
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Chang-Tong Yang | + 1851 word(s) | 1851 | 2022-02-15 07:37:42 | | | |
| 2 | Yvaine Wei | + 3 word(s) | 1854 | 2022-02-16 04:48:50 | | |
Video Upload Options
Nuclear imaging is a powerful non-invasive imaging technique that is rapidly developing in medical theranostics. Nuclear imaging requires radiolabeling isotopes for non-invasive imaging through the radioactive decay emission of the radionuclide. Nuclear imaging probes, commonly known as radiotracers, are radioisotope-labeled small molecules. Nanomaterials have shown potential as nuclear imaging probes for theranostic applications. By modifying the surface of nanomaterials, multifunctional radio-labeled nanomaterials can be obtained for in vivo biodistribution and targeting in initial animal imaging studies. Various surface modification strategies have been developed, and targeting moieties have been attached to the nanomaterials to render biocompatibility and enable specific targeting. Through integration of complementary imaging probes to a single nanoparticulate, multimodal molecular imaging can be performed as images with high sensitivity, resolution, and specificity.
1. Introduction
2. Challenges of Nuclear Imaging and the Role of Nanomaterials
3. Modifications of Nanomaterials for Nuclear Imaging
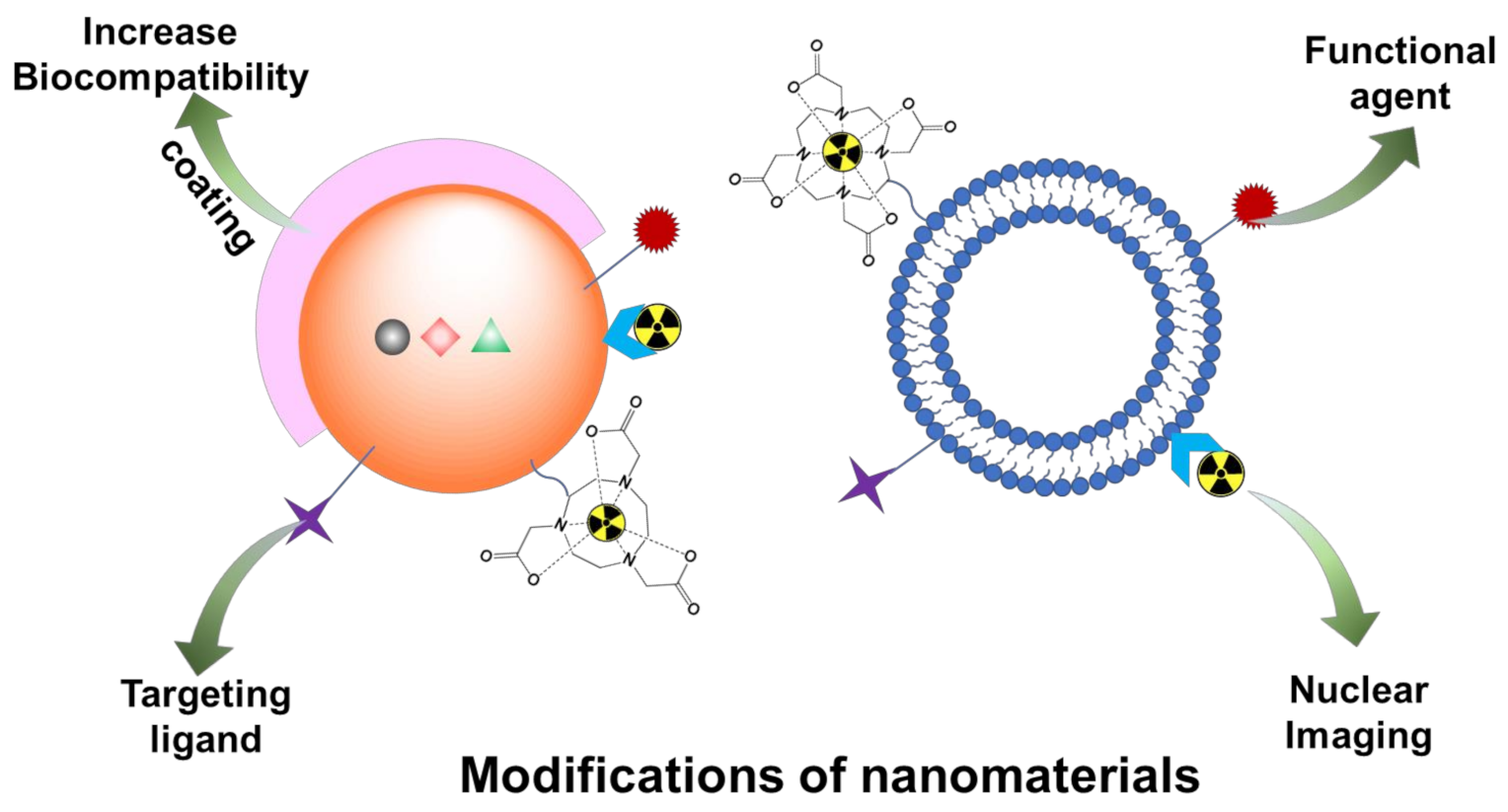
3.1. Coating
3.2. Active Targeting Moieties for Disease-Specific Receptors
4. Nanomaterials for Theranostic Nuclear Imaging Probes
5. Radiolabeled Exosomes for Nuclear Imaging
6. Conclusions
References
- Chen, K.; Chen, X. Design and development of molecular imaging probes. Curr. Top. Med. Chem. 2010, 10, 1227–1236.
