Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Luca Catalina | + 4789 word(s) | 4789 | 2022-02-14 10:06:30 | | | |
| 2 | Dean Liu | Meta information modification | 4789 | 2022-02-15 10:28:52 | | | | |
| 3 | Dean Liu | Meta information modification | 4789 | 2022-02-18 10:31:10 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Catalina, L. Effects of Exercise on the Autonomic Nervous System. Encyclopedia. Available online: https://encyclopedia.pub/entry/19443 (accessed on 07 February 2026).
Catalina L. Effects of Exercise on the Autonomic Nervous System. Encyclopedia. Available at: https://encyclopedia.pub/entry/19443. Accessed February 07, 2026.
Catalina, Luca. "Effects of Exercise on the Autonomic Nervous System" Encyclopedia, https://encyclopedia.pub/entry/19443 (accessed February 07, 2026).
Catalina, L. (2022, February 15). Effects of Exercise on the Autonomic Nervous System. In Encyclopedia. https://encyclopedia.pub/entry/19443
Catalina, Luca. "Effects of Exercise on the Autonomic Nervous System." Encyclopedia. Web. 15 February, 2022.
Copy Citation
The autonomic nervous system (ANS) has an important impact on health in general. In response to environmental demands, homeostatic processes are often compromised, therefore determining an increase in the sympathetic nervous system (SNS)’s functions and a decrease in the parasympathetic nervous system (PNS)’s functions.
autonomic nervous system
sympathetic
parasympathetic
immunity
1. Physical Exercise
Regular physical exercise is a key factor for the prevention of many chronic diseases [1]. Physical exercise (PE) can be used as a primary non-pharmacological clinical tool because it can improve antioxidant capacity, reduce oxidative stress and inflammation and increase energy efficiency. Depending on the volume, the intensity and the frequency of exercise, acute or chronic biochemical and physiological responses are induced.
The positive impact of physical activity is well known and has been studied by many researchers. But physical activity may also have negative impacts on the body, depending on the type of effort, the duration of the effort, and the individual characteristics of the person exerting the effort (age, gender, diseases, etc.). These negative impacts are less well studied and seem to be linked with the oxidative stress and inflammation induced by effort, mainly reflected in the increase in oxidants and decrease in antioxidants during physical activity. Due to the fact that the level of antioxidants in the body decreases with age, age is an important factor in the body’s response to oxidative stress.
2. Physical Exercise and Oxidative Stress
During exercise, an increase in respiration and oxygen uptake directed to the body’s vital organs take place. Increased oxygen consumption due to higher energy requirements results in increased levels of reactive oxygen and nitrogen species [2]. ROS and other free radicals produced cause oxidative stress at the level of vital organs and this causes cells to defend themselves using antioxidants. Antioxidants can be divided into endogenous antioxidants (glutathione; vitamins C, A and E; uric acid; and iron binding protein) and antioxidant enzymes (AOE) (superoxidase dismutase, CAT and glutathione peroxidase). AOE activity undergoes changes due to modifications in the consumption of oxygen in the body (oxidative stress). The systemic levels of antioxidant during exercise depend on the type, mode, intensity, frequency and duration of the exercise. During exercise, the blood flow is increased to the vital organs and muscles but is lowered to the liver, and this has an impact on antioxidant levels. The intra and extracellular transportation of glutathione is affected and the synthesis and degradation of glutathione is also affected. This explains why the efficacy of antioxidant systems differs after acute exercise and exercise training [3].
Over the years, studies conducted on the impact of PE on the body in general, but also on elderly people in particular, have shown a positive impact of PE on lowering the risk of age-related diseases. PE can impact the activity of antioxidants during effort, and this is one of the mechanisms considered to be implicated in lowering the risk of age-related diseases. Oxidative stress seems to reach higher levels during high-intensity acute exercises. This connection was studied by Vezzoli et al., with the aim of assessing the impact of high-intensity discontinuous training (HIDT) on oxidative stress and damage. Oxidative damage markers (thiobarbituric acid reactive substances, protein carbonyls, 8-hydroxy-2-deoxy-guanosine and total antioxidant capacity) were used to assess the participants before and after the training. There was no difference between the two groups regarding the levels of oxidative stress induced by exercise and the beneficial effects of training on redox homeostasis were similar [4]. High signaling could have an impact on sympathetic outflow and endothelium-dependent relaxation in relation to the increased expression of the genes implicated. High levels of ROS caused by effort induce the activation of antioxidant defenses and this causes a positive adaptation of both the CNS and PNS [4].
Yen et al. studied the impact of exercise training on a group of 42 patients undergoing chemotherapy for head and neck cancer because it has previously been shown that chemotherapy has a negative impact on fitness performance and can cause an increase oxidative stress. The patients were included in an eight-week exercise course that included aerobic and resistance exercises carried out three days a week. Blood pressure and heart rate were used to assess the exercise capacity and responses, showing an increase in the exercise capacity and an amelioration of exercise responses. Blood pressure at rest was decreased, with an increase 1 to 3 min after the physical exercise. Oxidative stress markers (8-hydroxy-20-deoxyguanosine, malondialdehyde, and carbonyl content) were also evaluated, along with total antioxidant capacity. The levels of oxidative stress were decreased, and the levels of antioxidants were increased. The results of the study show that in this category of patients training can decrease systemic oxidative stress and it also has a positive impact on exercise capacity and response [5].
Moderate physical activity has a positive impact on the body because it helps maintain the health of bones, muscles and joints; helps maintain normal levels of cholesterol and body weight; and also decreases levels of cholesterol and overweight. During this type of effort, the level of free radicals produced is moderate and the body can adapt. The body also tries to adapt during exhaustive physical activity but the levels of oxidants produced are much higher so this will cause an imbalance between oxidants and antioxidants resulting in oxidative damage (lipid oxidation, protein oxidation and DNA oxidation). This makes body more vulnerable to fatigue, injury and disease [6].
