
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Matteo Mario Carlà | + 2077 word(s) | 2077 | 2022-02-14 06:56:54 | | | |
| 2 | Bruce Ren | -1 word(s) | 2076 | 2022-02-16 09:25:11 | | |
Video Upload Options
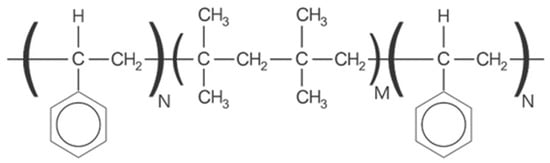
For moderate-to-severe glaucoma, trabeculectomy remains the “gold standard” intraocular pressure (IOP)-lowering treatment; nonetheless, this method requires extensive post-operative maintenance. Microinvasive glaucoma surgery (MIGS) treatments are designed to lessen intra- and post-operative care burden while offering an acceptable IOP decrease for individuals with mild to moderate glaucoma. The PreserFlo® MicroShunt (previously InnFocus MicroShunt) is an 8.5 mm glaucoma drainage device manufactured from poly(styrene-block-isobutylene-block-styrene) (SIBS), an extremely biocompatible and bioinert material. The lumen is narrow enough to prevent hypotony, but big enough to avoid being obstructed by sloughed cells or pigment. The device is implanted ab externo, as a stand-alone procedure or in conjunction with cataract surgery, with intraoperative mitomycin C, and a bleb is produced under the conjunctiva and Tenon’s capsule. The MicroShunt was CE-marked in 2012 and designed for primary open-angle glaucoma, the IOP of which remains uncontrolled after maximally tolerated topical treatment. Several clinical trials evaluating the MicroShunt’s long-term safety and effectiveness have been conducted, highlighting the effectiveness of the device over time, along with a tolerable safety profile.
1. Introduction
2. History of PreserFlo MicroShunt
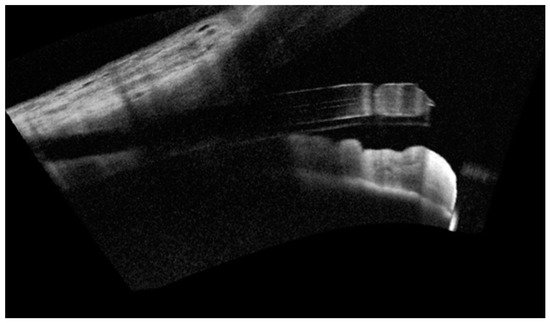
3. Surgical Technique
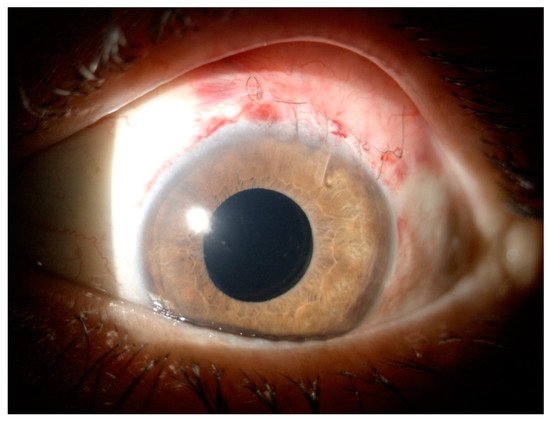
References
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The pathophysiology and treatment of glaucoma: A review. JAMA 2014, 311, 1901–1911.
- Jonas, J.; Aung, T.; Bourne, R.; Bron, A.; Ritch, R.; Panda-Jonas, S. Glaucoma. Lancet 2017, 390, 31461–31469.
- Gedde, S.J.; Feuer, W.J.; Shi, W.; Lim, K.S.; Barton, K.; Goyal, S.; Ahmed, I.I.K.; Brandt, J.; Primary Tube Versus Trabeculectomy Study, G. Treatment Outcomes in the Primary Tube Versus Trabeculectomy Study after 1 Year of Follow-up. Ophthalmology 2018, 125, 650–663.
- Gedde, S.J.; Schiffman, J.C.; Feuer, W.J.; Herndon, L.W.; Brandt, J.D.; Budenz, D.L.; Tube versus Trabeculectomy Study, G. Treatment outcomes in the Tube Versus Trabeculectomy (TVT) study after five years of follow-up. Am. J. Ophthalmol. 2012, 153, 789–803.e2.
