Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Michael Plank | + 4652 word(s) | 4652 | 2022-02-08 08:56:57 | | | |
| 2 | Rita Xu | -50 word(s) | 4602 | 2022-02-16 04:10:50 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Plank, M. TOR–PKA Interactions. Encyclopedia. Available online: https://encyclopedia.pub/entry/19482 (accessed on 07 February 2026).
Plank M. TOR–PKA Interactions. Encyclopedia. Available at: https://encyclopedia.pub/entry/19482. Accessed February 07, 2026.
Plank, Michael. "TOR–PKA Interactions" Encyclopedia, https://encyclopedia.pub/entry/19482 (accessed February 07, 2026).
Plank, M. (2022, February 16). TOR–PKA Interactions. In Encyclopedia. https://encyclopedia.pub/entry/19482
Plank, Michael. "TOR–PKA Interactions." Encyclopedia. Web. 16 February, 2022.
Copy Citation
TOR and PKA signaling are the major growth-regulatory nutrient-sensing pathways in S. cerevisiae. A number of experimental findings demonstrated a close relationship between these pathways: Both are responsive to glucose availability. Both regulate ribosome production on the transcriptional level and repress autophagy and the cellular stress response. Sch9, a major downstream effector of TORC1 presumably shares its kinase consensus motif with PKA, and genetic rescue and synthetic defects between PKA and Sch9 have been known for a long time.
TOR
PKA
signaling pathway interaction
kinase
substrate specificity
cross-talk
1. Introduction
Protein kinase A (PKA) and TOR signaling are two highly conserved signaling pathways that respond to nutrient and stress signals and regulate various responses that govern cellular growth. Numerous findings indicate a strong connection between the pathways, but no clear picture of the nature of this interplay has emerged. This work aims to critically review the literature on shared targets and direct cross-talk and to point out gaps in current knowledge that hinder a better understanding. Beyond providing a resource about specifics of the signaling systems discussed, the described modes of interaction are intended to serve as examples relevant for understanding signaling interplay in a wider context.
1.1. TOR Signaling
TOR signaling is one of the most central mechanisms that allows cells to adapt their growth to nutrient availability and also functions as a stress sensor. TOR signaling has been reviewed elsewhere [1][2][3][4][5][6][7][8][9][10][11] and therefore only a short summary is given here, in particular with respect to downstream functions that are shared with PKA signaling. The TOR functions explored in this review are mediated through TOR complex 1 (TORC1), and therefore “TOR signaling” will refer to signaling through TORC1 for the rest of this review. TORC1 exerts its physiological effects mainly through regulation of ribosome production, cell cycle progression and amino acid import and metabolism, as well as repression of autophagy and the cellular stress response [3][10].
Signaling downstream of TORC1 can be divided into two major branches, namely the PP2A and Sch9 branch. PP2A is a trimeric protein phosphatase, consisting of catalytic C subunit Pph21 or Pph22, scaffold A subunit Tpd3 and regulatory B subunit Cdc55 or Rts1 [12][13][14][15]. In addition, S. cerevisiae expresses a PP2A-like phosphatase (referred to as PP2ASit4), consisting of catalytic subunit Sit4 and either Sap155, Sap185 or Sap190 [16][17]. Both PP2A and PP2ASit4 activity are inhibited through Tap42 which forms complexes with Pph21/22 and Sit4 in a TORC1-dependent manner [18][19]. The tap42-11 mutant, which renders Tap42 temperature sensitive, but also rapamycin insensitive, is a frequently used experimental tool in this context [18]. When TORC1 is inactivated, PP2ASit4 induces a transcriptional program allowing the utilization of non-preferred nitrogen sources (among others through the transcription factors Gln3 and Gat1) and alters the profile of plasma membrane amino acid transporters [20][21][22][23].
The second major direct TORC1 target is the AGC kinase Sch9. Like other AGC kinases, it is basophilic and its limited number of known substrates suggest a preference for arginines and, to a lesser extent, lysines in the P-3 and P-2 positions [24][25]. It is phosphorylated by TORC1 on six serine and threonine residues near its C-terminus that reside within the so-called hydrophobic motif and turn motif [26]. In addition to the hydrophobic motif, AGC kinases generally require phosphorylation of their activation loop for full activity, which is catalyzed by the PDK1 homologs Pkh1/2 [27][28][29]. Sch9 is phylogenetically closely related to mammalian PKB/Akt and S6K [30] and, due to its ability to phosphorylate Rps6, is generally considered the functional homolog of S6K [26]. Several mechanisms through which Sch9 regulates ribosome biogenesis are discussed below.
1.2. PKA Signaling
PKA is a hetero-tetramer of two regulatory and two catalytic subunits. In S. cerevisiae, the regulatory subunit is encoded by BCY1 and the catalytic subunits by TPK1, TPK2 and TPK3, which are members of the AGC kinase family [31][32]. Specificity of PKA for phosphorylation of sites in R[RK]x[ST] (where x is any residue) motifs is well established [33][34]. Combinatorial deletions of the catalytic subunits demonstrated that only tpk1∆ tpk2∆ tpk3∆ strains are inviable, while the viability of double deletion strains suggests a high level of redundancy [31].
PKA is activated by the binding of cAMP to the regulatory subunits, triggering their dissociation from the catalytic subunits [35]. The second messenger cAMP is produced by adenylate cyclase Cyr1, which is activated via two routes: First, by the small G proteins Ras1 or Ras2, which are regulated by the guanine-nucleotide exchange factor Cdc25 and GTPase-activating proteins Ira1/2, and second via the G protein-coupled receptor Gpr1 and its G protein alpha subunit Gpa2 [36][37] (Figure 1). Both pathways are best known for their activation by glucose when added to cultures without a fermentable carbon source [38][39][40]. The low-affinity, high-capacity phosphodiesterase Pde1 and a high-affinity, low-capacity phosphodiesterase Pde2 are responsible for cAMP degradation [41][42]. Through upregulation of Pde1 activity and other negative feedback loops, the PKA pathway dampens its activity within minutes after glucose addition, resulting in a characteristic cAMP spike [43]. While glucose-triggered activation is the by far most studied scenario, activation of PKA in a cAMP-independent manner [44] and in response to nitrogen and other nutrients [45][46] has also been described. There is an increasing number of examples in which conveying the presence of these nutrients to PKA depends on nutrient transceptors, transporters that serve a role in signaling (see [47] for a review). PKA is also phosphorylated by the PDK homologs Pkh1/2 and undergoes autophosphorylation, but understanding of the regulatory roles of these modifications is limited [48][49].
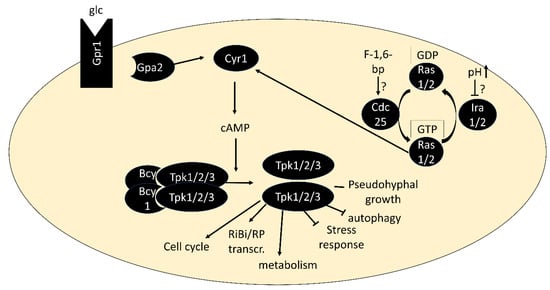
Figure 1. Core components of the PKA pathway. In its inactive form, PKA exists as a tetramer of two regulatory subunits (Bcy1) and two catalytic subunits (Tpk1, Tpk2 or Tpk3). Binding of cAMP to Bcy1 leads to dissociation of the complex and activation of the catalytic subunits. Two main routes of activation of adenylate cyclase Cyr1 exist: via the G protein-coupled receptor Gpr1 and its G protein alpha subunit Gpa2 and via the small GTPases Ras1/2.