The effects of professional training regarding redox balance were studied by Tong et al. In this study, 10 adolescent runners were included and the effects of a 21 km running time trial on the status of oxidants and antioxidants were evaluated twice in a year. The serum concentrations of thiobarbituric acid-reactive substances (TBARS), reduced glutathione (GSH), xanthine oxidase (XO), superoxide dismutase (SOD), catalase (CAT) and total antioxidant capacity (T-AOC) were determined before and 4 h after the 21 km run. The serum concentrations of TBARS and SOD were lower after the run, while XO, CAT, TAOC and GSH remained the same as before. At the subsequent evaluation the levels of TBARS and SOD were lower and XO and CAT levels were higher post-exercise. The results seem to show that professional training in this category of individuals does not interfere with the evolution of their antioxidant defense [7].
The autonomic nervous system seems to play an important role in the way an organism reacts to oxidative stress because it is related to a decrease in oxidative stress induced by physical effort. The divisions of the autonomic nervous systems include the internal organs, skin and muscles and controls their function by producing and secreting acetylcholine, adrenaline and noradrenaline. In this way, ANS is capable of influencing the response of the body to stress and inflammation [8]. The adaptation of the ANS is one of the ways in which the positive impact of exercise is achieved. The recommendations regarding moderate-intensity exercises for most people are 30 min/day 5 days/week. For people with diseases such as autonomic disorders, training should be carried out under expert supervision [9].
Exercises conducted on a daily basis can cause the ANS to adapt to parasympathetic dominance, which translates to a lower HR at rest. Nitric oxide seems to be associated with bradycardia induced by exercise and studies have shown that the transfer of nitric oxide synthase into the atrial wall has the same effect as the exercise-induced vagal phenotype. HRV and muscle sympathetic nerve activity (MSNA) are useful, objective and reliable methods used to assess the autonomic nervous system’s activity. HRV can be easily assessed with the help of an electrocardiograph. MSNA is used to directly determine the sympathetic nerve activity at the level of the peroneal nerve using microneurography and this is considered the “gold standard” when assessing the intensity of sympathetic outflow. In a review, A.J. Hautala et al. showed that regular aerobic fitness training can cause an increased cardiac vagal modulation of the heart rate and they also showed that normal or pathological functioning of the ANS causes individual responses to aerobic training. Individuals with high vagal modulation at the start of the training obtain greater improvements in their VO2 peak. The use of methods to assess and monitor the ANS can help optimize the exercises chosen for aerobic fitness [10].
Because studies show that in patients with obesity the activity of the sympathetic nervous system and oxidative stress are high, Li et al. investigated the impact that exercise had on four groups of rats with different diets. One of the groups received a high-fat diet for 12 weeks. Rats from the group with a high-fat diet and the ones from the group that received a regular diet were trained on a treadmill 5 days/week 60 min/day for eight weeks. The activity of the sympathetic nervous system was assessed by measuring the plasmatic levels of norepinephrine and oxidative stress was assessed by measuring the plasmatic and muscular levels of malondialdehyde, superoxide anion and F2-isoprostanes. The results showed that in the group of rats who underwent exercise training the activity of the sympathetic nervous system and oxidative stress was lower compared to the activity in the three other groups of rats [11].
Menopause has been identified as a risk for cardio-metabolic disorders and combined training (resistance exercises and aerobic exercises) seem to have a positive impact on this type of disorder. Conti et al. conducted a study with the purpose of evaluating the impact of combined training on blood pressure. Inflammation and oxidative stress were measured in ovariectomized rats suffering from hypertension with fructose overload. The rats included in the study were divided into three groups with different levels of exercise and blood pressure: sedentary but normotensive and sedentary or trained ovariectomized hypertensive rats with fructose overload. The combined training was performed for eight weeks with 40–60% maximal capacity output using treadmills and ladders on alternate days. Blood pressure was determined directly and oxidative stress and inflammation levels were determined using cardiac and renal tissues. The rats included in the third group had increased insulin resistance, cardiac inflammation and oxidative stress parameters. The combined training had a positive impact on atrial pressure, heart rate, sympathetic modulation and insulin resistance. Additionally, nitric oxide bioavailability was higher, TNF-α was reduced, high levels of IL-10 were identified in the cardiac tissue and high levels of antioxidants were present in the cardiac and renal tissue of the rats who underwent training. The conclusions of the study were that risk factors such as menopause can have a negative impact on oxidative stress and metabolic, autonomic, cardiovascular and inflammatory parameters and that combined training has a positive impact and can attenuate this dysfunction [12].
Obesity is another factor that can increase sympathetic activity and oxidative stress. Li et al., studied the role of exercise in decreasing sympathetic activity and oxidative stress in obese rats. The rats included in the study were divided in four groups (regular diet sedentary rats, regular diet exercise training rats, high-fat diet sedentary rats and high-fat diet exercise training rats). The rats included in the second and last group underwent a training program on a treadmill 60 min/day, 5 day/week for eight weeks. The plasma level of norepinephrine was used to assess the activity of the sympathetic nervous system and oxidative stress was assessed by measuring superoxide anion, F2-isoprostanen and MDA serum concentrations. The rats with a high-fat diet presented lower levels of norepinephrine and oxidative stress parameters, which suggests that exercise can attenuate the impact of the sympathetic nervous system and oxidative stress in obesity [13].
Because overload training (large volume or long-term exercise) causes oxidative distress, this will nullify the positive impact of the physical training on health outcomes. The kinds of physical exercises recommended due to the increased levels of antioxidant enzymes they generate are moderate exercises that can improve individuals’ physiological and functional capabilities. During this type of exercise, MAP kinases and NF-kappa B pathways are activated [14].
3. Physical Exercise and Anti-Inflammatory Effects
Skeletal muscle, the largest organ in the body, can produce myokines, firstly in the form of a sequence of pro-inflammatory cytokines (IL-1, IL-6, IL-8, IL-12, TNF-α, IFN-γ, VEGF and IL-1β) and then in the form of regulatory, anti-inflammatory cytokines (e.g., IL-2, IL-4, IL-10, IL-11, IL-13 and IL- 1ra), in response to contraction [15][16]. Myokines may be involved in mediating the health-beneficial effects of exercise and play important roles in protection against diseases associated with low-grade inflammation, insulin resistance and hyperlipidemia such as cardiovascular diseases, type 2 diabetes mellitus and cancer.
