
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Gilberto Igrejas | + 3624 word(s) | 3624 | 2021-04-22 07:48:38 | | | |
| 2 | Lily Guo | + 407 word(s) | 4031 | 2022-02-14 02:18:05 | | | | |
| 3 | Lily Guo | + 407 word(s) | 4031 | 2022-02-14 02:20:21 | | |
Video Upload Options
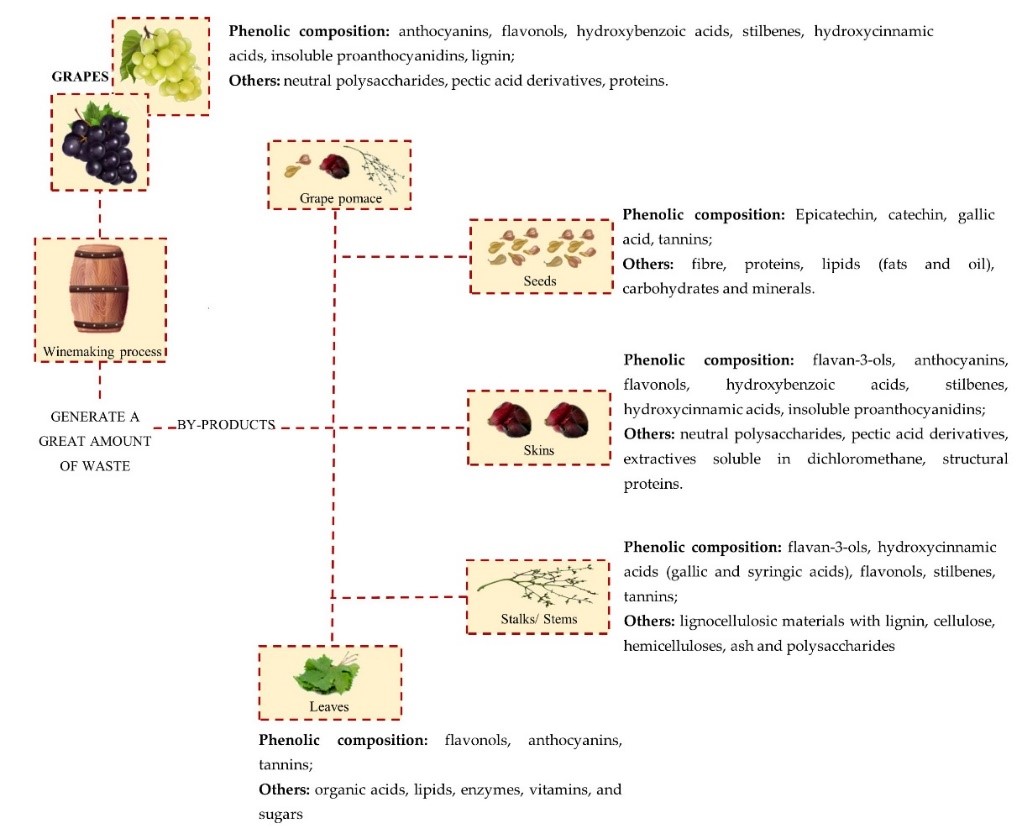
The emergence of antibiotic-resistance in bacteria has limited the ability to treat bacterial infections, besides increasing their morbidity and mortality at the global scale. The need for alternative solutions to deal with this problem is urgent and has brought about a renewed interest in natural products as sources of potential antimicrobials. The wine industry is responsible for the production of vast amounts of waste and by-products, with associated environmental problems. These residues are rich in bioactive secondary metabolites, especially phenolic compounds. Some phenolics are bacteriostatic/bactericidal against several pathogenic bacteria and may have a synergistic action towards antibiotics, mitigating or reverting bacterial resistance to these drugs. Complex phenolic mixtures, such as those present in winemaking residues (pomace, skins, stalks, leaves, and especially seeds), are even more effective as antimicrobials and could be used in combined therapy, thereby contributing to management of the antibiotic resistance crisis.
