Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | eugenia quiros roldan | + 2460 word(s) | 2460 | 2022-02-14 07:25:14 | | | |
| 2 | Vivi Li | -40 word(s) | 2420 | 2022-02-14 12:17:06 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Quiros Roldan, E. Omicron. Encyclopedia. Available online: https://encyclopedia.pub/entry/19415 (accessed on 16 January 2026).
Quiros Roldan E. Omicron. Encyclopedia. Available at: https://encyclopedia.pub/entry/19415. Accessed January 16, 2026.
Quiros Roldan, Eugenia. "Omicron" Encyclopedia, https://encyclopedia.pub/entry/19415 (accessed January 16, 2026).
Quiros Roldan, E. (2022, February 14). Omicron. In Encyclopedia. https://encyclopedia.pub/entry/19415
Quiros Roldan, Eugenia. "Omicron." Encyclopedia. Web. 14 February, 2022.
Copy Citation
The Coronavirus disease 2019 (COVID-19) pandemic poses a great threat to global public health. The original wild-type strain of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has genetically evolved, and several variants of concern (VOC) have emerged. On 26 November 2021, a new variant named Omicron (B.1.1.529) was designated as the fifth VOC, revealing that SARS-CoV-2 has the potential to go beyond the available therapies. The high number of mutations harboured on the spike protein make Omicron highly transmissible, less responsive to several of the currently used drugs, as well as potentially able to escape immune protection elicited by both vaccines and previous infection.
Omicron
B.1.1.529
variants of concern
SARS-CoV-2
COVID-19
1. Introduction
The Coronavirus disease 2019 (COVID-19) pandemic poses a great threat to global public health—more than 260 million confirmed cases have been reported, resulting in over 5 million deaths [1]. The original wild-type strain of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), identified at the end of 2019 in Wuhan, has since genetically evolved, and several variants have emerged. Until late 2021, four variants of concern (VOC) of SARS-CoV-2 had been described, including Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1), and Delta (B.1.617.2). On 26 November 2021, a new variant named Omicron (B.1.1.529) was designated the fifth VOC [2]. This new VOC harbours a significant number of mutations on the spike protein (S protein) and appears to be highly transmissible as well as potentially able to escape immune protection elicited by both vaccines and previous infection [2][3]. Although recent data suggest that Omicron has a less severe clinical presentation [4][5][6], it is still too early to conclude on the clinical impact of this variant. Furthermore, the increased transmissibility and the consequent exponential growth of Omicron cases have currently made the Omicron variant the protagonist of the ongoing new surge.
2. Virology and Pathogenesis
Phylogenetic studies reveal that the Omicron variant has likely diverged early from other SARS-CoV-2 strains [7] (Figure 1). It is even speculated that the Omicron variant might have been gestated in immunocompromised individuals (e.g., HIV patients coinfected by SARS-CoV-2) for a while [8]. However, according to recent studies based on nonsynonymous mutations analysis in Omicron open reading frame (ORF), the molecular spectrum of preoutbreak mutations is inconsistent with the rapid accumulation of mutations in humans. Moreover, the B.1.1 variants and human coronavirus hCoV-229E show the highest sequence similarities [9]. These results strongly support a trajectory in which the progenitor of Omicron experienced a reverse zoonotic event from humans to mice during the pandemic accumulating mutations [9].
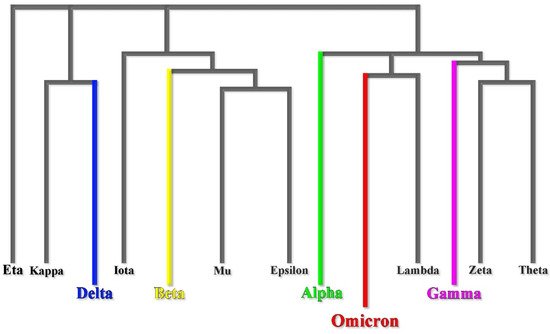
Figure 1. Omicron early divergence in a phylogenetic mutational tree.