- Gedde, S.J.; Herndon, L.W.; Brandt, J.D.; Budenz, D.L.; Feuer, W.J.; Schiffman, J.C.; Tube Versus Trabeculectomy Study, G. Postoperative complications in the Tube Versus Trabeculectomy (TVT) study during five years of follow-up. Am. J. Ophthalmol. 2012, 153, 804–814.e1.
- Gazzard, G.; Konstantakopoulou, E.; Garway-Heath, D.; Garg, A.; Vickerstaff, V.; Hunter, R.; Ambler, G.; Bunce, C.; Wormald, R.; Nathwani, N.; et al. Selective laser trabeculoplasty versus eye drops for first-line treatment of ocular hypertension and glaucoma (LiGHT): A multicentre randomised controlled trial. Lancet 2019, 393, 1505–1516.
- Saheb, H.; Ahmed, I.I.K. Micro-invasive glaucoma surgery: Current perspectives and future directions. Curr. Opin. Ophthalmol. 2012, 23, 96–104.
- Richter, G.M.; Coleman, A.L. Minimally invasive glaucoma surgery: Current status and future prospects. Clin. Ophthalmol. 2016, 10, 189–206.
- Kerr, N.M.; Wang, J.; Barton, K. Minimally invasive glaucoma surgery as primary stand-alone surgery for glaucoma. Clin. Exp. Ophthalmol. 2017, 45, 393–400.
- Pillunat, L.E.; Erb, C.; Junemann, A.G.M.; Kimmich, F. Micro-invasive glaucoma surgery (MIGS): A review of surgical procedures using stents. Clin. Ophthalmol. 2017, 11, 1583–1600.
- Parra, M.T.M.; Lopez, J.A.S.; Grau, N.S.L.; Ceausescu, A.M.; Santonja, J.J.P. XEN implant device versus trabeculectomy, either alone or in combination with phacoemulsification, in open-angle glaucoma patients. Graefe’s Arch. Clin. Exp. Ophthalmol. 2019, 257, 1741–1750.
- Grover, D.S.; Flynn, W.J.; Bashford, K.P.; Lewis, R.A.; Duh, Y.-J.; Nangia, R.S.; Niksch, B. Performance and safety of a new ab interno gelatin stent in refractory glaucoma at 12 months. Am. J. Ophthalmol. 2017, 183, 25–36.
- Pinchuk, L.; Riss, I.; Batlle, J.F.; Kato, Y.P.; Martin, J.B.; Arrieta, E.; Palmberg, P.; Parrish, R.K.; Weber, B.A.; Kwon, Y.; et al. The use of poly(styrene-block-isobutylene-block-styrene) as a microshunt to treat glaucoma. Regen. Biomater. 2016, 3, 137–142.
- Pinchuk, L.; Riss, I.; Batlle, J.F.; Kato, Y.P.; Martin, J.B.; Arrieta, E.; Palmberg, P.; Parrish, R.K.; Weber, B.A.; Kwon, Y.; et al. The development of a micro-shunt made from poly(styrene-block-isobutylene-block-styrene) to treat glaucoma. J. Biomed. Mater. Res. B 2017, 105, 211–221.
- Batlle, J.F.; Fantes, F.; Riss, I.; Pinchuk, L.; Alburquerque, R.; Kato, Y.P.; Arrieta, E.; Peralta, A.C.; Palmberg, P.; Parrish, R.K., 2nd; et al. Three-Year Follow-up of a Novel Aqueous Humor MicroShunt. J. Glaucoma 2016, 25, e58–e65.
- Pinchuk, L.; Wilson, G.J.; Barry, J.J.; Schoephoerster, R.T.; Parel, J.M.; Kennedy, J.P. Medical applications of poly(styrene-block-isobutylene-block-styrene) (“SIBS”). Biomaterials 2008, 29, 448–460.
- Parel, J.; Stoiber, J.; Fernandez, V. Optical properties and biocompatibility of a novel polymer for intraocular implants: Comparative study in the rabbit. Ophthalmic. Technol. 2004, XIV#5314-45, 24–25.
- Acosta, A.C.; Espana, E.M.; Yamamoto, H.; Davis, S.; Pinchuk, L.; Weber, B.A.; Orozco, M.; Dubovy, S.; Fantes, F.; Parel, J.M. A newly designed glaucoma drainage implant made of poly(styrene-b-isobutylene-b-styrene)—Biocompatibility and function in normal rabbit eyes. Arch. Ophthalmol.-Chic. 2006, 124, 1742–1749.