1.Introduction
2. Wine By-Products—Chemical Composition and Bioactive Compounds
| Phenolic Compounds | ||
|---|---|---|
| Flavonoids | Non-Flavonoids | |
Anthocyanidins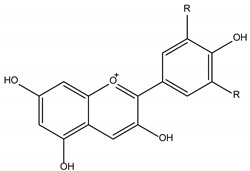 |
Phenolic Acids | |
Hydroxybenzoic acids derivates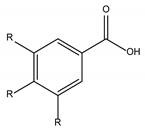 |
Hydroxycinnamic acids derivates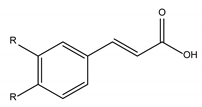 |
|
Flavonols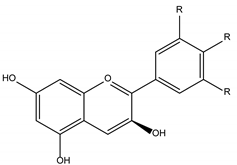 |
Stilbenes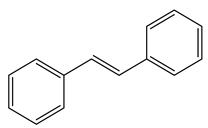 |
|
Flavan-3-ols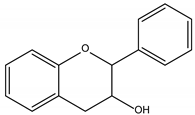 |
Coumarins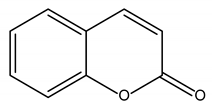 |
|
Flavones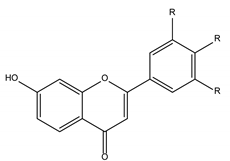 |
Lignans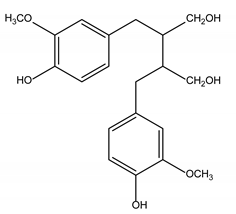 |
|
Chalcones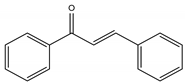 |
||
3. Wine By-Products as a Source of Compounds Possessing Antibacterial Properties
| Grape Variety | Country | By-Product | Extraction Solvent(s)/Methodologies | Target Bacteria | MIC Range (mg mL−1) | Reference | |
|---|---|---|---|---|---|---|---|
| Gram-Positive | Gram-Negative | ||||||
| Arinto | Portugal | Skins/Seeds | Water | B. cereus | E. coli S. Poona |
ND | [84] |
| Touriga Nacional | Portugal | Skins | Water:ethanol (50:50) |
B. cereus E. faecium L. monocytogenes S. epidermidis |
K. pneumoniae | 0.01–1.0 | [80] |
| Stems | E. faecium L. monocytogenes S. aureus S. epidermidis |
- | 0.05–0.1 | ||||
| Seeds | B. cereus E. faecalis E. faecium L. monocytogenes S. epidermidis S. aureus |
K. pneumoniae | 0.01–0.1 | ||||
| Preto Martinho | Skins | E. faecalis L. monocytogenes S. epidermidis S. aureus |
- | 0.01–0.075 | |||
| Stems | E. faecalis E. faecium L monocytogenes S. epidermidis |
K. pneumoniae | 0.025–0.1 | ||||
| Seeds | B. cereus E. faecalis L. monocytogenes S. epidermidis S. aureus |
K. pneumoniae | 0.001–0.01 | ||||
| Noble | USA | Skins | Methanol:water (70:30) |
S. aureus (ATCC 35548) S. aureus (ATCC 12600) S. aureus (ATCC 29247) |
S. sonnei | 226–903 | [81] |
| Seeds | 268–1069 | ||||||
| Carlos | Skins | 304–608 | |||||
| Seeds | 268–1069 | ||||||
| Grape Variety | Country | By-Product | Extraction Solvent(s)/Methodologies | Gram-Positive | Gram-Negative | MIC Range (mg mL−1) | Reference |
| Kujundžuša | Croatia | Skins | Ethanol:water (80:20) |
B. cereus S. aureus |
C. coli E. coli S. Infantis |
0.032–0.15 * | [83] |
| Rkaciteli | 0.014–0.20 * | ||||||
| Zlatarica | 0.042–0.59 * | ||||||
| Medna | 0.014–0.21 * | ||||||
| Kuč | 0.019–0.26 * | ||||||
| Maraština | 0.015–0.21 * | ||||||
| Debit | 0.025–0.25 * | ||||||
| Vranac | 0.16–0.23 * | ||||||
| Trnjac | 0.12–0.31 * | ||||||
| Rudežuša | 0.15–0.29 * | ||||||
| Merlot | 0.13–0.44 * | ||||||
| Babić | 0.08–0.42 * | ||||||
| Lain | 0.04–0.34 * | ||||||
| Plavina | 0.09–0.41 * | ||||||
| Autumn Royal | India | Leaf | Methanol | C. septicum S. aureus (MRSA) S. viridans |
E. coli Proteus spp. Pseudomonas spp. |
ND | [85] |
| Crimson | |||||||
| Thompson | |||||||
| Sundarkhani | |||||||
| Perlette | |||||||
| King’s Ruby | |||||||
| Viognier | USA | Pomace | Acetone:water (80:20) |