Important tools for studying PKA are strains in which the pathway is artificially activated, through BCY1 deletion or a single amino acid substitution in Ras1/2 (rasV19). These strains fail to grow on non-fermentable carbon sources and to accumulate storage carbohydrates, arrest in G0 or acquire heat-shock resistance like wild-type strains upon nutrient deprivation [32][50][51][52]. Growth of bcy1∆ strains on non-fermentable carbon sources is restored by deletion of any two of the TPK genes and a point-mutation of the third, denoted as tpkw (“wimpy”). These mutants were isolated from spontaneous revertants of strains carrying deletions of BCY1 and two TPK genes, which formed papillations after the parent strains had exhausted glucose on agar plates [53]. They form important tools for study as their remaining PKA activity can no longer be regulated by cAMP binding to Bcy1. Their capacity to accumulate glycogen upon nutrient exhaustion and utilize it upon nutrient repletion must therefore rely on signaling other than through PKA or on PKA regulation independent of cAMP [53].
Similar to TOR signaling, PKA has been implicated in the positive regulation of ribosome biogenesis and cell cycle progression and the repression of autophagy and the cellular stress response. It is also involved in pseudo-hyphal growth and meiosis. Further, PKA plays a major role in the regulation of metabolism; however, unlike for TOR signaling, this mainly evolves around the storage carbohydrates glycogen and trehalose, glycolysis and gluconeogenesis [1][11][54][55]. Several substrates regulated through direct phosphorylation by PKA have been identified [56][57][58][59].
Intriguingly, the inviability of the tpk1∆ tpk2∆ tpk3∆ triple deletion strain can be rescued by the additional deletion of YAK1, RIM15 or double deletion of MSN2 and MSN4 [60][61][62]. All these proteins, which are direct PKA substrates, play important roles in communicating stress signals and sending cells into quiescence, indicating that repression of these responses is the only essential PKA function [5][61][63][64][65].
Yak1, Msn2/4 and Rim15 are connected in a number of ways: while Yak1 phosphorylates and positively regulates Msn2/4 [66][67], Msn2/4 are conversely required for transcription of the YAK1 gene [62]. Similarly, Rim15 also appears to phosphorylate Msn2/4 and Rim15-dependent regulation of gene expression is to a large extent explained by Msn2/4 [68][69].
2. TOR–PKA Genetic Interactions
Findings about a genetic interaction between the TOR and PKA pathways pre-date even the discovery of TOR signaling itself, as overexpression of Sch9—later determined as the main TORC1 effector kinase—rescued a temperature-sensitive mutation of Cdc25 and deletions of components of the PKA pathway, including the catalytic subunits [70]. An overview of the many subsequently reported genetic interactions is given in Table 1.
Table 1. Genetic interactions of PKA and TOR signaling.
| Observed Phenotype | Reference | Interaction Type * |
|---|---|---|
| bcy1Δ rescues growth defect of sch9Δ | Toda, 1988 [70] | TOR + PKA |
| SCH9-overexpression rescues inviability of tpk1Δ tpk2Δ tpk3Δ, ras1Δ ras2Δ and cyr1Δ | Toda, 1988 [70] | PKA + TOR |
| SCH9 rescues temperature-sensitivity of cdc25-ts | Toda, 1988 [70] | PKA + TOR |
| ras2V19-, CDC25- or TPK1-overexpression increase rapamycin resistance of gat1Δ gln3Δ | Schmelzle, 2004 [71] | TOR + PKA |
| bcy1Δ increases rapamycin resistance of gat1Δ gln3Δ | Schmelzle, 2004 [71] | TOR + PKA |
| ira2Δ, bcy1Δ and rasV19 mutations increase rapamycin resistance | Zurita-Martinez, 2005 [72] | TOR + PKA |
| ras2Δ, tpk1Δ, tpk2Δ and tpk3Δ increase rapamycin sensitivity | Zurita-Martinez, 2005 [72] | TOR + PKA |
| tpk1Δ tpk2Δ tpk3Δ yak1Δ and tpk1Δ tpk2Δ tpk3Δ msn2Δ msn4Δ increase rapamycin sensitivity | Zurita-Martinez, 2005 [72] | TOR AND PKA |
| BCY1 and PDE2 overexpression rescue temperature sensitivity of kog1-ts | Araki, 2005 [73] | TOR -PKA |
| ras1Δ ras2-23 mutations increase rapamycin resistance (1.5–2.5 ng/mL) | Ramachandran, 2011 [74] | TOR -PKA |
| bcy1Δ increases rapamycin sensitivity (1.5 ng/mL) | Ramachandran, 2011 [74] | TOR -PKA |
| Expression of rasV19 causes rapamycin sensitivity (3 ng/mL) | Ramachandran, 2011 [74] | TOR -PKA |
| Overexpression of PDE2 causes rapamycin resistance (3 ng/mL) and rescues temperature-sensitivity of tor2-ts | Ramachandran, 2011 [74] | TOR -PKA |
| rasV19 mutant shows synthetic growth defect with tor1Δ and tor1Δ tor2-ts and with tor2-ts at non-permissive temperature | Ramachandran, 2011 [74] | TOR -PKA |
| Rapamycin treatment increases phosphorylation of PKA targets Srb9, Rim15 (after 2 h) and Cki1 (~2–3 h) | Ramachandran, 2011 [74] | TOR -| PKA |
| sch9Δ has increased basal trehalase activity during growth on glycerol, but magnitude of increase after glucose addition is decreased | Crauwels, 1997 [75] | TOR -| PKA; TOR -> PKA |
* The reported interaction is consistent with TOR + PKA: positive interaction w. possible epistasis of TOR over PKA. PKA + TOR: positive interaction w. possible epistasis of PKA over TOR. TOR AND PKA: positive interaction via AND gate. TOR -PKA: negative interaction. TOR -| PKA: negative interaction: TOR represses PKA. TOR -> PKA: positive interaction: TOR activates PKA.
After the discovery of TOR signaling, a series of experiments in the mid-2000s using rapamycin further strengthened the connection between PKA and TOR signaling: Deletion of BCY1 or overexpression of Cdc25, Tpk1 or an activated version of Ras (rasV19), all increased rapamycin resistance. These observations were most obvious in a gat1Δ gln3Δ background, indicating that PKA has the clearest effect on rescuing TOR inhibition when repression of the nitrogen discrimination pathway was rescued by independent means [71].
The gat1Δ gln3Δ mutations were, however, not strictly necessary, as deletion of IRA2 or BCY1 or the ras2V19 mutation caused rapamycin resistance in an otherwise wt strain, while deletion of RAS2 or of PKA catalytic subunits conferred rapamycin sensitivity [72].
Are these data consistent with a model in which PKA regulates TOR signaling or vice versa in a linear pathway? As the rescuing factor must act downstream or in parallel with the rescued factor, Sch9 should function downstream of PKA to rescue mutations in the PKA pathway, if assuming a linear connection [70]. In contrast, PKA should function downstream of TORC1 according to Schmelzle, 2004 [71] and Zurita-Martinez, 2005 [72]. The latter is not completely compelling, as temperature-sensitive or rapamycin-dependent inhibition may be incomplete and hyperactivation of an upstream function may alleviate a diminished downstream function. However, Toda, 1988 had also reported that hyperactivation of PKA via BCY1 deletion rescued the growth defect of sch9∆-strains [70].
It is therefore clear that a linear connection between PKA and TOR signaling cannot explain the experimental observations, and instead, a parallel placement of the pathways may be assumed. Independent of the wiring, all of the above studies reported a positive interaction between TOR and PKA signaling.
Conversely, antagonistic interactions have also been described: Araki et al. identified Pde2 and Bcy1 as suppressors of a temperature-sensitive mutation in the TORC1 subunit KOG1 (aka LAS24) [73]. A later study found that genetic manipulations activating the PKA pathway (bcy1∆ and expression of rasV19) increased rapamycin sensitivity, while ras1Δ ras2-23 mutants and cells overexpressing PDE2 were rapamycin resistant [74]. The latter also rescued the temperature sensitivity of a tor2-ts mutant, while the rasV19 mutation caused synthetic growth defects with partial inhibition of TORC1.