The Omicron variant is not structurally different from other already identified SARS-CoV-2 VOCs. The S protein remains a trimer, and each monomer is composed of two subunits (S1 and S2). The receptor-binding domain (RBD), which interacts with the ACE2 receptor, is located in the S1 subunit (Figure 2) [10].
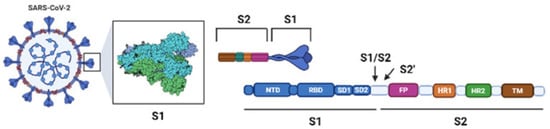
Figure 2. Schematic SARS-CoV-2 and its S protein.
While these aspects are shared among VOCs, Omicron genomic features are highly divergent. Recently, whole-genome examination and mutational analysis have found that the Omicron variant might be classified into two different lineages (BA.1 and BA.2). Six genome sequences of both BA.1 and BA.2 were analysed and compared to the original Wuhan strain, and 32 common mutations were found in both lineages, whilst 19 mutations were lineage-specific and considered “signature mutations” [11].
More specifically, 21 common mutations and 13 signature mutations have been found in the BA.1 lineage S-glycoprotein (34 mutations overall), whereas 7 signature mutations are described in the BA.2 lineage S-glycoprotein (28 mutations overall) [11].
Considering coding and noncoding regions, Omicron carries at least 60 mutations in total compared with the original Wuhan strain. Many of those mutations are shared with the previously described VOCs, whereas others are uniquely found in Omicron (Figure 3). Similarly, there are 6 deletions (in positions 69, 70, 143, 144, 145, and 211) in the Omicron variant, of which only the one in position 211 does not appear in the other VOCs [12]. Crucial implications in infectiousness and immune escape regard at least 36 mutations in the S protein; over 30 amino acid substitutions, 3 deletions and 1 insertion are recorded. Notably, 15 of the 30 amino acid substitutions are in the receptor-binding domain (RBD) [13]. Lastly, the Omicron variant shares the mutations at the furin cleavage region with the other VOC. During the S protein cleavage process, the mutations in positions 547, 655, 679 and 681 allow the formation of two subunits which boost the transmissibility of the virus [12].

Figure 3. Comparison among mutational profiles of the five already known variants of concern (VOCs).
The Omicron variant shows a three to four-fold increase in the number of mutations expressed on the spike protein compared with the other 4 VOCs [2]. Furthermore, seven common mutations (G142D, K417N, T478K, N501Y, D614G, H655Y, and P681H) and three signature BA.1 lineage mutations (ΔHV69del, T95I, and ΔYY144del) overlap Alpha, Beta, Gamma, and Delta VOC. These overlapping mutations have been previously associated with increased transmissibility, more efficient viral binding, as well as immune evasion. The D614G mutation correlates with a higher upper respiratory tract viral load and a younger age of the patients affected, and it has been found in all five VOCs already identified. Furthermore, the Omicron variant shares N501Y, which is believed to increase the binding affinity between the viral spike protein and the angiotensin-converting enzyme 2 (ACE2) receptor [2].
Regarding the structural proteins, three substitutions (D3G, Q19E, and A63T) involve the membrane, one substitution (T9I), the envelope and three substitutions, and a three-residue deletion of the nucleocapsid proteins [2].
Lastly, another crucial point regards the Omicron entry pathway, which might have implications on the clinical manifestations and disease severity. While the Delta variant replicated well in Calu-3 cells, which has robust TMPRSS2 (transmembrane serine protease 2) expression, the Omicron variant replicated poorly in this cell line, showing a weaker cell–cell fusion activity [14]. As a matter of fact, Camostat, which inhibits the TMPRSS2 pathway alone, significantly reduced only the Delta variant entry pathway. This observation supports the point that the Omicron variant infection is not enhanced by TMPRSS2 but is largely mediated by the endocytic pathway instead [14]. The inefficiency in using TMPRSS2 might explain the dramatically attenuated replication rate in Calu3 and Caco2 cells with a less severe lung pathology [15].
