach an elevation of 2000 m.a.s.l. [3]. J. turbinata has a scattered wide range of biogeographical distribution, extending from coastal lands to the peaks of the Moroccan Atlas Mountains [4]. The species thrives in extreme ecological conditions under semi-arid and arid ombotypes, and occupies a significant position in Moroccan vegetation. It is a pioneering species with a preponderant role in the dynamics of woodlands ecosystem [5]. The ecosystems where the species begins to appear are in the thermo-Mediterranean to the mountain Mediterranean bioclimatic belts, and it reaches its optimum conditions in the meso-Mediterranean and supra-Mediterranean belts [5][6][7]. Moreover, when rainfall is insufficient, J. turbinata may substitute either Tetraclinis articulate (Vahl) Mast. or Quercus ilex L. in continental locations.
Juniperus turbinata is a large shrub or a small tree; its taxonomic traits are distinguished by reddish shoots, scaly leaves, and turbinate cones when immature, but globose ones when mature. Its pollen sheds between October and November [8]. In terms of nomenclature, Juniperus turbinata is referenced as a subspecies of J. phoenicea in Basic Flora of Morocco by Fennane et al. [9] and Valdés et al. [10]. However, to the best of the knowledge, there are no explicit phenotypic descriptions of its cones, leaves, or seeds. Its biogeography in the Moroccan Atlas Mountains was described by Benabid [11], and recent works on the Moroccan taxon based on essential oil and morphological and genetical analysis on these populations confirmed the taxonomic rank of the species [12][13][14]. The description of the species started earlier in the Iberian Peninsula [15], whereas there is a great interest in data on its traits and intrapopulation variability along the biogeographic pattern.
Moroccan populations of J. turbinata were most likely reported in several studies under different names due to the difficulty of distinction of discriminatory traits in the genus Juniperus L. [16]. However, much confusion was eliminated with recent molecular studies [10][13][14][16][17][18] showing that the Moroccan population of red juniper has strong affinities with J. turbinata. The following cited names—(1) var. megalocarpa Maire [19] in the Essaouira dunes (south Atlantic coast in Morocco); (2) var. mollis Maire & Weiller in the high Atlas Mountains or J. phoenicea subsp. mediterranea Lebreton & Thivend [20][21]; (3) J. phœnicea subsp. lycia L. [11] for the population of Phoenician juniper in the Mahdia littoral Atlantic; and (4) chemovar montana Lebreton & Pérez de Paz [22]—were considered to be ambiguous names of J. turbinata.
Juniperus turbinata, is an arid-adapted plant due to its limited lumen area, bimodal radial growth pattern, and extended roots, which allow it to quickly utilize shallow water [23]. These characteristics provide the junipers more adaptation to drought stress than taller trees with broader tracheids, such as pines. Consequently, J. turbinata can be used for ecological restoration in low-potential-productivity environments such as semi-arid and arid regions [24]. Its pink-colored wood is hard and resinous with an aromatic fragrance, and is valued for small manufactured objects and decorative works, as are other juniper species [25]. Its wood is primarily used as a fuel and for the production of charcoal [26]. In addition, juniper essential oils have been used in cosmetics for centuries, and there is now interest in their pharmaceutical properties [27]. Some cultivars have been chosen for horticultural uses and have been planted in some rocky gardens [28].
2. Plant Material
The plant material was sampled in the north-eastern region of Morocco, from the coastal location at Saidia, Site 1; the semi-coastal location at Jerrada, Site 2; and the continental location at Figuig, Site 3 (Figure 1). Plant material was collected throughout the months of February and March of 2021. From each shrub/tree in each population, 10 samples of ripened cones and 10 samples of small sections of one-year-old branches with adult leaves were collected separately. For the current study, 280 cones and 280 shoots from 28 individual in each of the three populations were collected from the southern, typically sunny sections of the shrubs/tree crown at a height of (1–2.5 m). The measurements of characters were assessed on dry material under a stereomicroscope with a scaled ocular in 2021 [13]. The cones, shoots, and seeds were measured with to a precision of 0.1 mm. The cone scales were counted after soaking the cones in water for 24 h. The seeds were removed from the soaked cones and air dried before measuring. The thickness of terminal lateral shoot with leaves was measured along the side rather than diagonally, as described by Marcysiak et al. [29]. The three populations were compared on the basis of four characters of cones, two characters of leaves and shoots, three characters of seeds, and eight ratios.