Therefore, the same genetic manipulations, bcy1∆ and expression of rasV19 from a single copy plasmid, lead to opposite outcomes in the studies by Zurita-Martinez et al. 2005 [72] and Ramachandran and Herman 2011 [74]: rapamycin resistance vs. rapamycin sensitivity. In addition to the use of different strain backgrounds, the major difference between the experiments was the use of different rapamycin concentrations, with at least ten times less in the latter study. It is interesting to note that this study observed increased phosphorylation of known PKA substrates upon rapamycin treatment, albeit on a timescale of hours [74]. As will be detailed below, there is in contrast ample evidence for reduced phosphorylation of substrates shared by PKA and TORC1/Sch9 upon rapamycin treatment.
Researcher propose a model in which the main interaction between TOR and PKA signaling is positive via shared substrates, but a second layer of weak mutual inhibition also exists. The latter may arise due to feedback from shared substrates. If one pathway is already deleted or strongly inhibited, further loss of input to the shared targets through inhibition of the second pathway will result in lethality or severe growth defects. In contrast, if, for example, TORC1 is only mildly inhibited (e.g., via low rapamycin), the activity of shared targets will be lowered, but sufficient to support growth when also PKA signaling is reduced (e.g., by PDE2 overexpression). Negative feedback to TORC1 will be reduced, alleviating effects on TOR-unique targets and therefore resulting in rapamycin resistance. An analogous model may explain the observation that trehalase activity, generally considered a PKA-unique readout, was increased upon SCH9 deletion [75]. Further work will be needed to test the proposed antagonistic/feedback effects. Signaling through shared functions and targets, which is, in contrast, more firmly established, will be discussed next.
3. Shared Targets
3.1. Ribosome Production
An increased rate of ribosome production to provide the machinery for growth is a hallmark of rapidly growing cells, compared to slowly growing cells under nutrient-limited conditions [76]. Ribosome production involves all three RNA polymerases for the synthesis of rRNA, ribosomal proteins and assembly factors. Considering that approximately half of all Pol II transcription initiation events take place at ribosomal protein gene promoters in rapidly growing cells and that rRNA makes up the majority of cellular RNA, it is unsurprising that these energy-intensive events are highly regulated in response to nutrient and other environmental conditions [77].
Consequently, ribosomal protein (RP) and ribosome biogenesis (RiBi; including rRNA modifiers, assembly factors and subunits of RNA polymerases I and III [78][79]) genes are two groups of genes most strongly affected by carbon source shifts. RP and RiBi genes each form regulons, i.e., groups of genes that appear highly coordinated in their expression [78][79]. Approximately 116 RP genes and >200 RiBi genes exhibit a rapid increase in expression after glucose addition to glucose-depleted cultures [79][80][81], while glucose-starved cells dramatically reduce the level of RP and RiBi transcripts within 30 min [79].
3.1.1. Ribosome Biogenesis: Dot6/Tod6 and Stb3
Transcriptomic studies found that activation of the PKA pathway (e.g., via overexpression of activated Ras2 or activated Gpa2) recapitulated transcriptional changes induced by glucose addition to cultures without a fermentable carbon source for a large number of genes [81][82].
Conversely, the glucose-dependent transcriptional changes were largely blocked when simultaneously inhibiting PKA in one study. Despite its apparent genetic interaction with PKA, inhibition of Sch9 had little effect [82].
This observation was seemingly in stark contrast to a previous finding that a majority of genes induced by glucose addition in a wt strain were still induced in a tpkw strain in which cAMP-dependent regulation of PKA is disabled through BCY1 deletion [81]. This group of genes was strongly enriched in RiBi genes. Given its genetic interaction with PKA, TOR/Sch9 signaling was an obvious suspect for the redundant pathway [81].
An interesting temporal perspective on the contribution of PKA and TOR signaling to RiBi transcriptional regulation has recently been unveiled: after an initial phase upon glucose addition, in which PKA was the dominant factor necessary for RiBi gene transcription, a co-operative effect between the pathways, with TOR signaling gaining importance, was observed [83]. As inhibition of PKA and Sch9 by Zaman et al. was performed 20 min after glucose addition, much effect of PKA and little of Sch9 inhibition was observed since the PKA contribution was dominant at this timepoint [82].
At face value, this does not resolve the discrepancy to the study by Wang et al., as both early and late induction of RiBi genes in tpkw were observed here [81]. Invoking the earlier proposal of indirect negative TOR–PKA interaction, it can, however, not be ruled out that the temporal dynamics of TOR signaling in response to glucose are altered in this strain in adaptation to the mutations in the PKA pathway. These effects would be less obvious in the context of instantaneous inhibition used by Zaman, 2009 [82].
If the temporal observations by Kunkel et al. hold true for other transcript classes remains to be determined. Interestingly, as detailed in the following, the dynamics observed for RiBi genes are not adequately explained by current knowledge of their PKA- and TOR-dependent mechanisms of transcription regulation [83].
The architectures of Ribi gene promoters are distinct from the one of RP genes, with Rap1-binding sites only present in a small subset of RiBi-promoters [84]. Instead, RiBi-promoters are enriched in PAC (Polymerase A and C) and RRPE (rRNA processing element) motifs [80][81][85][86][87]. The PAC and RRPE elements are each found in approximately half of the RiBi-promoters and approximately one-quarter of the promoters contain both elements [79]. Both elements function in the binding of transcriptional repressors, Dot6 and its homolog Tod6 in the former, and Stb3 in the latter case [24][88]. These transcription factors exert their repressive role via recruitment of the Rpd3L histone deacetylase complex [24]. This is in agreement with observations that Rpd3L is recruited to a number of rapamycin-repressed genes upon rapamycin treatment [89] but in contradiction to earlier studies that found Rpd3L binding to be constitutive [90][91].
Double deletion of DOT6 and TOD6 led to a noticeable change in transcriptional repression after glucose and nitrogen starvation [92] and both proteins were dephosphorylated as early as 5 min after these starvations and upon various stresses, such as heat, oxidative and osmotic stress, as well as upon rapamycin treatment [93]. The same study demonstrated nuclear localization of Dot6 and Tod6 following stress, nitrogen- or glucose starvation.
It was shown recently that the levels of Dot6 and Tod6 are low when cells are grown in medium without a fermentable carbon source and only rise upon glucose addition and that early RiBi gene induction upon glucose addition occurred normally in a dot6∆ tod6∆ strain [83]. Therefore, the regulatory function of these transcription factors is likely relevant upon depletion of a fermentable carbon source rather than in the relief of repression upon encountering glucose. The mechanism of RiBi gene regulation in the latter transition is still unclear [83].
One of the early phospho-proteomics screens into TOR signaling found reduced phosphorylation of Dot6 upon rapamycin treatment, which was alleviated by mutations of Sch9 that rendered it active independent of TORC1 [94]. Similar observations were made for its paralog Tod6, but here hypo-phosphorylation was additionally alleviated by rapamycin-insensitive tap42-11. These observations were only partially reflected by gel-shift assays of Dot6, while Tod6 exhibited clear dephosphorylation, that depended on the inhibition of Sch9. Dot6 was also detected in a screen for proteins interacting with a substrate-trapping mutant of Tpk1 and subsequently shown to be phosphorylated by PKA in vitro [95]. Later, in vitro phosphorylation of Dot6/Tod6 by Sch9, as well as a decrease in Dot6/Tod6 phosphorylation after inhibition of Sch9 or PKA in vivo was also observed [24]. The consequence of DOT6 and/or TOD6 deletion for RiBi gene repression upon rapamycin treatment or analog-sensitive PKA inhibition have been evaluated, and a more prominent role was attributed to Tod6 downstream of TORC1 and to Dot6 downstream of PKA [92].