Regular PE, if guided correctly, can modulate neurobiological and neuroinflammatory mechanisms to generate anti-inflammatory responses, which are the key factors in improving overall health and controlling the persistent inflammation that is characteristic of chronic diseases.
It has been demonstrated that the plasma concentration of IL-6 increases in an exponential manner during muscular exercise [17]. The peak IL-6 level is reached at the end of the exercise or shortly thereafter [17]. IL-6 plays a fundamental role in the anti-inflammatory process resulting from exercise, and it presents both pro- and anti-inflammatory characteristics [18].
Tidball demonstrated that the proinflammatory response generated by PE plays a role in the recovery process of damaged muscles and that this response is associated with a complex situation in which inflammatory cells promote both injury and repair through the combined actions of free radicals, growth factors and chemokines. Muscle damage produces an inflammatory response in which neutrophils invade rapidly followed by macrophages, which coincide with muscle repair that involves the activation and proliferation of satellite cells followed by their differentiation. In contrast to other high-intensity PE studies that show the destructive role of high intensity exercise, it is showed that inflammatory cells can promote both injury and repair mechanisms [19].
Suzuki et al., documented the systemic kinetics of cytokines after PE, especially TNF-α and IL-1b, which induce cytokines in acute phase reactions. They found that the circulating concentration of these cytokines remains almost unchanged after exertion. Plasma interferon (IFN)-alpha and IFN-gamma remain unchanged, while IL-2 decreases and IL-8 increases after endurance exercises. They concluded that long-duration high-intensity PE suppresses the production of immunomodulatory cytokines [20][21].
Rokitzki et al., evaluated TNF-α levels immediately following a marathon run and discovered high values of TNF-α [22]. Moldoveanu et al. observed a 90% increase in plasma TNF-α following 3 h of endurance exercise at 60 to 65% of VO2max [23].
From the studies described, it is clear that the intensity, type and duration of exercise and the muscle mass involved in the exercise influence the secretion of cytokines into the circulation. Thus, high-intensity and long-duration PE can be dangerous from the point of view of the secreted inflammatory cytokines [24]. Concentric muscle contractions in contrast to eccentric exercises are associated with higher amounts of plasma IL 6. It was demonstrated that muscle damage is not required to increase plasma IL-6 during exercise [25].
Fibroblasts, myoblasts, endothelial cells and smooth muscle cells have been shown to be capable of producing IL-6. Skeletal muscle cells are capable of producing IL-6 in response to reactive oxygen species that are produced as a result of the oxidation of fat and glucose.
A small net release of IL-6 from the internal jugular vein has been reported, suggesting that the CNS may contribute to the IL-6 found in the circulation [26]. In the brain, IL-6 predominantly comes from activated astrocytes [27]. IL-6 levels in the plasma increase rapidly during exercise, whereas the production of IL-6 in the brain increases more slowly [28].
Levels of other cytokines that are expressed in the skeletal muscle following exercise, such as TNF-α and IL-1β, increase, but the circulating concentration of these cytokines does not change (or only increases slightly) [28]. Conversely, the circulating concentrations of IL-1 receptor antagonist (IL-1ra) and IL-10 increase markedly, but these cytokines are not expressed in skeletal muscle after exercise [28].
There are studies that show that physical exercise can alter the inflammatory mode of microglial cells. Microglia, the primary immune cells in the CNS, can be activated by the M1 (pro-inflammatory subtype) and M2 (anti-inflammatory subtype) pathways. The M1 secretes pro-inflammatory cytokines and free radicals that are toxic to the surrounding cells. The M2 secretes anti-inflammatory cytokines and promotes tissue healing by secreting trophic factors.
Sung et al. demonstrated that 30 min of treadmill exercise five days a week at speeds of up to 12 m/min could reduce microglial activation by decreasing the expression of the inflammatory enzyme iNOS. Exercise in mice can increase levels of the growth hormone insulin growth factor 1 (IGF-1) in the prefrontal cortex and hippocampus, which have an anti-inflammatory effect by stimulating the M2 macrophage phenotype [29]. Physical exercise can switch microglial cells from inflammatory M1 to anti-inflammatory M2 types.
Both adrenaline and cortisol rapidly increase during physical training and could be related anti-inflammatory pathways. β2-adrenergic receptor stimulation of microglia inhibits their activation by inhibiting NF-κB [30]. The levels of β2AR on the cell membrane of macrophages are downregulated following exercise in humans [31]. This β2AR downregulation occurs in over-trained subjects but not after moderate exercise. This implies that these receptors still function normally and thus inhibit the expression of pro-inflammatory cytokines such as IL-12 after moderate exercise [32]. Physical exercises can downregulate TNF and TRL4 and allow monocytes to enter an anti-inflammatory mode [33]. The inhibition of this pro-inflammatory response can restore hippocampal neurogenesis [34].
Exercise can lead to increased levels of neurotrophic factors, especially nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF) and insulin-like growth factor (IGF-1). BDNF plays many important roles in neuroplasticity, neuronal growth and differentiation. Physical exercise has been found to normalize BDNF. It has been suggested that higher aerobic fitness levels are associated with larger hippocampal volume and improved neuronal health and that acute aerobic exercise can induce increased BDNF levels in the peripheral blood [35]. Other studies have shown that acute stress and cortisol administration can lead to reduced BDNF levels [36].
Exercise can also increase endorphin levels. β-endorphins are endogenous opioid neuropeptides that play a role in relieving pain and inducing wellbeing. In the brain, β-endorphins are considered neurotransmitters as well as neuromodulators because they are more efficient and stable on more distant targets than other neurotransmitters. They are produced by pro-opiomelanocortin (POMC) cells in the hypothalamus and pituitary gland [37].
Hawkes demonstrated that PE can contribute to the natural production of endorphins. The phenomenon of “wellbeing” in which PE is involved is the consequence of three mechanisms by which endorphins are stimulated: the ‘runner’s high’, addiction to exercise and pain tolerance [38].