- Hussain, T.; Nguyen, Q.T. Molecular imaging for cancer diagnosis and surgery. Adv. Drug Deliv. Rev. 2014, 66, 90–100.
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science 2009, 324, 1029–1033.
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124.
- Wells, R.G. Instrumentation in molecular imaging. J. Nucl. Cardiol. 2016, 23, 1343–1347.
- Erdi, Y.E. Limits of Tumor Detectability in Nuclear Medicine and PET. Mol. Imaging Radionucl. Ther. 2012, 21, 23–28.
- Pellico, J.; Gawne, P.J.; de Rosales, R.T.M. Radiolabelling of nanomaterials for medical imaging and therapy. Chem. Soc. Rev. 2021, 50, 3355–3423.
- Wei, Y.; Tang, T.; Pang, H.-B. Cellular internalization of bystander nanomaterial induced by TAT-nanoparticles and regulated by extracellular cysteine. Nat. Commun. 2019, 10, 3646.
- Yang, C.-T.; Hattiholi, A.; Selvan, S.T.; Yan, S.X.; Fang, W.-W.; Chandrasekharan, P.; Koteswaraiah, P.; Herold, C.J.; Gulyás, B.; Aw, S.E.; et al. Gadolinium-based bimodal probes to enhance T1-Weighted magnetic resonance/optical imaging. Acta Biomater. 2020, 110, 15–36.
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71.
- Xia, Y.; Matham, M.V.; Su, H.; Padmanabhan, P.; Gulyás, B. Nanoparticulate Contrast Agents for Multimodality Molecular Imaging. J. Biomed. Nanotechnol. 2016, 12, 1553–1584.
- Kim, J.; Lee, N.; Hyeon, T. Recent development of nanoparticles for molecular imaging. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2017, 375, 20170022.
- Kraeber-Bodéré, F.; Barbet, J. Challenges in Nuclear Medicine: Innovative Theranostic Tools for Personalized Medicine. Front. Med. 2014, 1, 16.
- Chen, Z.; Haider, A.; Chen, J.; Xiao, Z.; Gobbi, L.; Honer, M.; Grether, U.; Arnold, S.E.; Josephson, L.; Liang, S.H. The Repertoire of Small-Molecule PET Probes for Neuroinflammation Imaging: Challenges and Opportunities beyond TSPO. J. Med. Chem. 2021, 64, 17656–17689.
- Farzin, L.; Sheibani, S.; Moassesi, M.E.; Shamsipur, M. An overview of nanoscale radionuclides and radiolabeled nanomaterials commonly used for nuclear molecular imaging and therapeutic functions. J. Biomed. Mater. Res. A 2019, 107, 251–285.
- Prashant, C.; Dipak, M.; Yang, C.-T.; Chuang, K.-H.; Jun, D.; Feng, S.-S. Superparamagnetic iron oxide—Loaded poly (lactic acid)-d-α-tocopherol polyethylene glycol 1000 succinate copolymer nanoparticles as MRI contrast agent. Biomaterials 2010, 31, 5588–5597.
- Dipak, M.; Prashant, C.; Yang, C.-T.; Chuang, K.-H.; Feng, S.-S.; Ding, J. Facile synthesis of water-stable fine magnetite nanoparticles for MRI and magnetic hyperthermia applications. Nanomedicine 2010, 5, 1571–1584.
- Fang, J.; Chandrasekharan, P.; Liu, X.-L.; Yang, Y.; Lv, Y.-B.; Yang, C.-T.; Ding, J. Manipulating the surface coating of ultra-small Gd2O3 nanoparticles for improved T1-weighted MR imaging. Biomaterials 2014, 35, 1636–1642.
- Lao, L.L.; Ramanujan, R.V. Magnetic and hydrogel composite materials for hyperthermia applications. J. Mater. Sci. Mater. Med. 2004, 15, 1061–1064.