- Fantes, F.; Acosta, A.; Carraway, J.; Pinchuk, L.; Weber, B.; Davis, S.; Arrieta, E.; Parel, J.M. An independent GLP evaluation of a new glaucoma drain, the MIDI. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3547.
- Arrieta, E.A.; Aly, M.; Parrish, R.; Dubovy, S.; Pinchuk, L.; Kato, Y.; Fantes, F.; Parel, J.M. Clinicopathologic correlations of poly-(styrene-b-isobutylene-b-styrene) glaucoma drainage devices of different internal diameters in rabbits. Ophthalmic Surg. Lasers Imaging 2011, 42, 338–345.
- Pinchuk, L.; Fantes, F.; Zhou, Y.; Martin, J.; Parel, J.M. The BPEI–InnFocus Glaucoma Drainage Implant–The MiDi: Concept, Physics and Pressure Control. Investig. Ophthalmol. Vis. Sci. 2006, 47, 36.
- Silber, S.; Colombo, A.; Banning, A.P.; Hauptmann, K.; Drzewiecki, J.; Grube, E.; Dudek, D.; Baim, D.S. Final 5-year results of the TAXUS II trial: A randomized study to assess the effectiveness of slow- and moderate-release polymer-based paclitaxel-eluting stents for de novo coronary artery lesions. Circulation 2009, 120, 1498–1504.
- Strickler, F.; Richard, R.; McFadden, S.; Lindquist, J.; Schwarz, M.C.; Faust, R.; Wilson, G.J.; Boden, M. In vivo and in vitro characterization of poly(styrene-b-isobutylene-b-styrene) copolymer stent coatings for biostability, vascular compatibility and mechanical integrity. J. Biomed. Mater. Res. A 2010, 92, 773–782.
- Sadruddin, O.; Pinchuk, L.; Angeles, R.; Palmberg, P. Ab externo implantation of the MicroShunt, a poly (styrene-block-isobutylene-block-styrene) surgical device for the treatment of primary open-angle glaucoma: A review. Eye Vis. (Lond) 2019, 6, 36.
- Morita, K.; Gao, Y.; Saito, Y.; Higashide, T.; Kobayashi, A.; Ohkubo, S.; Sugiyama, K. In vivo confocal microscopy and ultrasound biomicroscopy study of filtering blebs after trabeculectomy: Limbus-based versus fornix-based conjunctival flaps. J. Glaucoma 2012, 21, 383–391.
- An, D.; Morgan, W.H.; Yu, D.Y. Glymphatics and lymphatics in the eye and central nervous system. Clin. Exp. Ophthalmol. 2017, 45, 440–441.
- Jeon, T.Y.; Kim, H.J.; Kim, S.T.; Chung, T.Y.; Kee, C. MR imaging features of giant reservoir formation in the orbit: An unusual complication of Ahmed glaucoma valve implantation. AJNR Am. J. Neuroradiol. 2007, 28, 1565–1566.
- Wilkins, M.; Indar, A.; Wormald, R. Intraoperative mitomycin C for glaucoma surgery. Cochrane Database Syst. Rev. 2005.
- Martinez-de-la-Casa, J.M.; Saenz-Frances, F.; Morales-Fernandez, L.; Perucho, L.; Mendez, C.; Fernandez-Vidal, A.; Garcia-Saenz, S.; Sanchez-Jean, R.; Garcia-Feijoo, J. Clinical outcomes of combined Preserflo Microshunt implantation and cataract surgery in open-angle glaucoma patients. Sci. Rep. 2021, 11, 15600.
- Shaarawy, T. Glaucoma surgery: Taking the sub-conjunctival route. Middle East Afr. J. Ophthalmol. 2015, 22, 53–58.
- Beckers, H.J.M.; Aptel, F.; Webers, C.A.B.; Bluwol, E.; Martinez-de-la-Casa, J.M.; Garcia-Feijoo, J.; Lachkar, Y.; Mendez-Hernandez, C.D.; Riss, I.; Shao, H.; et al. Safety and Effectiveness of the PRESERFLO(R) MicroShunt in Primary Open-Angle Glaucoma: Results from a 2-Year Multicenter Study. Ophthalmol. Glaucoma 2021.