L. monocytogenes S. aureus |
- | 5.07–40.6 | [81] |
| Vidal Blanc | 15.6–250 | ||||||
| Cabernet Franc | 4.69–75 | ||||||
| Chambourcin | 18.8–75 | ||||||
| Bangalore blue | India | Seeds | Acetone:water:acetic acid (90:9.5:0.5) |
B. cereus B. coagulans B. subtilis S. aureus |
E. coli P. aeruginosa |
ND | [78] |
| Methanol:water:acetic acid (90:9.5:0.5) |
|||||||
| Palavá | Slovakia | Pomace | ethanol | B. ceresu S. aureus |
E. coli P. aeruginosa |
0.5–2 | [79] |
| Dornfelder | |||||||
| Narince | Turkey | Bagasse | Ethyl acetate:methanol:water (60:30:10) |
- | - | ND | [75] |
| Ethanol:water (95:5) |
|||||||
| Grape Variety | Country | By-Product | Extraction Solvent(s)/Methodologies | Gram-Positive | Gram-Negative | MIC Range (mg mL−1) | Reference |
| Narince | Turkey | Defatted seeds | Acetone:water:acetic acid (90:9.5:0.5) |
B. amyloliquefaciens B. brevis |
P. vulgaris P. aeruginosa |
ND | [75] |
| Ethyl acetate:methanol:water (60:30:10) |
|||||||
| Hasandede | Turkey | Defatted seeds | Acetone:water:acetic acid (90:9.5:0.5) |
B. cereus E. faecalis M. smegmatis S. aureus |
A. hydrophyla E. aerogenes E. coli K. pneumoniae P. vulgaris P. aeruginosa S. Enteritidis S. Typhimurium |
ND | [76] |
| Emir | |||||||
| Kalecic Karasi | |||||||
| Pinot Noir | New Zealand | Pomace | Acetone:water (50:50) |
S. aureus | E. coli | 0.39–25 | [77] |
| Ethanol:water (50:50) |
0.78–25 | ||||||
| Methanol:water (50:50) |
0.78–25 | ||||||
| Seeds | Acetone:water (50:50) |
0.39–25 | |||||
| Ethanol:water (50:50) |
0.78–25 | ||||||
| Methanol:water (50:50) |
0.195–25 | ||||||
| Skins | Acetone:water (50:50) |
0.39–25 | |||||
| Ethanol:water (50:50) |
0.78–25 | ||||||
| Methanol:water (50:50) |
12.5–25 | ||||||
| Grape Variety | Country | By-Product | Extraction Solvent(s)/Methodologies | Gram-Positive | Gram-Negative | MIC Range (mg mL−1) | Reference |
| Pinot Meunier | New Zealand | Pomace | Acetone:water (50:50) |
S. aureus | E. coli | 12.5–25 | [77] |
| Ethanol:water (50:50) |
12.5 | ||||||
| Methanol:water (50:50) |
12.5 | ||||||
| Seeds | Acetone:water (50:50) |
3.125–25 | |||||
| Ethanol:water (50:50) |
1.56–100 | ||||||
| Methanol:water (50:50) |
0.195–25 | ||||||
| Skins | Acetone:water (50:50) |
12.5–25 | |||||
| Ethanol:water (50:50) |
12.5–25 | ||||||
| Methanol:water (50:50) |
25 | ||||||
| Merlot | Brazil | Pomace | SFE-ethanol | B. cereus S. aureus |
E. coli P. aeruginosa |
0.007–0.012 | [86] |
| SOX-hexane | - | ||||||
| Syrah | Brazil | Pomace | SFE-ethanol | B. cereus S. aureus |
- | - | |
| SOX-hexane | B. cereus | 0.014 | |||||
4. Wine By-Products in the Combat against Antibiotic Resistance
- (i). Modification of the active sites in and on the bacterial cell, e.g., PBPs, topoisomerases [60];
- (ii). Inhibition of bacterial enzymes involved in antibiotic modification or degradation, e.g., β-lactamase inhibition [95];
- (iii). Increased membrane permeability [60]; and
- (iv). Inhibition of antibiotic efflux pumps, such as Tet(K) in S. aureus and S. epidermidis [60].