3. Epidemiology
Very recently, there was an alarming increase in COVID-19 cases in South Africa: in November 2021, the mean number of COVID-19 cases per day increased from 280 to 800 [2]. On 24 November 2021, the identification of a new SARS-CoV-2 variant, B.1.1.529, was reported by the southern African authorities to the World Health Organization (WHO) [16]. The first B.1.1.529 case was detected in specimens collected in Botswana on 11 November 2021 [17]. This VOC was soon promptly identified in multiple other nations, spreading to neighbouring countries such as Botswana, Namibia, Zimbabwe, Swaziland, and Mozambique [18]. Although several countries had arranged traveller restrictions for passengers from endemic areas, as of mid-December 2021, the Omicron variant accounted for the majority of new infections in the United States [16], and several studies showed that an international travel history within 14 days of symptom onset is not a must for Omicron community spreading [19]. However, according to analysis carried out by the National Wastewater Surveillance System, the detection of Omicron-associated mutations in community wastewater dates to at least a week before the first case was identified in the U.S. This suggests the possibility that variant tracking data from wastewater can be used as a complement to clinical testing for the early detection of emerging variants [20]. The second Omicron case in Europe was reported In North Italy in a patient travelling from Mozambique [21].
4. Diagnosis
The number of mutations involving epitopes of the Omicron variant have made several single-target molecular tests ineffective, raising the false negative rate results in patients infected by this VOC [22]. While Delta VOC is defined as S-gene positive, the Omicron variant carries the deletion at H69 and V70 in the spike gene, as does the Alpha VOC. This deletion in the spike protein results in an S-gene dropout and the inability of some SARS-CoV-2 molecular tests to detect the S-gene [23]. However, using multiple target molecular tests, the overall sensitivity should not be impacted [22]. Moreover, specimens that yield an S-gene target failure, when tested with these kits, might be used as a rapid proxy for the frequency of Omicron cases. New generation sequencing verification is always advised [24]: testing positive for SARS-CoV-2 with one of these tests does not mean an individual is infected with the omicron variant and, not every sample obtained from patients affected by the omicron variant displays a mutation that leads to a gene dropout [22].
Early data based on patient samples containing live viruses suggest that antigen tests, relying mainly on nucleocapsid proteins, can also detect the Omicron variant proteins but may have a reduced sensitivity [22]. Although these are encouraging preliminary results, it is crucial to underscore that antigen tests are generally less sensitive and less likely to pick up very early infections compared with molecular tests. Following the FDA’s long-standing rapid test recommendations, a negative antigenic test in a symptomatic person or with a high likelihood of infection due to exposure to a confirmed COVID-19 case requires, with a high degree of recommendation, a follow-up molecular testing [24].
5. Treatment
Currently, several therapeutic options approved under the FDA-issued Emergency Use Authorization (EUA) are available in COVID-19 management, including antiviral drugs (e.g., molnupiravir, nirmatrelvir, remdesivir), anti-SARS-CoV-2 monoclonal antibodies (e.g., bamlanivimab-etesevimab, casirivimab-imdevimab, and sotrovimab), anti-inflammatory drugs (e.g., dexamethasone), and immunomodulators agents (e.g., baricitinib, tocilizumab). For most of them, little data are available regarding their efficacy against the Omicron variant.
5.1. Monoclonal Antibodies
Every monoclonal antibody (mAb) against SARS-CoV-2 used so far binds to the virus’ S protein, preventing infection of human cells and reducing the risk of severe COVID-19 up to 85%. The shift in epitopes showed in the Omicron’s S protein (particularly within the RDB domain) raised concern about the effectiveness of some mAbs currently available in terms of susceptibility and potency [25].