Schwarz and Kindermann have shown that sustained PE increases the peripheral concentration of β-endorphins, which is directly related to the perception of pain and the mood of the individual. They analyzed the function of β-endorphins during PE by investigating changes in opioid concentrations in comparison with other stress hormones depending on the exercise intensity and duration. The results of the study showed that peripheral β-endorphin levels during exercise are directly influenced by the effort intensity and duration. In short term aerobic exercise, the degree of metabolic demand is a decisive factor for the release of β-endorphins that is correlated with the concentration of lactate, suggesting that endogenous opioids have a direct influence on acidosis tolerance and anaerobic capacity. The levels of β-endorphins are constant during endurance exercises because a state of equilibrium with lactate is induced and the levels of opioids increase exponentially one hour after exercise [39].
Anandamide (AEA), an endocannabinoid (eCB), is a fatty acid neurotransmitter and is an endogenous binder of the same receptors involved in the effects of cannabis. AEA participates in the body’s endocannabinoid system and mimics many of the pharmacological effects of Δ-9-tetrahydrocannabinol (THC). Just like THC, AEA has two main molecular targets in the form of cannabinoid CB1 and CB2 receptors [40].
An increasing number of studies support the idea that PE has a positive effect on cognitive function. In a recent randomized controlled study, Farinha et al. observed the beneficial effects of aquatic PE on cognitive function, body composition and functional fitness in elderly patients. The combined PE water-based exercise group showed more beneficial effects in terms of improving cognitive function variables than the aerobic interval PE group and continuous aerobic PE group [41].
The ANS is a vast network of nerves affecting every organ in the body and is responsible for maintaining the balance between body and mind. Health is a result of the harmonic interchange between the SNS and PNS branches of the ANS.
Acute stress response with predominant SNS activity is important for survival, performance and achieving various goals. However, when this activation becomes chronic it can be detrimental to people's health and wellbeing. Chronic stress leads to the dysregulation of ANS, causing SNS predominance and the non-involvement of the PN. This disorder is associated with neuroendocrine, cardiovascular, respiratory, digestive and psychiatric diseases (Figure 1).
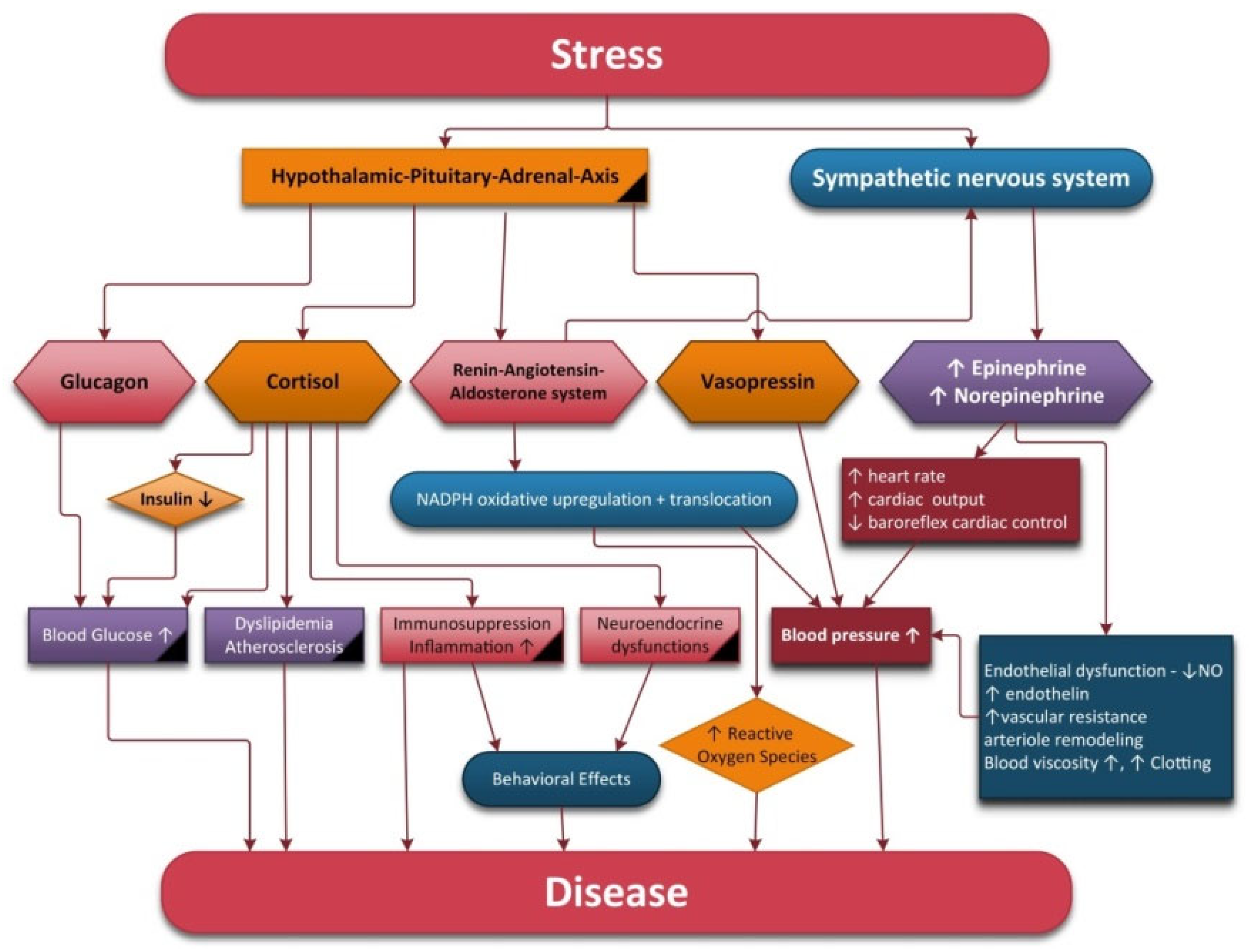
Figure 1. Physiological modifications under stimulation of the hypothalamic–pituitary–adrenal axis and sympathetic nervous system.