References
- Barba, F.J.; Zhu, Z.; Koubaa, M.; Sant’Ana, A.S.; Orlien, V. Green alternative methods for the extraction of antioxidant bioactive compounds from winery wastes and by-products: A review. Trends Food Sci. Technol. 2016, 49, 96–109.
- Brenes, A.; Viveros, A.; Chamorro, S.; Arija, I. Use of polyphenol-rich grape by-products in monogastric nutrition. A review. Anim. Feed Sci. Technol. 2016, 211, 1–17.
- Venkitasamy, C.; Zhao, L.; Zhang, R.; Pan, Z. Chapter 6. Grapes. In Integrated Processing Technologies for Food and Agricultural By-Products; Pan, Z., Zhang, R., Zicaria, S., Eds.; Academic Press/Elsevier Inc.: London, UK, 2019; pp. 131–164.
- Kalli, E.; Lappa, I.; Bouchagier, P.; Tarantilis, P.A.; Skotti, E. Novel application and industrial exploitation of winery by-products. Bioresour. Bioprocess. 2018, 5.
- Oliveira, M.; Duarte, E. Integrated approach to winery waste: Waste generation and data consolidation. Front. Environ. Sci. Eng. 2016, 10, 168–176.
- Christ, K.L.; Roger, L. Burritt. Critical environmental concerns in wine production: An integrative review. J. Clean. Prod. 2013, 53, 232–242.
- Bustamante, M.A.; Moral, R.; Paredes, C.; Pérez-Espinosa, A.; Moreno-Caselles, J.; Pérez-Murcia, M.D. Agrochemical characterisation of the solid by-products and residues from the winery and distillery industry. Waste Manag. 2008, 28, 372–380.
- Mendes, J.A.S.; Prozil, S.O.; Evtuguin, D.V.; Lopes, L.P.C. Towards comprehensive utilization of winemaking residues: Characterization of grape skins from red grape pomaces of variety Touriga Nacional. Ind. Crop. Prod. 2013, 43, 25–33.
- Nogales, R.; Cifuentes, C.; Benítez, E. Vermicomposting of winery wastes: A laboratory study. J. Environ. Sci. Health Part B Pestic. Food Contam. Agric. Wastes 2005, 40, 659–673.
- Brito, P.S.D.; Oliveira, A.S.; Rodrigues, L.F. Energy valorization of solid vines pruning by thermal gasification in a pilot plant. Waste Biomass Valorization 2014, 5, 181–187.
- Sánchez-Gómez, R.; Alonso, G.L.; Salinas, M.R.; Zalacain, A. Reuse of Vine-Shoots Wastes for Agricultural Purposes; Elsevier Inc.: London, UK, 2017; ISBN 9780128098714.
- Ruggieri, L.; Cadena, E.; Martínez-Blanco, J.; Gasol, C.M.; Rieradevall, J.; Gabarrell, X.; Gea, T.; Sort, X.; Sánchez, A. Recovery of organic wastes in the Spanish wine industry. Technical, economic and environmental analyses of the composting process. J. Clean. Prod. 2009, 17, 830–838.
- Kokkinomagoulos, E.; Kandylis, P. Sustainable exploitation of by-Products of vitivinicultural origin in winemaking. Proceedings 2020, 67, 5.
- Antonić, B.; Jančíková, S.; Dordević, D.; Tremlová, B. Grape pomace valorization: A systematic review and meta-analysis. Foods 2020, 7, 1627.
- Troilo, M.; Difonzo, G.; Paradiso, V.M.; Summo, C.; Caponio, F. Bioactive compounds from vine shoots, grape stalks, and wine lees: Their potential use in agro-food chains. Foods 2021, 5, 342.
- Mateo, J.J.; Maicas, S. Valorization of winery and oil mill wastes by microbial technologies. Food Res. Int. 2015, 73, 13–25.