Figure 1. The area of study in north-eastern Morocco, showing the three sites of sampling.
3. Assessment of Characters
Amongst 17 examined traits, 15 had normal frequency distribution. The length of seeds in continental region and the width of seeds in semi-continental region both showed an almost normal distribution frequency, allowing the use of parametric tests. The recta cones number and the scales cones number were not normally distributed in the three populations; they were used after arcsine transformation.
For each population and for all populations combined, ranges, means, standard deviations, and variation coefficients are presented in the Table 1 and Table 2. Significant variations in the means of the examined populations were identified in the cone diameter/number-of-seeds ratio, with a variation coefficient of about 44% for the three populations and approximately 19 to 35% in a specific population. The length/diameter ratio of cones was the most constant trait, with a variation coefficient of approximately 11% in all studied populations and 9 to 13% in one population.
Table 1. Results of ANOVA on the analyzed characters of cones, seeds, and shoots.
| Characters | Total | Coastline | Semi-Continental | Continental | Signification |
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | ||
| (1) Recta number of cones (4 or 6) | 4 ± 0.021 | 4.02 ± 0.80 a | 4.05 ± 0.50 b | 4.02 ± 0.24 b | *** |
| (2) Length of cone (mm) | 7.82 ± 0.040 | 8.78 ± 0.91 b | 7.94 ± 0.86 b | 6.73 ± 0.58 a | *** |
| (3) Diameter of cone (mm) “average of 2 independent measures of angle of 90°” | 7.05 ± 0.036 | 8.02 ± 0.77 c | 7.08 ± 0.6 b | 6.02 ± 0.56 a | *** |
| (4) Number of scales of cone | 6.76 ± 0.038 | 6.77 ± 1.31 b | 7.22 ± 1.04 c | 6.27 ± 0.73 a | *** |
| (5) Number of leaves per 5mm section of ultimate lateral branchlet | 22.45 ± 0.106 | 19.85 ± 1.81 a | 24.72 ± 2.32 c | 22.78 ± 2.85 b | *** |
| (6) Thickness of the ultimate lateral branchlet and leaves (mm) | 1.12 ± 0.004 | 1.19 ± 0.14 c | 1.09 ± 0.11 b | 1.06 ± 0.11 a | *** |
| (7) Number of seeds | 4.84 ± 0.048 | 3.86 ± 1.33 a | 5.94 ± 1.06 c | 4.71 ± 0.92 b | *** |
| (8) Length of seeds (mm) | 4.01 ± 0.025 | 4.76 ± 0.55 c | 3.82 ± 0.49 b | 3.42 ± 0.42 a | *** |
| (9) Width of seeds(mm) | 1.85 ± 0.014 | 2.05 ± 0.44 | 1.77 ± 0.29 | 1.73 ± 0.41 | ns |
| (10) Length/diameter (of cone) | 1.12 ± 0.004 | 1.09 ± 0.11 | 1.12 ± 0.1 | 1.12 ± 0.14 | ns |
| (11) Length/width (of seed) | 2.21 ± 0.013 | 2.39 ± 0.43 c | 2.19 ± 0.36 b | 2.03 ± 0.33 a | *** |
| (12) Cone diameter/number-of-seeds | 1.63 ± 0.024 | 2.33 ± 0.82 b | 1.22 ± 0.25 a | 1.32 ± 0.27 a | *** |
| (13) Cone diameter/width-of-seeds | 3.92 ± 0.026 | 4.05 ± 0.8 b | 4.08 ± 0.74 b | 3.61 ± 0.71 a | *** |
| (14) Thickness-of-branchlet/number-of-leaves | 0.05 ± 0.0003 | 0.60 ± 0.009 c | 0.04 ± 0.006 a | 0.05 ± 0.009 b | *** |
| (15) Cone diameter/recta-number-of-cone | 1.89 ± 0.021 | 2.39 ± 0.8 c | 1.77 ± 0.32 b | 1.50 ± 0.15 a | *** |
| (16) Cone length/number-of-leaves | 0.36 ± 0.0028 | 0.44 ± 0.05 c | 0.32 ± 0.04 b | 0.30 ± 0.5 a | *** |
| (17) Cone number-of-scales/cone length | 0.88 ± 0.0057 | 0.78 ± 0.16 a | 0.91 ± 0.16 b | 0.93 ± 0.13 b | *** |
SD: standard deviation; *** statistical significance level p < 0.00; a,b,c values with different letters are significantly (p < 0.05) different within rows; ns = not significant.