Physical exercise training is protective against cardiovascular diseases, obesity, metabolic syndrome and type 2 diabetes mellitus and is also effective to improving the performance of the autonomic nervous system.
Exercise is associated with reduced resting heart and respiratory rate and blood pressure; improved baroreflex, cardiac and endothelial function; increased skeletal muscle blood flow; and more effective redistribution of blood flow during exercise.
SNS is activated during PE but repeated physical training can reduce SNS activity and improve autonomic balance. It is generally believed that reductions in sympathetic outflow represent a major adaptation of exercise training. After exercise, slow breathing shifts the autonomic balance to parasympathetic dominance. The salutary effects of slow and deep breathing are mediated by an increase in tidal volume and the activation of the Hering–Breuer reflex, an inhibitory reflex triggered by lung stretch receptors and mediated by vagal afferents, which may increase baroreflex sensitivity [42]. In addition to stimulating the PNS, slow breathing also improves pulmonary ventilation, gas exchange and arterial oxygenation. Additionally, reduced SNS activity may be the result of a decrease in chemoreflex activity due to the reciprocal influences of the baroreflex and chemoreflex [43]. The NTS has been proposed as an integrating center for the baroreflex, chemoreflex and Hering-Breuer reflex and plays an important role in the effect of breathing on cardiovascular modulation.
During exercise, an increase in respiration and oxygen uptake take place with the purpose of directing a high quantity of O2 to the body’s vital organs. After the oxygen is used, a lot of ROS/NRS are produced. High levels of ROS induce the activation of antioxidant defenses and this will cause a positive adaptation of the nervous system. Training can decrease systemic oxidative stress and it also has a positive impact on antioxidant defenses. A single session of overload training (in large volume or over a long time period) can cause oxidative distress, leading to the loss of beneficial health outcomes related to physical activity. However, if training continues the body can adapt to the exhaustive physical activity by increasing the expression of antioxidant enzymes.
If oxidative stress is reduced or antioxidant capacity is increased with training then less inflammatory process will occur during training. Physical exercise is an efficient clinical tool that limits chronic inflammation using complex mechanisms to activate the immune system, which increases the level of anti-inflammatory cytokines and limits pro-inflammatory cytokine levels in the blood plasma and serum.
Physical activity, by enhancing the parasympathetic tone and activating the cholinergic anti-inflammatory pathway, may be a therapeutic strategy for reducing chronic inflammation and preventing many chronic diseases. If PE can produce inflammation during and after its execution, regular physical exercise training may be considered an anti-inflammatory therapy. Moreover, pro-inflammatory processes that occur after exercise may be vital for long-term adaptive responses to exercise training.
The SNS’s effects on glucose and lipid metabolism are mediated by circulating glucagon, epinephrine, direct sympathetic liver innervation, adipose tissue and skeletal muscle. The effects of the PNS on glucose and lipid metabolism are mediated by insulin and direct parasympathetic innervation of the liver. Generally, sympathetic stimulation produces catabolic effects while parasympathetic stimulation produces anabolic effects.
Sympathetic stimulation leads to hepatic glucose production by activating glycogenolysis in fed states and gluconeogenesis in fasted states. In addition, hormones, such as insulin or leptin, may indicate the peripheral metabolic state, the hypothalamus being the principal site for the integration of autonomic function in the control of appetite regulation and glucose and lipid metabolism [44]. Insulin stimulates glucose and free fatty acid uptake, inhibits lipolysis, promotes the reesterification of fatty acids to triglycerides and stimulatese lipogenesis [44]. Additionally, insulin increases protein synthesis, cell proliferation and growth and enhances SNS activity. The solitary and ambiguous nuclei in the brainstem and the dorsomedial, ventromedial, paraventricular, supraoptic and arcuate nucleii in the hypothalamus are all sensitive to insulin levels. Intracerebroventricular injection of insulin increases MSNA, which shows that insulin may act as a direct mediator of sympathetic overdrive in metabolic syndrome [45].
Several studies have shown that SNS hyperactivity precedes and predicts the appearance of impaired glucose metabolism and insulin resistance. On the other hand, hyperinsulinemia can contribute to chronic sympathetic activation. Impaired parasympathetic regulation of glucose is a risk factor for chronic hyperglycemia and insulin resistance [46]. Insulin resistance promotes ROS accumulation, DNA damage and mitochondrial and endothelial dysfunction and can also exacerbate inflammatory responses. The secretion of leptin, a hormone produced primarily by adipose tissue that acts on specific receptors in the hypothalamus to decrease appetite, is increased by insulin, corticosteroids, TNF-alpha and estrogens and is decreased by androgens, growth hormone, catecholamines and free fatty acids [47]. Leptin promotes weight loss by increasing energy expenditure through stimulation of the SNS in thermogenic brown adipose tissue and in non-thermogenic organs (heart, kidney, adrenal medulla). A deficit of leptin can lead to obesity, insulin resistance and glucose tolerance impairment. Increased plasma leptin levels found in obesity may reflect a high fat mass and partial resistance to leptin. Weight-loss via PE reduces leptin levels and raises insulin sensitivity.
In summary, PE causes lower HPA axis, SNS, oxidative stress and inflammatory activity and increased PNS activity. In addition, PE also contributes to greater cardiovascular and respiratory function, insulin sensitivity and neuroplasticity and higher levels of neurotrophic factors, which may all contribute to the beneficial effects of regular exercise (Figure 2).
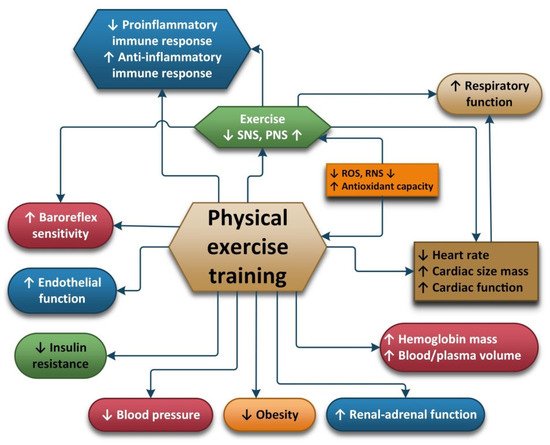
Figure 2. Pathophysiological changes under regular physical training.