Bamlanivimab–etesevimab (LY-CoV016-LY-CoV555) is a cocktail of 2 antibodies. Both bind to an overlapping epitope in the RBD of the SARS-CoV-2 S protein, targeting both the open and closed conformation increasing the neutralisation rate and blocking the attachment to the human ACE2 receptor. Similarly, casirivimab-imdevimab (REGN-CoV2), authorised as an intravenous or subcutaneous injection, reduces the risk of severe COVID-19 by binding distinct epitopes of the S protein [10]. The neutralisation potency of both cocktails is greatly reduced in the Omicron variant [25][26]. In vitro analysis using Vero-TMPRSS2 and Vero-hACE2-TMPRSS2 cells demonstrated a complete loss of inhibitory activity against this VOC [27]. As the Delta VOC still represents a major concern, bamlanivimab-etesevimab and casirivimab-imdevimab are still considered treatment options, but since Omicron is resistant to both, SARS-CoV-2 genotyping might be recommended before initiating mAbs treatment [28]. Even the promising regdanvimab (CT-P59) seems likely to be ineffective against the Omicron variant. Through the measurement of the median fluorescence intensity of the signal, regdanvimab displayed a strong reduction in its binding to Omicron infected cells when compared with Delta [29].
Sotrovimab (GSK4182136 or S309) binds to a cryptic RBD epitope shared between SARS-CoV and SARS-CoV-2 where, apparently, fewer mutations have occurred [25]. Since this mAb does not overlap with ACE2 on the RBD binding interface, its neutralising activity does not directly interfere with ACE2 binding [30]. Sotrovimab seems to be active against the Omicron variant, but its neutralising potency results dropped 3-fold [31]. High hopes come from a recently found RBD-specific antibody called bebtelovimab (LY-CoV1404) which potentially neutralises both the authentic SARS-CoV-2 virus (B.1.1.7, B.1.351 and B.1.617.2) and VOCs (B.1.1.7, B.1.351, B.1.617.2, B.1.427/B.1.429, P.1, and B.1.526), including Omicron (B.1.1.529). In this preliminary analysis, the binding and neutralising activity seems to be unaffected by the most common mutations presented by already known VOCs, making bebtelovimab a viable therapeutic agent for the treatment of the Omicron variant [32]. Lastly, from preliminary analysis, another newly synthesised mAb known as DXP-604 seems to be insensitive to the K417N single site change in the Omicron variant RBD. However, when K417N is combined with other mutations (S477N, Q493R, G496S, Q498R, N501Y, and Y505H), the DXP-604’s binding affinity against Omicron RBD is largely decreased (nearly 30-fold reduction compared with the wild-type strain) [26]. A panel of over 30 mAbs against Omicron are currently being tested with the primary goal of identifying those that retain a neutralising activity against this VOC and perhaps also modelling a new generation of mAbs [33].
5.2. Antivirals
Regulatory authorities are continuously evaluating already approved drugs, and hundreds of medicines with newly identified targets have been proposed [34]. The effect of approved drugs and those under investigation against the Omicron variant remains to be further investigated.
Remdesivir was the first FDA-approved drug in COVID-19 treatment. It blocks viral replication by acting as a nucleoside analogue inhibiting the RNA dependent RNA polymerase (RdRp) of Coronavirdae. Although no definitive study has been carried out on the Omicron variant, typical mutations in the RdRp associated with remdesivir resistance (e.g., V557L, V473F, N491S, F480L/S/C, P323L, or E802D) have not been described in this VOC yet [35]. However, new evidence on remdesivir intracellular signalling pathways is currently emerging as the possible implications on herpesviruses (HHV8 and EBV) reactivation [36]. Notwithstanding, a very low level of overall remdesivir resistance is described [37].