Table 2. Results of descriptive statistics on the analyzed characters of cones, seeds, and shoots. Coastline: Coas; semi-continental: Smc; continental: Con.
| Characters | Total | Coas | Smc | Con | p Value of Tukey t-Test | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Max | CV (%) |
Min | Max | CV (%) |
Min | Max | CV (%) |
Min | Max | CV (%) |
Coas/ Smc |
Coas/ Con |
Smc/ Con |
|
| Recta number of cones (4 or 6) | 4 | 6 | 15.82 | 4 | 6 | 22.99 | 4 | 6 | 12.35 | 4 | 6 | 5.97 | 0.000 | 0.000 | 0.79 |
| Length of cone (mm) | 5 | 15 | 14.83 | 5.70 | 11.50 | 10.36 | 5 | 15 | 10.83 | 5.50 | 9.5 | 8.62 | 0.000 | 0.000 | 0.000 |
| Diameter of cone (mm) “average of 2 independent measures of angle of 90°” | 2.2 | 10 | 14.96 | 6 | 10 | 9.60 | 5.6 | 8.80 | 9.04 | 2.2 | 7.8 | 9.30 | 0.000 | 0.000 | 0.000 |
| Number of scales of cone | 4 | 13 | 16.67 | 4 | 13 | 19.35 | 4 | 9 | 14.40 | 5 | 9 | 1.64 | 0.000 | 0.000 | 0.000 |
| Number of leaves per 5 mm section of ultimate lateral branchlet | 10 | 29 | 13.80 | 15 | 24 | 9.12 | 19 | 29 | 9.39 | 10 | 28 | 12.52 | 0.000 | 0.000 | 0.000 |
| Thickness of the ultimate lateral branchlet and leaves (mm) | 0.13 | 1.66 | 12.06 | 0.85 | 1.66 | 11.76 | 0.13 | 1.43 | 10.09 | 0.74 | 1.41 | 10.38 | 0.000 | 0.000 | 0.015 |
| Number of seeds | 2 | 9 | 29.09 | 2 | 9 | 34.46 | 3 | 9 | 17.85 | 2 | 7 | 19.53 | 0.000 | 0.000 | 0.000 |
| Length of seeds (mm) | 2.18 | 6 | 18.70 | 2.4 | 6 | 11.55 | 2.47 | 5.52 | 12.83 | 2.18 | 5.12 | 12.28 | 0.000 | 0.000 | 0.000 |
| Width of seeds(mm) | 0.88 | 6.3 | 22.29 | 1.13 | 5.16 | 21.46 | 0.88 | 2.58 | 16.38 | 0.88 | 6.30 | 23.70 | 0.000 | 0.000 | 0.39 |
| Length/diameter (of cone) | 0.77 | 3.18 | 10.68 | 0.78 | 1.57 | 9.17 | 0.77 | 1.76 | 8.93 | 0.92 | 3.18 | 12.50 | 0.025 | 0.027 | 1 |
| Length/width (of seed) | 0.56 | 4 | 17.92 | 0.91 | 3.83 | 17.15 | 1.42 | 4 | 15.53 | 0.56 | 3.09 | 16.26 | 0.000 | 0.000 | 0.000 |
| Cone diameter/number-of-seeds | 0.37 | 4.5 | 44.19 | 0.94 | 4.50 | 35.19 | 0.76 | 2.33 | 19.67 | 0.37 | 2.90 | 20.45 | 0.000 | 0.000 | 0.071 |
| Cone diameter/width-of-seeds | 0.83 | 7.02 | 19.76 | 1.55 | 6.67 | 19.75 | 2.6 | 7.02 | 17.40 | 0.83 | 5.71 | 19.67 | 0.97 | 0.000 | 0.000 |
| Thickness-of-branchlet/number-of-leaves | 0.01 | 0.09 | 21.29 | 0.04 | 0.09 | 15.00 | 0.01 | 0.06 | 15.00 | 0.03 | 0.09 | 22.50 | 0.000 | 0.000 | 0.000 |
| Cone diameter/recta-number-of-cone | 0.55 | 4.5 | 33.38 | 1.33 | 4.50 | 33,47 | 1.08 | 4 | 18.08 | 0.55 | 2.37 | 10.00 | 0.000 | 0.000 | 0.000 |
| Cone length/number-of-leaves | 0.19 | 0.68 | 23.27 | 0.32 | 0.63 | 11,36 | 0.19 | 0.68 | 12.50 | 0.21 | 0.65 | 16.67 | 0.000 | 0.000 | 0.000 |
| Cone number-of-scales/cone length | 0.44 | 1.6 | 19.09 | 0.44 | 1.57 | 20,51 | 0.53 | 1.6 | 16.48 | 0.67 | 1.53 | 13.98 | 0.000 | 0.000 | 0.26 |
The measured characters correlated with each other at a statistically very significant level of p < 0.01. The number of correlations was different; hence, some features in one population were strongly correlated, whereas in others, the correlation was insignificant or negligible. The strongest positive correlations were found between length and cone diameter, with a Pearson’s r value of 0.81 (p < 0.01); and length of cone and length of seed, with a Pearson’s r value of 0.74 (p < 0.01). Length of cone showed a low, but significant correlation with width of seed, with a Pearson’s r value of 0.52 (p < 0.01), while the correlation of diameter of cone with width of seed has a Pearson’s r value of 0.38 (p < 0.01), and correlation of number of seeds with number of cone scales has an r value of 0.20 (p < 0.01).