- Gómez-Brandón, M.; Lores, M.; Insam, H.; Domínguez, J. Strategies for recycling and valorization of grape marc. Crit. Rev. Biotechnol. 2019, 39, 437–450.
- Ali, K.; Maltese, F.; Choi, Y.H.; Verpoorte, R. Metabolic constituents of grapevine and grape-derived products. Phytochem. Rev. 2010, 9, 357–378.
- Graça, A.; Corbet-Milward, J.; Schultz, H.R.; Ozer, C.; de la Fuente, M. Managing By-Products of Vitivinicultural Origin; OIV–International Organization of Vine and Wine: Paris, France, 2018; 16p.
- Lelario, F.; Scrano, L.; De Franchi, S.; Bonomo, M.G.; Salzano, G.; Milan, S.; Bufo, S.A. Identification and antimicrobial activity of most representative secondary metabolites from different plant species. Chem. Biol. Technol. Agric. 2018, 5, 13.
- Barbieri, R.; Coppo, E.; Marchese, A.; Daglia, M.; Sobarzo-Sánchez, E.; Nabavi, S.F.; Nabavi, S.M. Phytochemicals for human disease: An update on plant-derived compounds antibacterial activity. Microbiol. Res. 2017, 196, 44–68.
- Gorlenko, C.L.; Kiselev, H.Y.; Budanova, E.V.; Zamyatnin, A.A., Jr.; Ikryannikova, L.N. Plant secondary metabolites in the battle of drugs and drug-resistant bacteria: New heroes or worse clones of antibiotics? Antibiotics 2020, 9, 170.
- Abegaz, B.M.; Kinfe, H.H. Secondary metabolites, their structural diversity, bioactivity, and ecological functions: An overview. Phys. Sci. Rev. 2019, 4.
- Swallah, M.S.; Sun, H.; Affoh, R.; Fu, H.; Yu, H. Antioxidant potential overviews of secondary metabolites (polyphenols) in fruits. Int. J. Food Sci. 2020, 9081686.
- Lipiński, K.; Mazur, M.; Antoszkiewicz, Z.; Purwin, C. Polyphenols in monogastric nutrition—A review. Ann. Anim. Sci. 2017, 17, 41–58.
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18B, 820–897.
- Ambriz-Pérez, D.L.; Leyva-López, N.; Erick, P.; Gutierrez-Grijalva, E.P.; Heredia, J.B. Phenolic compounds: Natural alternative in inflammation treatment. A Review. Cogent Food Agric. 2016, 2, 1131412.
- Roleira, F.M.; Tavares-da-Silva, E.J.; Varela, C.L.; Costa, S.C.; Silva, T.; Garrido, J.; Borges, F. Plant derived and dietary phenolic antioxidants: Anticancer properties. Food Chem. 2015, 183, 235–258.
- Salehi, B.; Vlaisavljevic, S.; Adetunji, C.O.; Adetunji, J.B.; Kregiel, D.; Antolaki, H.; Pawlikowska, E.; Uprety, Y.; Mileski, K.S.; Devkota, H.P.; et al. Plants of the genus Vitis: Phenolic compounds, anticancer properties and clinical relevance. Trends Food Sci. Technol. 2019, 91, 362–379.
- Al Shukor, N.; Van Camp, J.; Gonzalez, G.B.; Staljanssens, D.; Struijs, K.; Zotti, M.J.; Raes, K.; Smagghe, G. Angiotensin-converting enzyme inhibitory effects by plant phenolic compounds: A study of structure activity relationships. J. Agric. Food Chem. 2013, 61, 11832–11839.
- Rempe, C.S.; Burris, K.P.; Lenaghan, S.C.; Stewart, C.N., Jr. The potential of systems biology to discover antibacterial mechanisms of plant phenolics. Front. Microbiol. 2017, 8, 422.
- Averilla, J.N.; Oh, J.; Kim, H.J.; Kim, J.S.; Kim, J.-S. Potential health benefits of phenolic compounds in grape processing by-products. Food Sci. Biotechnol. 2019, 28, 1607–1615.
- Miklasińska-Majdanik, M.; Kępa, M.; Wojtyczka, R.D.; Idzik, D.; Wąsik, T.J. Phenolic compounds diminish antibiotic resistance of Staphylococcus aureus clinical strains. Int. J. Environ. Res. Public Health 2018, 15, 2321.