A further transcriptional repressor, Stb3, that forms part of the RPD3L complex [96] was also found to be phosphorylated by PKA and Sch9 in vitro [24][97]. Stb3 phosphorylation also decreased upon Sch9 inhibition in vivo and Stb3 was recruited to both RiBi and RP-promoters upon Sch9 inhibition, which correlated with the recruitment of RPD3L [24]. A growth defect caused by Stb3 overexpression was mitigated by the deletion of PPH22, but more direct involvement of PP2A in the regulation of Stb3 is lacking [98]. Stb3 is regulated via subcellular localization as glucose addition to post-log phase cells triggered its export from the nucleus within minutes, while rapamycin addition to log-phase cells had the opposite effect [98] (Figure 2).
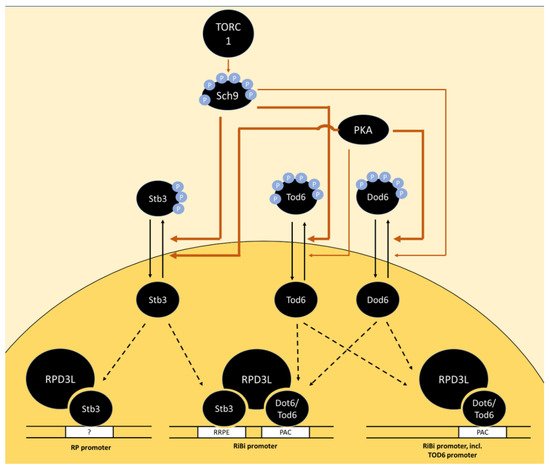
Figure 2. Model of the regulation of RP and RiBi genes via the transcriptional repressors Stb3, Tod6 and Dot6 downstream of TORC1 and PKA. In the absence of phosphorylation by Sch9 and PKA, the transcription factors Stb3, Tod6 and Dot6 bind specific promoter elements upstream of a subset of RP and RiBi genes, triggering their repression via the recruitment of histone deacetylase complex RPD3L. The promoter element bound by Stb3 in RP promoters is marked with a question mark as RP promoters generally do not contain RRPE sequences and the mode of interaction with these promoters is unclear. The thickness of arrows indicates a potentially stronger relative contribution of PKA than Sch9 on Dot6 phosphorylation and vice versa for Tod6 phosphorylation. Potential dephosphorylation of Tod6 by PP2A is omitted as direct evidence is lacking.
The phosphorylation sites on Dot6, Tod6 and Stb3, which are presumably directly phosphorylated by PKA and Sch9 (and potentially dephosphorylated by PP2A), have not yet been accurately mapped. Dot6 and Tod6 are among the proteins with the highest number of PKA motifs in yeast. Mutation of six phosphorylation sites in R[R/K]x[S/T] motifs on Tod6 and four sites in R[R/K]x[S/T] and one in an Rxx[S/T] motif on Dot6 caused a decrease, but not complete abrogation of phosphorylation by Sch9 in vitro [24]. The identity of sites phosphorylated by PKA on Dot6 and Tod6 has not been reported to date. The RRxS motif at S283 in Stb3 is conserved in yeast species from S. cerevisiae to C. albicans and was therefore proposed as a functionally important target in PKA signaling [97]. Further phospho sites with PKA consensus motifs exist on Stb3 but show a lower degree of evolutionary conservation. Signal obtained from an antibody directed against the RRxS-motif on purified Stb3 was completely lost after Sch9 inhibition, indicating that Sch9 targets the same motif on this protein. Surprisingly, inhibition of PKA alone did not cause a clear change in signal [24]. Further, the addition of cAMP to a cyr1∆ strain was not sufficient to reverse Stb3 nuclear localization under conditions of glucose depletion or rapamycin treatment [98]. In contrast, glucose re-addition to the same glucose-depleted strain (without cAMP) triggered Stb3 cytoplasmic localization [98]. Therefore, cAMP neither is necessary nor sufficient for Stb3 translocation. Together, these data suggest that TOR signaling overrides PKA-dependent regulation of Stb3 under the conditions tested.
3.1.2. Ribosomal Protein Production: Ifh1, Crf1 and Spf1
The second major regulon in ribosome production are transcripts coding for ribosomal proteins (RP). As for RiBi genes, rapamycin treatment causes severe repression of this regulon [21][99]. The main transcription factors involved in RP gene transcription are Rap1, Hmo1 [100], Sfp1 and Fhl1 with its co-factors Ifh1 and Crf1 [101][102][103]. Of these, Rap1, Sfp1 and Fhl1-Ifh1/Crf1 have been linked to the TOR and PKA pathways [101][102][104][105][106]. Additionally, Stb3, functions as a transcriptional repressor also of RP genes, albeit apparently not via the binding of RRPE promoter motifs [24][88] (Figure 2). PKA-dependent regulation of RP genes induced by Rap1 has been shown in a number of experiments [106][107]: RP-transcripts were up-regulated in a Rap1-dependent manner in a BCY1 deletion strain and the presence of the Rap1 binding site in its promoter was sufficient to confer an increase in a reporter transcript in a bcy1∆ strain compared to wt [108]. While phosphorylation of Rap1 via PKA cannot be completely ruled out, more recent models suggest, Rap1 serves as a recruiting factor for proteins regulated by PKA. These binding partners may include Sfp1 and Fhl1 and its co-factors [107].
While 127 of the 138 RP genes harbor Rap1-binding sites, approximately a half are bound by Fhl1 [101][107]. Fhl1 is not apparently regulated by PKA or TOR, instead, regulation occurs on the level of its co-regulators Ifh1 and Crf1. The level of the Ifh1 bound to RP genes drops upon nutrient depletion or rapamycin treatment and increases when cells resume growth upon encountering improved nutrient conditions, while Fhl1 remains constitutively associated with the promoters [101][102][103]. The interaction of Fhl1 and Ifh1 depends on the forkhead-associated (FHA) domain of Fhl1 [101][102], a domain previously reported to interact with phosphorylated sequences [109]. It is, therefore, reasonable to posit that Fhl1-Ifh1 interaction depends on Ifh1 phosphorylation. Martin, 2004 provided a detailed description of how transcriptional activator Ifh1 and repressor Crf1 compete for binding to Fhl1 [104]. They observed a rapamycin-triggered increase in Crf1 phosphorylation, causing its nuclear translocation and therefore RP gene repression. As this process could be prevented by mutations that render PKA signaling hyperactive, they suggested that TORC1 acts upstream of PKA in this respect [104]. They further found that protein kinase Yak1 is required for Crf1 nuclear translocation. As Yak1 is a known direct PKA, but not TORC1/Sch9 target, this is also consistent with the idea of TORC1 regulating PKA.
Surprisingly, regulation of RP gene transcription by Crf1 appears to be yeast strain dependent, as Zhao et al. observed no effect of CRF1 deletion on RP transcription in the W303, while they did observe the effect in the TB50 strain [110]. The Yak1-dependent mechanism also appears to be at odds with the finding by others that RP gene transcription is still repressed by rapamycin in strains lacking Yak1 [72]. Researcher propose that this is explained by a secondary mechanism that acts on longer timescales. Indeed, remaining RP gene inhibition in strains lacking CRF1 was observed also in the original study by Martin, 2004. What this second mechanism may be is not yet clear. Finally, to date, only CK2 has been shown to phosphorylate Ifh1 and Crf1 in a manner relevant for Fhl1 binding [111][112]. Mechanistic details if and how PKA and TORC1 regulate Fhl1-dependent RP transcription are therefore still lacking.
Sfp1 is a transcription activator of RP genes and deletion strains of SFP1 are marked by a striking small cell size phenotype [113]. While Sfp1 binds promoters of other gene groups as well, this appears to be mechanistically different [114].