4. Differences between Populations
4.1. Descriptive Statistics and ANOVA
The mean values of J. turbinata characters differed between the three populations at a statistically significant level (p < 0.05) (Table 1). The continental population had the smallest trait sizes (10 of the 17 tested characters had the lowest values). Hence, the continental population had the smallest cone diameter (6.02 mm) compared to the coastline population (8.31 mm), while the mean number of scales in the continental cones was 6.27, compared to the semi-continental cones with 7.22. The semi-continental population showed the greatest number of leaves and seeds (24.72 and 5.94, respectively); however, in the coastline population, the number was the lowest, with 19.85 and 3.86, respectively. The coastal population had the longest seeds (4.76 mm) and the highest seed length/width ratio (2.39), compared to the continental population with a seed length of 3.42mm and a seed length/width ratio of 2.03 (Table 1). The most different ratios were related to the cone dimensions: the cone diameter/recta number, with a value of 1.5 in continental population (in comparison to the coastline population, which had a level of 2.39), and the value of cone length/number-of-leaves, with 0.30 in the continental population and 0.44 in the coastline region. The shape of cones (length/diameter-of-cone) and the shape of seeds (length/width-of-seed) were rather similar among the three populations, and the mean value of the cone diameter/width-of-seeds ratio did not differ significantly (Table 1). The three populations could be distinguished from each other at a statistically significant level (p < 0.05). In Tukey’s test (Table 2) semi-continental and continental populations were closer, sharing 12 characters amongst the 17 studied (Table 2).
4.2. Discriminant Canonical Analysis
Researchers used Wilk’s lambda to assess if the canonical discriminant functions participate significantly to discriminate traits. Of the traits, 14 of 17 were used after removing the most redundant ones. Results show that every trait discriminated at a statistically significant level except the length/diameter-of-cone ratio, with p = 0.028. The overall results of the discriminant analysis based on Wilks’ lambda give: the cone diameter/number-of-seeds ratio—Wilks’ λ = 0.06; p < 0.001; the number of seeds—Wilks’ λ = 0.12; p < 0.001; the thickness-of-shoot/number-of-leaves ratio—Wilks’ λ = 0.19; p < 0.001; and the number of leaves per 5mm section—Wilks’ λ = 0.2; p < 0.001. The efficiency parameters of the canonical discriminant analysis are (i) Function 1 through Function 2 (Wilks’ Lambda = 0.000; Chi-Square = 428.6; df = 34: p = 0.001); and (ii) Function 2 (Wilks’ Lambda = 0.116; Chi-Square = 157.2; df = 16: p = 0.000). The populations of J. turbinata formed three groups in the space of Function 1 and Function 2 of the discriminant canonical analysis; the semi-continental and coastline populations were separated from the continental population mostly by the first discriminant function, which explained 84% of the total variation. The coastline population was separated from the semi-continental and continental populations by the second discriminant function, which explained 16% of total variation. (Figure 2). Function 1 was determined mostly by the following traits: cone diameter/number-of-seeds; length/width (of seed), cone length/number-of-leaves, number of scales of cone; and Function 2 was determined by: number of seeds, number of scales of cone, thickness-of-shoot/number-of-leaves, and number of leaves per 5mm section of ultimate lateral branchlet (Figure 2).