The promotion of PE in medicine and its influence on human health has led to the assimilation of the notion of “Exercise is Medicine” [48]. At the moment, a current research topic also looks at how estrogen/progestogen secretion is influenced by physical activity [49].
Besides the promotion of physical exercise, having an antioxidant-rich diet with healthy eating habits can prevent oxidative stress and inflammation. The beneficial effects of endogenous antioxidants are improved by PE, but exogenous antioxidants such as vitamins, omega 3 fatty acids, polyphenols, coenzyme Q10, alpha lipoic acid, etc., can also have a positive effect on athletic performance. In recent years, many studies have been focused on the search for natural compounds that can reduce oxidative stress and inflammation. It has been observed that people consuming Mediterranean or Asian diets have a reduced risk of developing neurodegenerative diseases. It has been found that these specific diets contain high amounts of different phenolic compounds that are found in green tea, extra virgin olive oil, resveratrol, curcuminoids, fruits and aromatic herbs, which may have a preventive effect against inflammation and oxidative stress [50]. It has also been demonstrated that a diet poor in antioxidants can lead to increases in oxidative stress during intensive short-term exercise. Supplementation with multivitamins before and during a marathon has been found to prevent an increase in lipid peroxidation [51].
Taherkhani and colleagues demonstrated that increased levels of ROS and cytokines in the body act as a double-edged sword. In addition to the destructive effects of oxidative stress, they can promote processes that create various adaptations in the body, such as increasing protein synthesis, activating insulin signaling and mitochondrial biogenesis and positive regulation of antioxidants [52]. Additionally, they point out that the effect of antioxidant supplements on improving oxidative stress and inflammatory cytokines is somewhat ambiguous [51]. On the other hand, it has been shown that sulforaphane can counteract oxidative stress and suppress inflammation by decreasing NF-kB leves [53]. Similarly, terpenoids can reduce oxidative stress by stimulating the nuclear factor erythroid-2/heme oxygenase-1 pathway and to decreasing NF-kB levels [54]. Choi et al. reported that the daily consumption of 1.5 L of electrolyte-reduced water was effective at reducing measures of basal oxidative stress [55]. Cannataro et al. demonstrated that consuming a ketogenic diet and microRNAs, particularly miR-30a-5p, are correlated with antioxidant homeostasis [56]. Ruhee et al. investigated the efficacy of sulforaphane on macrophages and proved that cells previously exposed to sulforaphane displayed attenuated oxidative stress and inflammation due to reductions in nitric oxide and cytokine expression [57]. Similar antioxidant effects were observed after the administration of alpha-lipoic acid by Andreeva-Gateva [58].
PE is considered a valuable non-pharmacological therapy, but it must be included in a lifestyle strategy designed to enhance overall wellbeing, in which diet also plays an important role. This combination can beneficially influence oxidative stress and inflammation levels [59].
References
- U.S. Department of Health and Human Services. Physical Activity Guidelines for Americans, 2nd ed.; U.S. Department of Health and Human Services: Washington, DC, USA, 2018.
- Djordjevic, D.; Cubrilo, D.; Macura, M.; Barudzic, N.; Djuric, D.; Jakovljevic, V. The influence of training status on oxidative stress in young male handball players. Mol. Cell. Biochem. 2011, 351, 251–259.
- Somani, S.M.; Husain, K. Influence of exercise-induced oxidative stress on the central nervous system. In Handbook of Oxidants and Antioxidants in Exercise; Sen, C.K., Packer, L., Hanninen, O., Eds.; Elsevier: Amsterdam, The Netherlands, 2000; art IX, Chapter 26.
- Vezzoli, A.; Pugliese, L.; Marzorati, M.; Serpiello, F.R.; la Torre, A.; Porcelli, S. Time-Course Changes of Oxidative Stress Response to High-Intensity Discontinuous Training versus Moderate-Intensity Continuous Training in Masters Runners. PLoS ONE 2014, 9, e87506.
- Yen, C.-J.; Hung, C.-H.; Tsai, W.-M.; Cheng, H.-C.; Yang, H.-L.; Lu, Y.-J.; Tsai, K.-L. Effect of Exercise Training on Exercise Tolerance and Level of Oxidative Stress for Head and Neck Cancer Patients Following Chemotherapy. Front. Oncol. 2020, 10, 1536.
- Finkler, M.; Lichtenberg, D.; Pinchuk, I. The relationship between oxidative stress and exercise. J. Basic Clin. Physiol. Pharmacol. 2014, 25, 1–11.
- Tong, K.T.; Kong, Z.; Lin, H.; Lippi, G.; Zhang, H.; Nie, J. Serum Oxidant and Antioxidant Status Following an All-Out 21-km Run in Adolescent Runners Undergoing Professional Training—A One-Year Prospective Trial. Int. J. Mol. Sci. 2013, 14, 15167–15178.
- Hendrix, J.; Nijs, J.; Ickmans, K.; Godderis, L.; Ghosh, M.; Polli, A. The Interplay between Oxidative Stress, Exercise, and Pain in Health and Disease: Potential Role of Autonomic Regulation and Epigenetic Mechanisms. Antioxidants 2020, 9, 1166.
- Fu, Q.; Levine, B.D. Exercise and the autonomic nervous system. Handb. Clin. Neurol. 2013, 117, 147–160.
- Hautala AJKiviniemi, A.M.; Tulppo, M.P. Individual responses to aerobic exercise: The role of the autonomic nervous system. Neurosci. Biobehav. Rev. 2009, 33, 107–115.
- Li, G.; Liu, J.-Y.; Zhang, H.-X.; Li, Q.; Zhang, S.W. Exercise Training Attenuates Sympathetic Activation and Oxidative Stress in Diet-Induced Obesity. Physiol. Res. 2015, 64, 355–367.