Sfp1 was associated with TOR signaling in a microscopic screen as it localized from the nucleus to the cytoplasm upon rapamycin treatment [115]. Importantly, PKA signaling can override TORC1 effects to some extent as hyperactivated PKA (bcy1∆) largely counteracted the rapamycin-induced cytoplasmic relocalization. In which way do PKA and TOR signaling converge on Sfp1? Sfp1 phosphorylation was reduced upon rapamycin treatment and, in vitro phosphorylation by TORC1 was observed, while no phosphorylation was detected in an in vitro kinase assay with Sch9 [105]. This renders Sfp1 one of only a handful of direct TORC1 substrates. Seven potential TORC1 phosphorylation sites on Sfp1 were explored and their mutation almost completely abrogated further dephosphorylation upon rapamycin treatment [105]. Sfp1 was also phosphorylated by bovine PKA in vitro, but phosphorylation sites were not determined [97]. An RRxS motif at a site different from the TORC1-dependent sites and conserved in multiple yeast species was proposed to constitute an important PKA site [97]. Regulation of Sfp1 through common sites by PKA and TORC1 is therefore unlikely.
References
- Zaman, S.; Lippman, S.I.; Zhao, X.; Broach, J.R. How Saccharomyces Responds to Nutrients. Annu. Rev. Genet. 2008, 42, 27–81.
- Broach, J.R. Nutritional Control of Growth and Development in Yeast. Genetics 2012, 192, 73–105.
- Loewith, R.; Hall, M.N. Target of Rapamycin (TOR) in Nutrient Signaling and Growth Control. Genetics 2011, 189, 1177–1201.
- Eltschinger, S.; Loewith, R. TOR Complexes and the Maintenance of Cellular Homeostasis. Trends Cell Biol. 2016, 26, 148–159.
- De Virgilio, C. The Essence of Yeast Quiescence. FEMS Microbiol. Rev. 2012, 36, 306–339.
- González, A.; Hall, M.N. Nutrient Sensing and TOR Signaling in Yeast and Mammals. EMBO J. 2017, 36, 397–408.
- De Virgilio, C.; Loewith, R. The TOR Signalling Network from Yeast to Man. Int. J. Biochem. Cell Biol. 2006, 38, 1476–1481.
- Inoki, K.; Guan, K.-L. Complexity of the TOR Signaling Network. Trends Cell Biol. 2006, 16, 206–212.
- Soulard, A.; Cohen, A.; Hall, M.N. TOR Signaling in Invertebrates. Curr. Opin. Cell Biol. 2009, 21, 825–836.
- De Virgilio, C.; Loewith, R. Cell Growth Control: Little Eukaryotes Make Big Contributions. Oncogene 2006, 25, 6392–6415.
- Conrad, M.; Schothorst, J.; Kankipati, H.N.; Van Zeebroeck, G.; Rubio-Texeira, M.; Thevelein, J.M. Nutrient Sensing and Signaling in the Yeast Saccharomyces cerevisiae. FEMS Microbiol. Rev. 2014, 38, 254–299.
- Healy, A.M.; Zolnierowicz, S.; Stapleton, A.E.; Goebl, M.; DePaoli-Roach, A.A.; Pringle, J.R. CDC55, a Saccharomyces cerevisiae Gene Involved in Cellular Morphogenesis: Identification, Characterization, and Homology to the B Subunit of Mammalian Type 2A Protein Phosphatase. Mol. Cell. Biol. 1991, 11, 5767–5780.
- Van Zyl, W.; Huang, W.; Sneddon, A.A.; Stark, M.; Camier, S.; Werner, M.; Marck, C.; Sentenac, A.; Broach, J.R. Inactivation of the Protein Phosphatase 2A Regulatory Subunit A Results in Morphological and Transcriptional Defects in Saccharomyces cerevisiae. Mol. Cell. Biol. 1992, 12, 4946–4959.
- Shu, Y.; Yang, H.; Hallberg, E.; Hallberg, R. Molecular Genetic Analysis of Rts1p, a B’ Regulatory Subunit of Saccharomyces cerevisiae Protein Phosphatase 2A. Mol. Cell. Biol. 1997, 17, 3242–3253.
- Sneddon, A.A.; Cohen, P.T.; Stark, M.J. Saccharomyces cerevisiae Protein Phosphatase 2A Performs an Essential Cellular Function and Is Encoded by Two Genes. EMBO J. 1990, 9, 4339–4346.
- Sutton, A.; Immanuel, D.; Arndt, K.T. The SIT4 Protein Phosphatase Functions in Late G1 for Progression into S Phase. Mol. Cell. Biol. 1991, 11, 2133–2148.
- Luke, M.M.; Della Seta, F.; Di Como, C.J.; Sugimoto, H.; Kobayashi, R.; Arndt, K.T. The SAP, a New Family of Proteins, Associate and Function Positively with the SIT4 Phosphatase. Mol. Cell. Biol. 1996, 16, 2744–2755.
- Di Como, C.J.; Arndt, K.T. Nutrients, via the Tor Proteins, Stimulate the Association of Tap42 with Type 2A Phosphatases. Genes Dev. 1996, 10, 1904–1916.
- Jiang, Y.; Broach, J.R. Tor Proteins and Protein Phosphatase 2A Reciprocally Regulate Tap42 in Controlling Cell Growth in Yeast. EMBO J. 1999, 18, 2782–2792.
- Cooper, T.G. Transmitting the Signal of Excess Nitrogen in Saccharomyces cerevisiae from the Tor Proteins to the GATA Factors: Connecting the Dots. FEMS Microbiol. Rev. 2002, 26, 223–238.
- Cardenas, M.E.; Cutler, N.S.; Lorenz, M.C.; Di Como, C.J.; Heitman, J. The TOR Signaling Cascade Regulates Gene Expression in Response to Nutrients. Genes Dev. 1999, 13, 3271–3279.
- Beck, T.; Hall, M.N. The TOR Signalling Pathway Controls Nuclear Localization of Nutrient-Regulated Transcription Factors. Nature 1999, 402, 689–692.
- Schmidt, A.; Beck, T.; Koller, A.; Kunz, J.; Hall, M.N. The TOR Nutrient Signalling Pathway Phosphorylates NPR1 and Inhibits Turnover of the Tryptophan Permease. EMBO J. 1998, 17, 6924–6931.
- Huber, A.; French, S.L.; Tekotte, H.; Yerlikaya, S.; Stahl, M.; Perepelkina, M.P.; Tyers, M.; Rougemont, J.; Beyer, A.L.; Loewith, R. Sch9 Regulates Ribosome Biogenesis via Stb3, Dot6 and Tod6 and the Histone Deacetylase Complex RPD3L. EMBO J. 2011, 30, 3052–3064.
- Soulard, A.; Cremonesi, A.; Moes, S.; Schütz, F.; Jenö, P.; Hall, M.N. The Rapamycin-Sensitive Phosphoproteome Reveals That TOR Controls Protein Kinase A toward Some but Not All Substrates. Mol. Biol. Cell 2010, 21, 3475–3486.
- Urban, J.; Soulard, A.; Huber, A.; Lippman, S.; Mukhopadhyay, D.; Deloche, O.; Wanke, V.; Anrather, D.; Ammerer, G.; Riezman, H.; et al. Sch9 Is a Major Target of TORC1 in Saccharomyces cerevisiae. Mol. Cell 2007, 26, 663–674.
- Pearce, L.R.; Komander, D.; Alessi, D.R. The Nuts and Bolts of AGC Protein Kinases. Nat. Rev. Mol. Cell Biol. 2010, 11, 9–22.
- Liu, K.; Zhang, X.; Lester, R.L.; Dickson, R.C. The Sphingoid Long Chain Base Phytosphingosine Activates AGC-Type Protein Kinases in Saccharomyces cerevisiae Including Ypk1, Ypk2, and Sch9. J. Biol. Chem. 2005, 280, 22679–22687.
- Casamayor, A.; Torrance, P.D.; Kobayashi, T.; Thorner, J.; Alessi, D.R. Functional Counterparts of Mammalian Protein Kinases PDK1 and SGK in Budding Yeast. Curr. Biol. CB 1999, 9, 186–197.