Molnupiravir is an isopropylester prodrug of the nucleoside analogue β-d-N4-hydroxycytidine (NHC), which increases the frequency of viral RNA mutations impairing SARS-CoV-2 replication. NHC-triphosphate, the active form of molnupiravir, is incorporated in viral RNA instead of cytidine triphosphate or uridine triphosphate. Moreover, this modified RNA, when used as a template, directs the incorporation of either G or A, leading to mutated RNA products [38]. Concerns regarding the possible mutational effect on uninfected human cells derive from in vitro studies on mammalian cells, which need further investigations [39]. Molnupiravir is orally available and, when administered early in non-hospitalised, unvaccinated adults with mild to moderate COVID-19, it reduces the overall hospitalisation and death risk by approximately 31% [40]. No data on the Omicron variant are yet available, but molnupiravir is expected to retain activity against all SARS-CoV-2 VOCs [34].
The antiviral drug nirmatrelvir (PF-07321332) will soon be available: it inhibits the SARS-CoV-2 protease (essential for viral replication) and is coadministered with ritonavir to slow its metabolism, allowing for longer persistence and higher drug concentrations [41][42]. Preliminary data on patients at high risk for severe COVID-19 show reduced hospital admissions and deaths by 80–90% [43]. The main problem is linked to ritonavir, a potent cytochrome and P-glycoprotein inhibitor, raising concerns about drug–drug interactions. The combination is expected to retain activity against all the SARS-CoV-2 variants, including the new Omicron [34].
The few data available about antiviral drugs suggest that Omicron remains sensitive to the approved antiviral drugs and several drug candidates with new mechanisms of action, such as nafamostat, camostat, or aprotinin [44].
References
- World Health Organization (WHO). Coronavirus Disease (COVID-19) Pandemic. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed on 24 January 2022).
- He, X.; Hong, W.; Pan, X.; Lu, G.; Wei, X. SARS-CoV-2 Omicron Variant: Characteristics and Prevention. MedComm 2021, 2, 838–845.
- Zhang, L.; Li, Q.; Liang, Z.; Li, T.; Liu, S.; Cui, Q.; Nie, J.; Wu, Q.; Qu, X.; Huang, W.; et al. The Significant Immune Escape of Pseudotyped SARS-CoV-2 Variant Omicron. Emerg. Microbes Infect. 2022, 11, 1–5.
- UK Health Security Agency. SARS-CoV-2 Variants of Concern and Variants under Investigation in England. Technical Briefing: Update on Hospitalisation and Vaccine Effectiveness for Omicron VOC-21 NOV-01 (B.1.1.529). Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1045619/Technical-Briefing-31-Dec-2021-Omicron_severity_update.pdf (accessed on 9 January 2022).
- Jassat, W.; Karim, S.A.; Mudara, C.; Welch, R.; Ozougwu, L.; Groome, M.; Govender, N.; von Gottberg, A.; Wolter, N.; Blumberg, L.; et al. Clinical Severity of COVID-19 Patients Admitted to Hospitals in Gauteng, South Africa During the Omicron-Dominant Fourth Wave. SSRN Electron. J. 2021.
- Sheikh, A.; Kerr, S.; Mcmenamin, J.; Robertson, C. Severity of Omicron Variant of Concern and Vaccine Effectiveness against Symptomatic Disease: National Cohort with Nested Test Negative Design Study in Scotland. Available online: https://www.research.ed.ac.uk/en/publications/severity-of-omicron-variant-of-concern-and-vaccine-effectiveness-?fbclid=IwAR1qHNz_yVl6KVtg7oq0XESOX-j9o5m9i9cxIE1r11LYZ787xdHHwj8nF_Q (accessed on 24 January 2022).
- Kupferschmidt, K. Where Did ‘Weird’ Omicron Come From? Science 2021, 374, 1179.
- Kumar, S.; Thambiraja, T.S.; Karuppanan, K.; Subramaniam, G. Omicron and Delta Variant of SARS-CoV-2: A Comparative Computational Study of Spike Protein. J. Med. Virol. 2021.