- Conti, F.F.; de Oliveira Brito, J.; Bernardes, N.; Dias, D.d.S.; Malfitano, C.; Morris, M.; Llesuy, S.F.; Irigoyen, M.-C.; de Angelis, K. Positive effect of combined exercise training in a model of metabolic syndrome and menopause: Autonomic, inflammatory, and oxidative stress evaluations. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 309, R1532–R1539.
- Cikrikcioglu, M.A.; Hursitoglu, M.; Erkal, H.; Kınas, B.E.; Sztajzel, J.; Cakirca, M.; Arslan, A.G.; Erek, A.; Halac, G.; Tukek, T. Oxidative stress and autonomic nervous system functions in restless legs syndrome. Eur. J. Clin. Investig. 2011, 41, 734–742.
- Cobley, J.N. How exercise induces oxidative eustress. In Oxidative Stress; Academic Press: Cambridge, MA, USA, 2020; pp. 447–462.
- Pedersen, B.K.; Ostrowski, K.; Rohde, T.; Bruunsgaard, H. The cytokine response to strenuous exercise. Can. J. Physiol. Pharmacol. 1998, 76, 505–511.
- Pedersen, M.; Lexell, J.; Deierborg, T. Effects of physical exercise on neuroinflammation, neuroplasticity, neurodegeneration, and behavior. Neurorehabil. Neural Repair 2015, 29, 577–589.
- Fischer, C.P.; Hiscock, N.; Basu, S.; Vessby, B.; Kallner, A.; Sjöberg, L.B.; Febbraio, M.A.; Pedersen, B.K. Supplementation with vitamins C and E inhibits the release of interleukin-6 from contracting human skeletal muscle. J. Physiol. 2004, 558, 633–645.
- Pedersen, B.K.; Steensberg, A.; Schjerling, P. Muscle-derived interleukin-6: Possible biological effects. J. Physiol. 2001, 536, 329–337.
- Tidball, J.G. Inflammatory processes in muscle injury and repair. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, R345–R353.
- Suzuki, K.; Nakaji, S.; Yamada, M.; Totsuka, M.; Sato, K.; Sugawara, K. Systemic inflammatory response to exhaustive exercise. Cytokine kinetics. Exerc. Immunol. Rev. 2002, 8, 6–48.
- Suzuki, K. Chronic inflammation as an immunological abnormality and effectiveness of exercise. Biomolecules 2019, 9, 223.
- Rokitzki, L.; Logemann, E.; Sagredos, A.N.; Murphy, M.; Wetzel-Roth, W.; Keul, J. Lipid peroxidation and antioxidative vitamins under extreme endurance stress. Acta Physiol. Scand. 1994, 151, 149–158.
- Dufaux, B.; Order, U. Plasma elastase-1-antitrypsin, neopterin, tumor necrosis factor, and soluble interleukin-2 receptor after prolonged exercise. Int. J. Sports Med. 1989, 10, 434–438.
- Moldoveanu, A.I.; Shephard, R.J.; Shek, P.N. The cytokine response to physical activity and training. Sports Med. 2001, 31, 115–144.
- Fischer, C.P. Interleukin-6 in acute exercise and training: What is the biological relevance? Exerc. Immunol. Rev. 2006, 12, 6–33.
- Hellsten, Y.; Frandsen, U.; Orthenblad, N.; Sjodin, N.; Richter, E.A. Xanthine oxidase in human skeletal muscle following eccentric exercise: A role of inflammation. J. Physiol. 1997, 498, 239–248.
- Nybo, L.; Nielsen, B.; Pedersen, B.K.; Moller, K.; Secher, N.H. Interleukin-6 release from the human brain during prolonged exercise. J. Physiol. 2002, 542, 991–995.
- Van Wagoner, N.J.; Benveniste, E.N. Interleukin-6 expression and regulation in astrocytes. J. Neuroimmunol. 1999, 100, 124–139.
- Sung, Y.-H.; Kim, S.-C.; Hong, H.-P.; Park, C.-Y.; Shin, M.-S.; Kim, C.-J.; Seo, J.-H.; Kim, C.-Y.; Kim, D.-J.; Cho, H.-J. Treadmill exercise ameliorates dopaminergic neuronal loss through suppressing microglial activation in Parkinson’s disease mice. Life Sci. 2012, 91, 1309–1316.
- Duman, C.H.; Schlesinger, L.; Terwilliger, R.; Russell, D.S.; Newton, S.S.; Duman, R.S. Peripheral insulin-like growth factor-I produces antidepressant-like behavior and contributes to the effect of exercise. Behav. Brain Res. 2009, 198, 366–371.
- Qian, L.; Wu, H.M.; Chen, S.H.; Zhang, D.; Ali, S.F.; Peterson, L.; Wilson, B.; Lu Ru Hong, J.-S.; Flood, P.M. Beta2-adrenergic receptor activation prevents rodent dopaminergic neurotoxicity by inhibiting microglia via a novel signaling pathway. J. Immunol. 2011, 186, 4443–4454.
- Fragala, M.S.; Kraemer, W.J.; Mastro, A.M.; Denegar, C.R.; Volek, J.S.; Häkkinen, K.; Anderson, J.M.; Lee, E.C.; Maresh, C.M. Leukocyte beta2-adrenergic receptor expression in response to resistance exercise. Med. Sci. Sports Exerc. 2011, 43, 1422–1432.
- Fry, A.C.; Schilling, B.K.; Weiss, L.W.; Chiu, L.Z. Beta2-adrenergic receptor downregulation and performance decrements during high-intensity resistance exercise overtraining. J. Appl. Physiol. 2006, 101, 1664–1672.
- Radom-Aizik, S.; Zaldivar, F.P., Jr.; Haddad, F.; Cooper, D.M. Impact of brief exercise on circulating monocyte gene and microRNA expression: Implications for atherosclerotic vascular disease. Brain Behav. Immun. 2014, 39, 121–129.
- Monje, M.L.; Toda, H.; Palmer, T.D. Inflammatory blockade restores adult hippocampal neurogenesis. Science 2003, 302, 1760–1765.