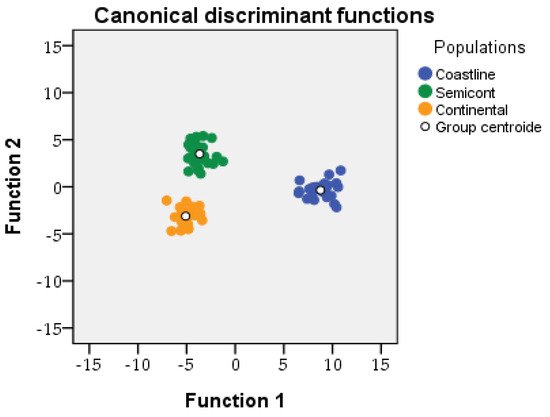
Figure 2. Results of the canonical discriminant analysis including n = 84 individuals of the three populations.
References
- Farjon, A. A Handbook of the World’s Conifers; EJ Brill: Leiden, The Netherlands; Boston, MA, USA, 2010; 1073p.
- Romo, A.; Mazur, M.; Salva-Catarineu, M.; Boraty’nski, A. A re-evaluated taxon: Genetic values and phenotypical characters support the recognition of the Canary Island juniper of the phoenicea group at a specific level. Phytotaxa 2019, 40, 64–70.
- Quézel, P.; Gast, M. Genévriers. In Encyclopédie Berbère; online; Peeters Publishers: Louvain, Belgium, 1998; p. 20.
- Quézel, P.; Médail, F. Écologie et Biogéographie des Forêts du Bassin Méditerranéen; Éditions scientifiques; Elsevier: Amsterdam, The Netherlands, 2003; 574p.
- Benabid, A.; Fennane, M. Connaissance sur la Végétation du Maroc. Phytogéographie, Phytosociologie et séries de végétation. Lazaroa 1994, 14, 21–97.
- Benabid, A. Grands écosystèmes naturels marocains, équilibre de fonctionnement, Perturbation, préservation et restauration. In Grande Encyclopédie du Maroc; GEM: Rabat, Morocco, 1986; pp. 117–190.
- Medail, F.; Quezel, P. The phytogeographical significance of S.W. Morocco compared to the Canary Islands. Plant Ecol. 1999, 140, 221–244.
- Adams, R.P. Junipers of the World: The genus Juniperus, 4th ed.; Trafford Publishing: Trafford, UK, 2014.
- Fennane, M.; Ibn Tattou, M.; Mathez, J.; Ouyahya, A.; El Oualidi, J. Flore Pratique du Maroc; Institut scientifique, Université Mohammed V: Rabat, Morocco, 1999; Volume 1, 558p.
- Valdés, B.; Rejdali, M.; EI Kadmiri, A.A.; Jury, J.L.; Montserrat, J.M. Catalogue des Plantes Vasculaires du Nord du Maroc, Incluant des Clés D’identification; Consejo Superior de Investigaciones: Madrid, Spain, 2002; Volume 1, 497p.
- Benabid, A. Flore et Ecosystèmes du Maroc. Évaluation et Préservation de la Biodiversité; Ibis Press: Paris, France; Librairies et éditions Kalila Wa Dimna: Rabat, Morocco, 2000; 359p.
- Adams, R.P.; Nguyen, S.; Achak, N. Geographic variation in Juniperus phoenicea (Cupressaceae) from the Canary Islands, Morocco and Spain based on RAPDs analysis. Phytologia 2006, 88, 270–278.
- Mazur, M.; Klajbor, K.; Kielich, M.; Sowińska, M.; Romo, A.; Montserrat, J.M.; Boratyński, A. Intra-specific differentiation of Juniperus phoenicea in the western Mediterranean region revealed in morphological multivariate analysis. Dendrobiology 2010, 63, 21–31.
- Dzialuk, A.; Mazur, M.; Boratyńska, K.; Montserrat, J.M.; Romo, A.; Boratyński, A. Population genetic structure of Juniperus phoenicea (Cupressaceae) in the western Mediterranean Basin: Gradient of diversity on a broad geographical scale. Ann. Sci. 2011, 68, 1341–1350.