- Wei, C.; Shan, K.-J.; Wang, W.; Zhang, S.; Huan, Q.; Qian, W. Evidence for a Mouse Origin of the SARS-CoV-2 Omicron Variant. J. Genet. Genom. 2021, 48, 1111–1121.
- Quiros-Roldan, E.; Amadasi, S.; Zanella, I.; Degli Antoni, M.; Storti, S.; Tiecco, G.; Castelli, F. Monoclonal Antibodies against SARS-CoV-2: Current Scenario and Future Perspectives. Pharmaceuticals 2021, 14, 1272.
- Majumdar, S.; Sarkar, R. Mutational and Phylogenetic Analyses of the Two Lineages of the Omicron Variant. J. Med. Virol. 2022.
- Fantini, J.; Yahi, N.; Colson, P.; Chahinian, H.; la Scola, B.; Raoult, D. The Puzzling Mutational Landscape of the SARS-2-variant Omicron. J. Med. Virol. 2022.
- National Center for Immunization and Respiratory Diseases (NCIRD). Science Brief: Omicron (B.1.1.529) Variant. 2020. Available online: https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/scientific-brief-omicron-variant.html#:~:text=On%20December%201%2C%202021%2C%20the,the%20days%20preceding%20symptom%20onset (accessed on 24 January 2022).
- Zhao, H.; Lu, L.; Peng, Z.; Chen, L.-L.; Meng, X.; Zhang, C.; Ip, J.D.; Chan, W.-M.; Chu, A.W.-H.; Chan, K.-H.; et al. SARS-CoV-2 Omicron Variant Shows Less Efficient Replication and Fusion Activity When Compared with Delta Variant in TMPRSS2-Expressed Cells. Emerg. Microbes Infect. 2022, 11, 277–283.
- Shuai, H.; Chan, J.F.-W.; Hu, B.; Chai, Y.; Yuen, T.T.-T.; Yin, F.; Huang, X.; Yoon, C.; Hu, J.-C.; Liu, H.; et al. Attenuated Replication and Pathogenicity of SARS-CoV-2 B.1.1.529 Omicron. Nature 2022.
- World Health Organization (WHO). Enhancing Response to Omicron SARS-CoV-2 Variant: Technical Brief and Priority Actions for Member States. Available online: https://www.who.int/publications/m/item/enhancing-readiness-for-omicron-(b.1.1.529)-technical-brief-and-priority-actions-for-member-states (accessed on 24 January 2022).
- Jansen, L.; Tegomoh, B.; Lange, K.; Showalter, K.; Figliomeni, J.; Abdalhamid, B.; Iwen, P.C.; Fauver, J.; Buss, B.; Donahue, M. Investigation of a SARS-CoV-2 B.1.1.529 (Omicron) Variant Cluster—Nebraska, November–December 2021. MMWR. Morb. Mortal. Wkly. Rep. 2021, 70, 1782–1784.
- Kannan, S.; Shaik Syed Ali, P.; Sheeza, A. Omicron (B.1.1.529)—Variant of Concern—Molecular Profile and Epidemiology: A Mini Review. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 8019–8022.
- Lee, J.J.; Choe, Y.J.; Jeong, H.; Kim, M.; Kim, S.; Yoo, H.; Park, K.; Kim, C.; Choi, S.; Sim, J.; et al. Importation and Transmission of SARS-CoV-2 B.1.1.529 (Omicron) Variant of Concern in Korea, November 2021. J. Korean Med. Sci. 2021, 36, e346.
- Kirby, A.E.; Welsh, R.M.; Marsh, Z.A.; Yu, A.T.; Vugia, D.J.; Boehm, A.B.; Wolfe, M.K.; White, B.J.; Matzinger, S.R.; Wheeler, A.; et al. Notes from the Field: Early Evidence of the SARS-CoV-2 B.1.1.529 (Omicron) Variant in Community Wastewater—United States, November–December 2021. MMWR. Morb. Mortal. Wkly. Rep. 2022, 71, 103–105.