- Van Dam, T.J.P.; Zwartkruis, F.J.T.; Bos, J.L.; Snel, B. Evolution of the TOR Pathway. J. Mol. Evol. 2011, 73, 209–220.
- Toda, T.; Cameron, S.; Sass, P.; Zoller, M.; Wigler, M. Three Different Genes in S. Cerevisiae Encode the Catalytic Subunits of the CAMP-Dependent Protein Kinase. Cell 1987, 50, 277–287.
- Toda, T.; Cameron, S.; Sass, P.; Zoller, M.; Scott, J.D.; McMullen, B.; Hurwitz, M.; Krebs, E.G.; Wigler, M. Cloning and Characterization of BCY1, a Locus Encoding a Regulatory Subunit of the Cyclic AMP-Dependent Protein Kinase in Saccharomyces cerevisiae. Mol. Cell. Biol. 1987, 7, 1371–1377.
- Kemp, B.E.; Bylund, D.B.; Huang, T.S.; Krebs, E.G. Substrate Specificity of the Cyclic AMP-Dependent Protein Kinase. Proc. Natl. Acad. Sci. USA 1975, 72, 3448–3452.
- Shabb, J.B. Physiological Substrates of CAMP-Dependent Protein Kinase. Chem. Rev. 2001, 101, 2381–2412.
- Matsumoto, K.; Uno, I.; Oshima, Y.; Ishikawa, T. Isolation and Characterization of Yeast Mutants Deficient in Adenylate Cyclase and CAMP-Dependent Protein Kinase. Proc. Natl. Acad. Sci. USA 1982, 79, 2355–2359.
- Kraakman, L.; Lemaire, K.; Ma, P.; Teunissen, A.W.; Donaton, M.C.; Van Dijck, P.; Winderickx, J.; de Winde, J.H.; Thevelein, J.M. A Saccharomyces cerevisiae G-Protein Coupled Receptor, Gpr1, Is Specifically Required for Glucose Activation of the CAMP Pathway during the Transition to Growth on Glucose. Mol. Microbiol. 1999, 32, 1002–1012.
- Tatchell, K.; Chaleff, D.T.; DeFeo-Jones, D.; Scolnick, E.M. Requirement of Either of a Pair of Ras-Related Genes of Saccharomyces cerevisiae for Spore Viability. Nature 1984, 309, 523–527.
- Mazón, M.J.; Gancedo, J.M.; Gancedo, C. Inactivation of Yeast Fructose-1,6-Bisphosphatase. In Vivo Phosphorylation of the Enzyme. J. Biol. Chem. 1982, 257, 1128–1130.
- Purwin, C.; Leidig, F.; Holzer, H. Cyclic AMP-Dependent Phosphorylation of Fructose-1,6-Bisphosphatase in Yeast. Biochem. Biophys. Res. Commun. 1982, 107, 1482–1489.
- Thevelein, J.M. Fermentable Sugars and Intracellular Acidification as Specific Activators of the RAS-Adenylate Cyclase Signalling Pathway in Yeast: The Relationship to Nutrient-Induced Cell Cycle Control. Mol. Microbiol. 1991, 5, 1301–1307.
- Nikawa, J.; Sass, P.; Wigler, M. Cloning and Characterization of the Low-Affinity Cyclic AMP Phosphodiesterase Gene of Saccharomyces cerevisiae. Mol. Cell. Biol. 1987, 7, 3629–3636.
- Sass, P.; Field, J.; Nikawa, J.; Toda, T.; Wigler, M. Cloning and Characterization of the High-Affinity CAMP Phosphodiesterase of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 1986, 83, 9303–9307.
- Ma, P.; Wera, S.; Van Dijck, P.; Thevelein, J.M. The PDE1-Encoded Low-Affinity Phosphodiesterase in the Yeast Saccharomyces cerevisiae Has a Specific Function in Controlling Agonist-Induced CAMP Signaling. Mol. Biol. Cell 1999, 10, 91–104.
- Peeters, T.; Louwet, W.; Gelade, R.; Nauwelaers, D.; Thevelein, J.M.; Versele, M. Kelch-Repeat Proteins Interacting with the G Protein Gpa2 Bypass Adenylate Cyclase for Direct Regulation of Protein Kinase A in Yeast. Proc. Natl. Acad. Sci. USA 2006, 103, 13034–13039.
- Conrad, M.; Kankipati, H.N.; Kimpe, M.; Van Zeebroeck, G.; Zhang, Z.; Thevelein, J.M. The Nutrient Transceptor/PKA Pathway Functions Independently of TOR and Responds to Leucine and Gcn2 in a TOR-Independent Manner. FEMS Yeast Res. 2017, 17, fox048.
- Thevelein, J.M.; Beullens, M. Cyclic AMP and the Stimulation of Trehalase Activity in the Yeast Saccharomyces cerevisiae by Carbon Sources, Nitrogen Sources and Inhibitors of Protein Synthesis. J. Gen. Microbiol. 1985, 131, 3199–3209.
- Steyfkens, F.; Zhang, Z.; Van Zeebroeck, G.; Thevelein, J.M. Multiple Transceptors for Macro- and Micro-Nutrients Control Diverse Cellular Properties Through the PKA Pathway in Yeast: A Paradigm for the Rapidly Expanding World of Eukaryotic Nutrient Transceptors up to Those in Human Cells. Front. Pharmacol. 2018, 9, 191.
- Haesendonckx, S.; Tudisca, V.; Voordeckers, K.; Moreno, S.; Thevelein, J.M.; Portela, P. The Activation Loop of PKA Catalytic Isoforms Is Differentially Phosphorylated by Pkh Protein Kinases in Saccharomyces cerevisiae. Biochem. J. 2012, 448, 307–320.
- Steichen, J.M.; Iyer, G.H.; Li, S.; Saldanha, S.A.; Deal, M.S.; Woods, V.L.; Taylor, S.S. Global Consequences of Activation Loop Phosphorylation on Protein Kinase A. J. Biol. Chem. 2010, 285, 3825–3832.
- Marshall, M.S.; Gibbs, J.B.; Scolnick, E.M.; Sigal, I.S. Regulatory Function of the Saccharomyces cerevisiae RAS C-Terminus. Mol. Cell. Biol. 1987, 7, 2309–2315.
- Cannon, J.F.; Tatchell, K. Characterization of Saccharomyces cerevisiae Genes Encoding Subunits of Cyclic AMP-Dependent Protein Kinase. Mol. Cell. Biol. 1987, 7, 2653–2663.
- Matsumoto, K.; Uno, I.; Ishikawa, T. Control of Cell Division in Saccharomyces cerevisiae Mutants Defective in Adenylate Cyclase and CAMP-Dependent Protein Kinase. Exp. Cell Res. 1983, 146, 151–161.
- Cameron, S.; Levin, L.; Zoller, M.; Wigler, M. CAMP-Independent Control of Sporulation, Glycogen Metabolism, and Heat Shock Resistance in S. Cerevisiae. Cell 1988, 53, 555–566.
- Uno, I.; Matsumoto, K.; Ishikawa, T. Characterization of a Cyclic Nucleotide Phosphodiesterase-Deficient Mutant in Yeast. J. Biol. Chem. 1983, 258, 3539–3542.
- Santangelo, G.M. Glucose Signaling in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 2006, 70, 253–282.
- Dihazi, H.; Kessler, R.; Eschrich, K. Glucose-Induced Stimulation of the Ras-CAMP Pathway in Yeast Leads to Multiple Phosphorylations and Activation of 6-Phosphofructo-2-Kinase. Biochemistry 2003, 42, 6275–6282.
- Portela, P.; Howell, S.; Moreno, S.; Rossi, S. In Vivo and in Vitro Phosphorylation of Two Isoforms of Yeast Pyruvate Kinase by Protein Kinase A. J. Biol. Chem. 2002, 277, 30477–30487.