- Bae, Y.H.; Park, K. Targeted drug delivery to tumors: Myths, reality and possibility. J. Control. Release 2011, 153, 198–205.
- Brannon-Peppas, L.; Blanchette, J.O. Nanoparticle and targeted systems for cancer therapy. Adv. Drug Deliv. Rev. 2012, 64, 206–212.
- Velikyan, I. Prospective of 68Ga-Radiopharmaceutical Development. Theranostics 2014, 4, 47–80.
- Augustine, R.; Al Mamun, A.; Hasan, A.; Salam, S.A.; Chandrasekaran, R.; Ahmed, R.; Thakor, A.S. Imaging cancer cells with nanostructures: Prospects of nanotechnology driven non-invasive cancer diagnosis. Adv. Colloid Interface Sci. 2021, 294, 102457.
- Aranda-Laraa, L.; Morales-Avilab, E.; Luna-Gutiérrezc, M.A.; Olivé-Alvarezd, E.; Isaac-Olivéa, K. Radiolabeled liposomes and lipoproteins as lipidic nanoparticles for imaging and therapy. Chem. Phys. Lipids 2020, 230, 104934.
- Chen, K.; Li, Z.-B.; Wang, H.; Cai, W.; Chen, X. Dual-modality optical and positron emission tomography imaging of vascular endothelial growth factor receptor on tumor vasculature using quantum dots. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 2235–2244.
- Hall, M.A.; Kwon, S.; Robinson, H.; Lachance, P.-A.; Azhdarinia, A.; Ranganathan, R.; Price, R.E.; Chan, W.; Sevick-Muraca, E.M. Imaging prostate cancer lymph node metastases with a multimodality contrast agent. Prostate 2012, 72, 129–146.
- Harding, C.V.; Heuser, J.E.; Stahl, P.D. Exosomes: Looking back three decades and into the future. J. Cell Biol. 2013, 200, 367–371.
- Pan, B.T.; Teng, K.; Wu, C.; Adam, M.; Johnstone, R.M. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J. Cell Biol. 1985, 101, 942–948.
- Isola, A.L.; Chen, S. Exosomes: The Messengers of Health and Disease. Curr. Neuropharmacol. 2017, 15, 157–165.
- Raposo, G.; Nijman, H.W.; Stoorvogel, W.; Liejendekker, R.; Harding, C.V.; Melief, C.J.M.; Geuze, H.J. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 1996, 183, 1161–1172.
- Dai, J.; Su, Y.; Zhong, S.; Cong, L.; Liu, B.; Yang, J.; Tao, Y.; He, Z.; Chen, C.; Jiang, Y. Exosomes: Key players in cancer and potential therapeutic strategy. Signal Transduct. Target. Ther. 2020, 5, 145.
- Zhao, Y.; Liu, P.; Tan, H.; Chen, X.; Wang, Q.; Chen, T. Exosomes as Smart Nanoplatforms for Diagnosis and Therapy of Cancer. Front. Oncol. 2021, 11, 3364.
- Zitvogel, L.; Regnault, A.; Lozier, A.; Wolfers, J.; Flament, C.; Tenza, D.; Ricciardi-Castagnoli, P.; Raposo, G.; Amigorena, S. Eradication of established murine tumors using a novel cell-free vaccine: Dendritic cell derived exosomes. Nat. Med. 1998, 4, 594–600.
- Deep, G. Exosomes-based biomarker discovery for diagnosis and prognosis of prostate cancer. Front. Biosci. 2017, 22, 1682–1696.
- Tai, Y.-L.; Chen, K.-C.; Hsieh, J.-T.; Shen, T.-L. Exosomes in cancer development and clinical applications. Cancer Sci. 2018, 109, 2364–2374.
- Khan, A.A.; de Rosales, R.T.M. Radiolabelling of Extracellular Vesicles for PET and SPECT imaging. Nanotheranostics 2021, 5, 256–274.
- Di Rocco, G.; Baldari, S.; Toietta, G. Towards Therapeutic Delivery of Extracellular Vesicles: Strategies for In Vivo Tracking and Biodistribution Analysis. Stem Cells Int. 2016, 2016, 1–12.
- Royo, F.; Cossío, U.; de Angulo, A.R.; Llop, J.; Falcon-Perez, J.M. Modification of the glycosylation of extracellular vesicles alters their biodistribution in mice. Nanoscale 2019, 11, 1531–1537.
- Kim, D.H.; Kothandan, V.K.; Kim, H.W.; Kim, K.S.; Kim, J.Y.; Cho, H.J.; Lee, Y.-K.; Lee, D.-E.; Hwang, S.R. Noninvasive Assessment of Exosome Pharmacokinetics In Vivo: A Review. Pharmaceutics 2019, 11, 649.