- Micheli, V.; Bracchitta, F.; Rizzo, A.; Mancon, A.; Mileto, D.; Lombardi, A.; Stefanelli, P.; Gismondo, M.R. First Identification of the New SARS-CoV-2 Omicron Variant (B.1.1.529) in Italy. Clin. Infect. Dis. 2022.
- U.S. Food & Drug Administration. SARS-CoV-2 Viral Mutations: Impact on COVID-19 Tests. Available online: https://www.fda.gov/medical-devices/coronavirus-covid-19-and-medical-devices/sars-cov-2-viral-mutations-impact-covid-19-tests#omicronvariantimpact (accessed on 24 January 2022).
- Scott, L.; Hsiao, N.; Moyo, S.; Singh, L.; Tegally, H.; Dor, G.; Maes, P.; Pybus, O.G.; Kraemer, M.U.G.; Semenova, E.; et al. Track Omicron’s Spread with Molecular Data. Science 2021, 374, 1454–1455.
- Thomas, E.; Delabat, S.; Carattini, Y.L.; Andrews, D.M. SARS-CoV-2 and Variant Diagnostic Testing Approaches in the United States. Viruses 2021, 13, 2492.
- Kozlov, M. Omicron Overpowers Key COVID Antibody Treatments in Early Tests. Nature 2021.
- Cao, Y.; Wang, J.; Jian, F.; Xiao, T.; Song, W.; Yisimayi, A.; Huang, W.; Li, Q.; Wang, P.; An, R.; et al. Omicron Escapes the Majority of Existing SARS-CoV-2 Neutralizing Antibodies. Nature 2021.
- VanBlargan, L.A.; Errico, J.M.; Halfmann, P.J.; Zost, S.J.; Crowe, J.E.; Purcell, L.A.; Kawaoka, Y.; Corti, D.; Fremont, D.H.; Diamond, M.S. An Infectious SARS-CoV-2 B.1.1.529 Omicron Virus Escapes Neutralization by Therapeutic Monoclonal Antibodies. Nat. Med. 2022.
- Wilhelm, A.; Widera, M.; Grikscheit, K.; Toptan, T.; Schenk, B.; Pallas, C.; Metzler, M.; Kohmer, N.; Hoehl, S.; Helfritz, F.A.; et al. Reduced Neutralization of SARS-CoV-2 Omicron Variant by Vaccine Sera and Monoclonal Antibodies. medRxiv 2021.
- Planas, D.; Saunders, N.; Maes, P.; Guivel-Benhassine, F.; Planchais, C.; Buchrieser, J.; Bolland, W.-H.; Porrot, F.; Staropoli, I.; Lemoine, F.; et al. Considerable Escape of SARS-CoV-2 Omicron to Antibody Neutralization. Nature 2021.
- Chen, J.; Wang, R.; Gilby, N.B.; Wei, G.-W. Omicron Variant (B.1.1.529): Infectivity, Vaccine Breakthrough, and Antibody Resistance. J. Chem. Inf. Model. 2022, 62, 412–422.
- Fang, F.; Shi, P.-Y. Omicron: A Drug Developer’s Perspective. Emerg. Microbes Infect. 2022, 11, 208–211.
- Westendorf, K.; Wang, L.; Žentelis, S.; Foster, D.; Vaillancourt, P.; Wiggin, M.; Lovett, E.; van der Lee, R.; Hendle, J.; Pustilnik, A.; et al. LY-CoV1404 (Bebtelovimab) Potently Neutralizes SARS-CoV-2 Variants. bioRxiv 2022.
- Cameroni, E.; Bowen, J.E.; Rosen, L.E.; Saliba, C.; Zepeda, S.K.; Culap, K.; Pinto, D.; VanBlargan, L.A.; de Marco, A.; di Iulio, J.; et al. Broadly Neutralizing Antibodies Overcome SARS-CoV-2 Omicron Antigenic Shift. Nature 2021.