- Schepers, W.; Van Zeebroeck, G.; Pinkse, M.; Verhaert, P.; Thevelein, J.M. In Vivo Phosphorylation of Ser21 and Ser83 during Nutrient-Induced Activation of the Yeast Protein Kinase A (PKA) Target Trehalase. J. Biol. Chem. 2012, 287, 44130–44142.
- Rittenhouse, J.; Moberly, L.; Marcus, F. Phosphorylation in Vivo of Yeast (Saccharomyces cerevisiae) Fructose-1,6-Bisphosphatase at the Cyclic AMP-Dependent Site. J. Biol. Chem. 1987, 262, 10114–10119.
- Garrett, S.; Broach, J. Loss of Ras Activity in Saccharomyces cerevisiae Is Suppressed by Disruptions of a New Kinase Gene, YAKI, Whose Product May Act Downstream of the CAMP-Dependent Protein Kinase. Genes Dev. 1989, 3, 1336–1348.
- Reinders, A.; Bürckert, N.; Boller, T.; Wiemken, A.; De Virgilio, C. Saccharomyces cerevisiae CAMP-Dependent Protein Kinase Controls Entry into Stationary Phase through the Rim15p Protein Kinase. Genes Dev. 1998, 12, 2943–2955.
- Smith, A.; Ward, M.P.; Garrett, S. Yeast PKA Represses Msn2p/Msn4p-Dependent Gene Expression to Regulate Growth, Stress Response and Glycogen Accumulation. EMBO J. 1998, 17, 3556–3564.
- Garrett, S.; Menold, M.M.; Broach, J.R. The Saccharomyces cerevisiae YAK1 Gene Encodes a Protein Kinase That Is Induced by Arrest Early in the Cell Cycle. Mol. Cell. Biol. 1991, 11, 4045–4052.
- Görner, W.; Durchschlag, E.; Martinez-Pastor, M.T.; Estruch, F.; Ammerer, G.; Hamilton, B.; Ruis, H.; Schüller, C. Nuclear Localization of the C2H2 Zinc Finger Protein Msn2p Is Regulated by Stress and Protein Kinase A Activity. Genes Dev. 1998, 12, 586–597.
- Lee, P.; Paik, S.-M.; Shin, C.-S.; Huh, W.-K.; Hahn, J.-S. Regulation of Yeast Yak1 Kinase by PKA and Autophosphorylation-Dependent 14-3-3 Binding. Mol. Microbiol. 2011, 79, 633–646.
- Lee, P.; Cho, B.-R.; Joo, H.-S.; Hahn, J.-S. Yeast Yak1 Kinase, a Bridge between PKA and Stress-Responsive Transcription Factors, Hsf1 and Msn2/Msn4. Mol. Microbiol. 2008, 70, 882–895.
- Malcher, M.; Schladebeck, S.; Mösch, H.-U. The Yak1 Protein Kinase Lies at the Center of a Regulatory Cascade Affecting Adhesive Growth and Stress Resistance in Saccharomyces cerevisiae. Genetics 2011, 187, 717–730.
- Lee, P.; Kim, M.S.; Paik, S.-M.; Choi, S.-H.; Cho, B.-R.; Hahn, J.-S. Rim15-Dependent Activation of Hsf1 and Msn2/4 Transcription Factors by Direct Phosphorylation in Saccharomyces cerevisiae. FEBS Lett. 2013, 587, 3648–3655.
- Cameroni, E.; Hulo, N.; Roosen, J.; Winderickx, J.; De Virgilio, C. The Novel Yeast PAS Kinase Rim 15 Orchestrates G0-Associated Antioxidant Defense Mechanisms. Cell Cycle 2004, 3, 462–468.
- Toda, T.; Cameron, S.; Sass, P.; Wigler, M. SCH9, a Gene of Saccharomyces cerevisiae That Encodes a Protein Distinct from, but Functionally and Structurally Related to, CAMP-Dependent Protein Kinase Catalytic Subunits. Genes Dev. 1988, 2, 517–527.
- Schmelzle, T.; Beck, T.; Martin, D.E.; Hall, M.N. Activation of the RAS/Cyclic AMP Pathway Suppresses a TOR Deficiency in Yeast. Mol. Cell. Biol. 2004, 24, 338–351.
- Zurita-Martinez, S.A.; Cardenas, M.E. Tor and Cyclic AMP-Protein Kinase A: Two Parallel Pathways Regulating Expression of Genes Required for Cell Growth. Eukaryot. Cell 2005, 4, 63–71.
- Araki, T.; Uesono, Y.; Oguchi, T.; Toh-E, A. LAS24/KOG1, a Component of the TOR Complex 1 (TORC1), Is Needed for Resistance to Local Anesthetic Tetracaine and Normal Distribution of Actin Cytoskeleton in Yeast. Genes Genet. Syst. 2005, 80, 325–343.
- Ramachandran, V.; Herman, P.K. Antagonistic Interactions between the CAMP-Dependent Protein Kinase and Tor Signaling Pathways Modulate Cell Growth in Saccharomyces cerevisiae. Genetics 2011, 187, 441–454.
- Crauwels, M.; Donaton, M.C.; Pernambuco, M.B.; Winderickx, J.; de Winde, J.H.; Thevelein, J.M. The Sch9 Protein Kinase in the Yeast Saccharomyces cerevisiae Controls CAPK Activity and Is Required for Nitrogen Activation of the Fermentable-Growth-Medium-Induced (FGM) Pathway. Microbiology 1997, 143, 2627–2637.
- Rudra, D.; Warner, J.R. What Better Measure than Ribosome Synthesis? Genes Dev. 2004, 18, 2431–2436.
- Warner, J.R. The Economics of Ribosome Biosynthesis in Yeast. Trends Biochem. Sci. 1999, 24, 437–440.
- Gasch, A.P.; Spellman, P.T.; Kao, C.M.; Carmel-Harel, O.; Eisen, M.B.; Storz, G.; Botstein, D.; Brown, P.O. Genomic Expression Programs in the Response of Yeast Cells to Environmental Changes. Mol. Biol. Cell 2000, 11, 4241–4257.
- Jorgensen, P.; Rupes, I.; Sharom, J.R.; Schneper, L.; Broach, J.R.; Tyers, M. A Dynamic Transcriptional Network Communicates Growth Potential to Ribosome Synthesis and Critical Cell Size. Genes Dev. 2004, 18, 2491–2505.
- Slattery, M.G.; Heideman, W. Coordinated Regulation of Growth Genes in Saccharomyces cerevisiae. Cell Cycle 2007, 6, 1210–1219.
- Wang, Y.; Pierce, M.; Schneper, L.; Güldal, C.G.; Zhang, X.; Tavazoie, S.; Broach, J.R. Ras and Gpa2 Mediate One Branch of a Redundant Glucose Signaling Pathway in Yeast. PLoS Biol. 2004, 2, e128.
- Zaman, S.; Lippman, S.I.; Schneper, L.; Slonim, N.; Broach, J.R. Glucose Regulates Transcription in Yeast through a Network of Signaling Pathways. Mol. Syst. Biol. 2009, 5, 245.
- Kunkel, J.; Luo, X.; Capaldi, A.P. Integrated TORC1 and PKA Signaling Control the Temporal Activation of Glucose-Induced Gene Expression in Yeast. Nat. Commun. 2019, 10, 3558.
- Bosio, M.C.; Fermi, B.; Spagnoli, G.; Levati, E.; Rubbi, L.; Ferrari, R.; Pellegrini, M.; Dieci, G. Abf1 and Other General Regulatory Factors Control Ribosome Biogenesis Gene Expression in Budding Yeast. Nucleic Acids Res. 2017, 45, 4493–4506.
- Fingerman, I.; Nagaraj, V.; Norris, D.; Vershon, A.K. Sfp1 Plays a Key Role in Yeast Ribosome Biogenesis. Eukaryot. Cell 2003, 2, 1061–1068.