- Mazur, M.; Boratynska, K.; Marcysiak, K.; Gomez, D.; Tomaszewski, D.; Didukh, Y.; Boratynski, A. Phenotypical variability of Juniperus phoenicea (Cupressaceae) from three distant localities on Iberian Peninsula. Acta Soc. Bot. Pol. 2003, 72, 71–78.
- Pavon, D.; Véla, E.; Médail, F. Are Meditterranean trees well known? Juniperus turbinata (Cupressaceae) a common but misunderstood taxons. Ecol. Med. 2021, 46, 77–104.
- Sánchez-Gómez, P.; Jiménez, J.F.; Cánovas, J.L. Genetic structure and phylogeography of Juniperus phoenicea complex throughout Mediterranean and Macaronesian regions: Different stories in one. Ann. For. Sci. 2018, 75, 1–12.
- Montserrat, S.C.; Angel, R.; Małgorzata, M.; Zielińska, M.; Minissale, P.; Dönmez, A.A.; Boratyńska, K.; Boratyński, A. Past, present, and future geographic range of the relict Mediterranean and Macaronesian Juniperus phoenicea complex. Ecol. Evol. 2021, 11, 5075–5095.
- Maire, R. Flore de l’Afrique du Nord; Paul Lechevalier Editore: Paris, France, 1952; Volume 1, pp. 114–115.
- Lebreton, P.; Thivend, S. Sur une sous-espèce du Genévrier de Phénicie Juniperus phoenicea L., définie à partir de critères biochimiques Naturalia monspeliensia Sér: Université de Montpelier, France. Bot 1981, 45, 1–12.
- Fennane, M.; Ibn Tattou, M. Flore Vasculaire du Maroc. Inventaire et Chorologie; Institut scientifique, Université Mohammed V: Rabat, Morocco, 2005; Volume 1, 483p.
- Lebreton, P.; Pérez de Paz, P.L. Définition du genévrier de Phénicie (Juniperus phoenicea), reconsidéré à ses limites biogéographiques: Méditerranée orientale (Crète et Chypre) et Atlantiques (Îles Canaries). Bull. Mens. Soc. linn. Lyon. 2001, 70, 73–92.
- Camarero, J.J.; Valeriano, C.; Gazol, A.; Colangelo, M.; Sánchez-Salguero, R. Climate Differently Impacts the Growth of Coexisting Trees and Shrubs under Semi-Arid Mediterranean Conditions. Forests 2021, 12, 381.
- García Morote, F.A.; Andrés Abellán, M.; Rubio, E.; Pérez Anta, I.; García Saucedo, F.; López Serrano, F.R. Stem CO2 Efflux as an Indicator of Forests’ Productivity in Relict Juniper Woodlands (Juniperus thurifera L.) of Southern Spain. Forests 2021, 12, 1340.
- Goldstein, G.M.; Simonetti, M. Watschinger, Guida al Riconoscimento Delgi Alberi d’Europa; Arnold Mondadori Editore: Milano, Italy, 1985.
- Farjon, A. World Checklist and Bibliography of Conifers, 2nd ed.; Kew: The Royal Botanic Gardens; The University of Chicago Press: Chicago, IL, USA, 2001; 316p.
- Kavetsou, E.; Pitterou, I.; Katopodi, A.; Petridou, G.; Adjali, A.; Grigorakis, S.; Detsi, A. Preparation, Characterization, and Acetylcholinesterase Inhibitory Ability of the Inclusion Complex of β-Cyclodextrin–Cedar (Juniperus phoenicea) Essential Oil. Micro 2021, 1, 250–266.
- Acuña-Míguez, B.; Valladares, F.; Martín-Forés, I. Both Mature Patches and Expanding Areas of Juniperus thurifera Forests Are Vulnerable to Climate Change But for Different Reasons. Forests 2020, 11, 960.
- Marcysiak, K.; Mazur, M.; Romo, A.; Montserrat, J.M.; Didukh, Y.; Boratyńska, K.; Jasińska, A.; Kosiński, P.; Boratyński, A. Numerical taxonomy of Juniperus thurifera, J. excelsa and J. foetidissima (Cupressaceae) basedon phenotypical characters. Bot. J. Linn. Soc. 2007, 155, 483–495.
More
Information
Subjects:
Area Studies
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Entry Collection:
Environmental Sciences
Revisions:
2 times
(View History)
Update Date:
16 Feb 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