- Kontoghiorghes, G.J.; Fetta, S.; Kontoghiorghe, C.N. The Need for a Multi-Level Drug Targeting Strategy to Curb the COVID-19 Pandemic. Front. Biosci.-Landmark 2021, 26, 1723–1736.
- Chakraborty, C.; Bhattacharya, M.; Sharma, A.R. Emerging Mutations in the SARS-CoV-2 Variants and Their Role in Antibody Escape to Small Molecule-Based Therapeutic Resistance. Curr. Opin. Pharmacol. 2022, 62, 64–73.
- Chen, J.; Dai, L.; Kendrick, S.; Post, S.R.; Qin, Z. The Anti-COVID-19 Drug Remdesivir Promotes Oncogenic Herpesviruses Reactivation through Regulation of Intracellular Signaling Pathways. Antimicrob. Agents Chemother. 2022.
- Focosi, D.; Maggi, F.; McConnell, S.; Casadevall, A. Very Low Levels of Remdesivir Resistance in SARS-COV-2 Genomes after 18 Months of Massive Usage during the COVID19 Pandemic: A GISAID Exploratory Analysis. Antivir. Res. 2022, 198, 105247.
- Kabinger, F.; Stiller, C.; Schmitzová, J.; Dienemann, C.; Kokic, G.; Hillen, H.S.; Höbartner, C.; Cramer, P. Mechanism of Molnupiravir-Induced SARS-CoV-2 Mutagenesis. Nat. Struct. Mol. Biol. 2021, 28, 740–746.
- Zhou, S.; Hill, C.S.; Sarkar, S.; Tse, L.V.; Woodburn, B.M.D.; Schinazi, R.F.; Sheahan, T.P.; Baric, R.S.; Heise, M.T.; Swanstrom, R. β-d-N4-Hydroxycytidine Inhibits SARS-CoV-2 Through Lethal Mutagenesis but Is Also Mutagenic to Mammalian Cells. J. Infect. Dis. 2021, 224, 415–419.
- Jayk Bernal, A.; Gomes da Silva, M.M.; Musungaie, D.B.; Kovalchuk, E.; Gonzalez, A.; Delos Reyes, V.; Martín-Quirós, A.; Caraco, Y.; Williams-Diaz, A.; Brown, M.L.; et al. Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients. N. Engl. J. Med. 2021, 386, 509–520.
- Mahase, E. Covid-19: Pfizer’s Paxlovid Is 89% Effective in Patients at Risk of Serious Illness, Company Reports. BMJ 2021, 375, n2713.
- Owen, D.R.; Allerton, C.M.N.; Anderson, A.S.; Aschenbrenner, L.; Avery, M.; Berritt, S.; Boras, B.; Cardin, R.D.; Carlo, A.; Coffman, K.J.; et al. An Oral SARS-CoV-2 Mpro Inhibitor Clinical Candidate for the Treatment of COVID-19. Science 2021, 374, 1586–1593.
- Pfizer Pfizer Announces Additional Phase 2/3 Study Results Confirming Robust Efficacy of Novel COVID-19 Oral Antiviral Treatment Candidate in Reducing Risk of Hospitalization or Death. Available online: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-announces-additional-phase-23-study-results#:~:text=These%20results%20were%20consistent%20with,three%20days%20of%20symptom%20onset (accessed on 24 January 2022).
- Bojkova, D.; Widera, M.; Ciesek, S.; Wass, M.N.; Michaelis, M.; Cinatl, J. Reduced Interferon Antagonism but Similar Drug Sensitivity in Omicron Variant Compared to Delta Variant of SARS-CoV-2 Isolates. Cell Res. 2022, 1–3.
More
Information
Subjects:
Allergy
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
889
Revisions:
2 times
(View History)
Update Date:
14 Feb 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