- Dequard-Chablat, M.; Riva, M.; Carles, C.; Sentenac, A. RPC19, the Gene for a Subunit Common to Yeast RNA Polymerases A (I) and C (III). J. Biol. Chem. 1991, 266, 15300–15307.
- Hughes, J.D.; Estep, P.W.; Tavazoie, S.; Church, G.M. Computational Identification of Cis-Regulatory Elements Associated with Groups of Functionally Related Genes in Saccharomyces cerevisiae. J. Mol. Biol. 2000, 296, 1205–1214.
- Liko, D.; Slattery, M.G.; Heideman, W. Stb3 Binds to Ribosomal RNA Processing Element Motifs That Control Transcriptional Responses to Growth in Saccharomyces cerevisiae. J. Biol. Chem. 2007, 282, 26623–26628.
- Humphrey, E.L.; Shamji, A.F.; Bernstein, B.E.; Schreiber, S.L. Rpd3p Relocation Mediates a Transcriptional Response to Rapamycin in Yeast. Chem. Biol. 2004, 11, 295–299.
- Kurdistani, S.K.; Robyr, D.; Tavazoie, S.; Grunstein, M. Genome-Wide Binding Map of the Histone Deacetylase Rpd3 in Yeast. Nat. Genet. 2002, 31, 248–254.
- Rohde, J.R.; Cardenas, M.E. The Tor Pathway Regulates Gene Expression by Linking Nutrient Sensing to Histone Acetylation. Mol. Cell. Biol. 2003, 23, 629–635.
- Lippman, S.I.; Broach, J.R. Protein Kinase A and TORC1 Activate Genes for Ribosomal Biogenesis by Inactivating Repressors Encoded by Dot6 and Its Homolog Tod6. Proc. Natl. Acad. Sci. USA 2009, 106, 19928–19933.
- Hughes Hallett, J.E.; Luo, X.; Capaldi, A.P. State Transitions in the TORC1 Signaling Pathway and Information Processing in Saccharomyces cerevisiae. Genetics 2014, 198, 773–786.
- Huber, A.; Bodenmiller, B.; Uotila, A.; Stahl, M.; Wanka, S.; Gerrits, B.; Aebersold, R.; Loewith, R. Characterization of the Rapamycin-Sensitive Phosphoproteome Reveals That Sch9 Is a Central Coordinator of Protein Synthesis. Genes Dev. 2009, 23, 1929–1943.
- Deminoff, S.J.; Howard, S.C.; Hester, A.; Warner, S.; Herman, P.K. Using Substrate-Binding Variants of the CAMP-Dependent Protein Kinase to Identify Novel Targets and a Kinase Domain Important for Substrate Interactions in Saccharomyces cerevisiae. Genetics 2006, 173, 1909–1917.
- Kasten, M.M.; Stillman, D.J. Identification of the Saccharomyces cerevisiae Genes STB1-STB5 Encoding Sin3p Binding Proteins. Mol. Gen. Genet. MGG 1997, 256, 376–386.
- Budovskaya, Y.V.; Stephan, J.S.; Deminoff, S.J.; Herman, P.K. An Evolutionary Proteomics Approach Identifies Substrates of the CAMP-Dependent Protein Kinase. Proc. Natl. Acad. Sci. USA 2005, 102, 13933–13938.
- Liko, D.; Conway, M.K.; Grunwald, D.S.; Heideman, W. Stb3 Plays a Role in the Glucose-Induced Transition from Quiescence to Growth in Saccharomyces cerevisiae. Genetics 2010, 185, 797–810.
- Powers, T.; Walter, P. Regulation of Ribosome Biogenesis by the Rapamycin-Sensitive TOR-Signaling Pathway in Saccharomyces cerevisiae. Mol. Biol. Cell 1999, 10, 987–1000.
- Hall, D.B.; Wade, J.T.; Struhl, K. An HMG Protein, Hmo1, Associates with Promoters of Many Ribosomal Protein Genes and throughout the RRNA Gene Locus in Saccharomyces cerevisiae. Mol. Cell. Biol. 2006, 26, 3672–3679.
- Schawalder, S.B.; Kabani, M.; Howald, I.; Choudhury, U.; Werner, M.; Shore, D. Growth-Regulated Recruitment of the Essential Yeast Ribosomal Protein Gene Activator Ifh1. Nature 2004, 432, 1058–1061.
- Rudra, D.; Zhao, Y.; Warner, J.R. Central Role of Ifh1p-Fhl1p Interaction in the Synthesis of Yeast Ribosomal Proteins. EMBO J. 2005, 24, 533–542.
- Wade, J.T.; Hall, D.B.; Struhl, K. The Transcription Factor Ifh1 Is a Key Regulator of Yeast Ribosomal Protein Genes. Nature 2004, 432, 1054–1058.
- Martin, D.E.; Soulard, A.; Hall, M.N. TOR Regulates Ribosomal Protein Gene Expression via PKA and the Forkhead Transcription Factor FHL1. Cell 2004, 119, 969–979.
- Lempiäinen, H.; Uotila, A.; Urban, J.; Dohnal, I.; Ammerer, G.; Loewith, R.; Shore, D. Sfp1 Interaction with TORC1 and Mrs6 Reveals Feedback Regulation on TOR Signaling. Mol. Cell 2009, 33, 704–716.
- Klein, C.; Struhl, K. Protein Kinase A Mediates Growth-Regulated Expression of Yeast Ribosomal Protein Genes by Modulating RAP1 Transcriptional Activity. Mol. Cell. Biol. 1994, 14, 1920–1928.
- Bosio, M.C.; Negri, R.; Dieci, G. Promoter Architectures in the Yeast Ribosomal Expression Program. Transcription 2011, 2, 71–77.
- De Sanctis, V.; La Terra, S.; Bianchi, A.; Shore, D.; Burderi, L.; Di Mauro, E.; Negri, R. In Vivo Topography of Rap1p-DNA Complex at Saccharomyces cerevisiae TEF2 UAS(RPG) during Transcriptional Regulation. J. Mol. Biol. 2002, 318, 333–349.
- Durocher, D.; Jackson, S.P. The FHA Domain. FEBS Lett. 2002, 513, 58–66.
- Zhao, Y.; McIntosh, K.B.; Rudra, D.; Schawalder, S.; Shore, D.; Warner, J.R. Fine-Structure Analysis of Ribosomal Protein Gene Transcription. Mol. Cell. Biol. 2006, 26, 4853–4862.
- Kim, M.S.; Hahn, J.-S. Role of CK2-Dependent Phosphorylation of Ifh1 and Crf1 in Transcriptional Regulation of Ribosomal Protein Genes in Saccharomyces cerevisiae. Biochim. Biophys. Acta BBA Gene Regul. Mech. 2016, 1859, 1004–1013.
- Rudra, D.; Mallick, J.; Zhao, Y.; Warner, J.R. Potential Interface between Ribosomal Protein Production and Pre-RRNA Processing. Mol. Cell. Biol. 2007, 27, 4815–4824.
- Jorgensen, P.; Nishikawa, J.L.; Breitkreutz, B.-J.; Tyers, M. Systematic Identification of Pathways That Couple Cell Growth and Division in Yeast. Science 2002, 297, 395–400.
- Albert, B.; Kos-Braun, I.C.; Henras, A.K.; Dez, C.; Rueda, M.P.; Zhang, X.; Gadal, O.; Kos, M.; Shore, D. A Ribosome Assembly Stress Response Regulates Transcription to Maintain Proteome Homeostasis. eLife 2019, 8, e45002.
- Marion, R.M.; Regev, A.; Segal, E.; Barash, Y.; Koller, D.; Friedman, N.; O’Shea, E.K. Sfp1 Is a Stress- and Nutrient-Sensitive Regulator of Ribosomal Protein Gene Expression. Proc. Natl. Acad. Sci. USA 2004, 101, 14315–14322.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
817
Revisions:
2 times
(View History)
Update Date:
16 Feb 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