- Schmolesky, M.T.; Webb, D.L.; Hansen, R.A. The effects of aerobic exercise intensity and duration on levels of brain-derived neurotrophic factor in healthy men. J. Sports Sci. Med. 2013, 12, 502–511.
- Pluchino, N.; Russo, M.; Santoro, A.N.; Litta, P.; Cela, V.; Genazzani, A.R. Sterioid hormones and BDNF. Neuroscience 2013, 239, 271–279.
- Smyth, D.G. 60 years of POMC: Lipotropin and beta-endorphin: A perspective. J. Mol. Endocrinol. 2016, 56, T13–T25.
- Hawkes, C.H. Endorphins: The basis of pleasure? J. Neurol. Neurosurg. Psychiatry 1992, 55, 247–250.
- Dietrich, A.; McDaniel, W.F. Endocannabinoids and exercise. Br. J. Sports Med. 2004, 38, 536–541.
- Thomas, R.; Johnsen, L.K.; Geertsen, S.S.; Christiansen, L.; Ritz, C.; Roig, M.; Lundbye-Jensen, J. Acute Exercise and Motor Memory Consolidation: The Role of Exercise Intensity. PLoS ONE 2016, 11, e0159589.
- Mahalakshmi, B.; Maurya, N.; Lee, S.D.; Bharath Kumar, V. Possible Neuroprotective Mechanisms of Physical Exercise in Neurodegeneration. Int. J. Mol. Sci. 2020, 21, 5895.
- Zou, Y.; Zhao, X.; Hou, Y.Y.; Liu, T.; Wu, Q.; Huang, Y.H.; Wang, X.H. Meta-analysis of effects of voluntary slow breathing exercises for control of heart rate and blood pressure in patients with cardiovascular diseases. Am. J. Cardiol. 2017, 120, 148–153.
- Bernardi, L.; Porta, C.; Spicuzza, L.; Bellwon, J.; Spadacini, G.; Frey, A.W.; Yeung, L.Y.C.; Sanderson, J.E.; Pedretti, R.; Tramarin, R. Slow breathing increases arterial baroreflex sensitivity in patients with chronic heart failure. Circulation 2002, 105, 143–145.
- Straznicky, N.E.; Nestel, P.J.; Esler, M. Autonomic Nervous System: Metabolic Function. Encycl. Neurosci. 2010, 951–959.
- Johnson, M.S.; DeMarco, V.G.; Whaley-Connell, A.; Sowers, J.R. Insuline resistance and the autonomic nervous system. In Primer on the Autonomic Nervous System, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2012.
- Robertson, D.; Biaggioni, I.; Burnstock, G.; Low, P.A.; Paton, J.F.R. (Eds.) Primer on the Autonomic Nervous System; Mayo Clinic, Elsevier Inc.: Rochester, MN, USA, 2012; ISBN 978-0-12-386525-0.
- Borer, K.T. Counter regulation of insulin by leptin as key component of autonomic regulation of body weight. World J. Diabetes 2014, 5, 606–629.
- Rocha-Rodrigues, S.; Sousa, M.; Lourenço Reis, P.; Leão, C.; Cardoso-Marinho, B.; Massada, M.; Afonso, J. Bidirectional Interactions between the Menstrual Cycle, Exercise Training, and Macronutrient Intake in Women: A Review. Nutrients 2021, 13, 438.
- Roberts, L.; Suzuki, K. Exercise and Inflammation. Antioxidants 2019, 8, 155.
- Machefer, G.; Groussard, C.; Vincent, S.; Zouhal, H.; Faure, H.; Cillard, J.; Radák, Z.; Gratas Delamarche, A. Multivitamin-mineral supplementation prevents lipid peroxidation during “the Marathon des Sables”. J. Am. Coll. Nutr. 2007, 26, 111–120.
- Taherkhani, S.; Suzuki, K.; Castell, L. A Short Overview of Changes in Inflammatory Cytokines and Oxidative Stress in Response to Physical Activity and Antioxidant Supplementation. Antioxidants 2020, 9, 886.
- Ruhee, R.T.; Suzuki, K. The Integrative Role of Sulforaphane in Preventing Inflammation, Oxidative Stress and Fatigue: A Review of a Potential Protective Phytochemical. Antioxidants 2020, 9, 521.
- Proshkina, E.; Plyusnin, S.; Babak, T.; Lashmanova, E.; Maganova, F.; Koval, L.; Platonova, E.; Shaposhnikov, M.; Moskalev, A. Terpenoids as Potential Geroprotectors. Antioxidants 2020, 9, 529.
- Choi, Y.A.; Lee, D.H.; Cho, D.-Y.; Lee, Y.-J. Outcomes Assessment of Sustainable and Innovatively Simple Lifestyle Modification at theWorkplace-Drinking Electrolyzed-ReducedWater (OASIS-ERW): A Randomized, Double-Blind, Placebo-Controlled Trial. Antioxidants 2020, 9, 564.
- Cannataro, R.; Caroleo, M.C.; Fazio, A.; La Torre, C.; Plastina, P.; Gallelli, L.; Lauria, G.; Cione, E. Ketogenic Diet and microRNAs Linked to Antioxidant Biochemical Homeostasis. Antioxidants 2019, 8, 269.
- Ruhee, R.T.; Ma, S.; Suzuki, K. Sulforaphane Protects Cells against Lipopolysaccharide-Stimulated Inflammation in Murine Macrophages. Antioxidants 2019, 8, 577.
- Andreeva-Gateva, P.; Traikov, L.; Sabit, Z.; Bakalov, D.; Tafradjiiska-Hadjiolova, R. Antioxidant Effect of Alpha-Lipoic Acid in 6-Hydroxydopamine Unilateral Intrastriatal Injected Rats. Antioxidants 2020, 9, 122.
- Roberts, L.A.; Suzuki, K. Anti-Inflammatory and Antioxidant Effects of Dietary Supplementation and Lifestyle Factors. Antioxidants 2021, 10, 371.
More
Information
Subjects:
Sport Sciences; Rehabilitation
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
6.1K
Revisions:
3 times
(View History)
Update Date:
21 Feb 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