- Vinholes, J.; Silva, B.M.; Silva, L.R. Hydroxycinnamic acids (HCAS): Structure, biological properties and health effects. In Advances in Medicine and Biology, 8th ed.; Berhardt, L.V., Ed.; Nova Science Publishers: New York, NY, USA, 2010; Volume 88, pp. 105–130.
- Devesa-rey, R.; Vecino, X.; Varela-alende, J.L.; Barral, M.T.; Cruz, J.M.; Moldes, A.B. Valorization of winery waste vs. the costs of not recycling. Waste Manag. 2011, 31, 2327–2335.
- Sirohi, R.; Tarafdar, A.; Singh, S.; Negi, T.; Gaur, V.K.; Gnansounou, E.; Bharathiraja, B. Green processing and biotechnological potential of grape pomace: Current trends and opportunities for sustainable biorefinery. Bioresour. Technol. 2020, 314, 123771.
- Bordiga, M.; Travaglia, F.; Locatelli, M. Valorisation of grape pomace: An approach that is increasingly reaching its maturity—A review. Int. J. Food Sci. Technol. 2019, 54, 933–942.
- Pavić, V.; Kujundžić, T.; Kopić, M.; Jukić, V.; Braun, U.; Schwander, F.; Drenjančević, M. Effects of Defoliation on phenolic concentrations, antioxidant and antibacterial activity of grape skin extracts of the varieties Blaufränkisch and Merlot (Vitis vinifera L.). Molecules 2019, 24, 2444.
- Hassan, Y.I.; Kosir, V.; Yin, X.; Ross, K.; Diarra, M.S. Grape pomace as a promising antimicrobial alternative in feed: A critical review. J. Agric. Food Chem. 2019, 67, 9705–9718.
- Dávila, I.; Robles, E.; Egüés, I.; Labidi, J.; Gullón, P. The Biorefinery Concept for the Industrial Valorization of Grape Processing By-Products. In Handbook of Grape Processing By-Products. Sustainable Solutions; Galanakis, C.M., Ed.; Academic Press/Elsevier: London, UK, 2017; pp. 29–53.
- Deng, Q.; Penner, M.H.; Zhao, Y. Chemical composition of dietary fiber and polyphenols of five different varieties of wine grape pomace skins. Food Res. Int. 2011, 44, 2712–2720.
- Hogervorst, J.C.; Miljić, U.; Puškaš, V. Extraction of Bioactive Compounds from Grape Processing By-Products. In Handbook of Grape Processing By-Products. Sustainable Solutions; Galanakis, C.M., Ed.; Academic Press/Elsevier: London, UK, 2017; pp. 105–135.
- Muhlack, R.A.; Potumarthi, R.; Jeffery, D.W. Sustainable wineries through waste valorisation: A review of grape marc utilisation for value-added products. Waste Manag. 2018, 72, 99–118.
- Nanni, A.; Parisi, M.; Colonna, M. Wine by-products as raw materials for the production of biopolymers and of natural reinforcing fillers: A critical review. Polymers 2021, 13, 381.
- Spigno, G.; Marinoni, L.; Garrido, G.D. State of the Art in Grape Processing By-Products. In Handbook of Grape Processing By-Products. Sustainable Solutions; Galanakis, C.M., Ed.; Academic Press/Elsevier: London, UK, 2017; pp. 1–27.
- Beres, C.; Costa, G.N.S.; Cabezudo, I.; da Silva-James, N.K.; Teles, A.S.C.; Cruz, A.P.G.; Mellinger-Silva, C.; Tonon, R.V.; Cabral, L.M.C.; Freitas, S.P. Towards integral utilization of grape pomace from winemaking process: A review. Waste Manag. 2017, 68, 581–594.
- Ma, Z.F.; Zhang, H. Phytochemical constituents, health benefits, and industrial applications of grape seeds: A mini-review. Antioxidants 2017, 6, 71.
- Domínguez, J.; Sanchez-Hernandez, J.C.; Lores, M. Vermicomposting of Winemaking By-Products. In Handbook of Grape Processing By-Products. Sustainable Solutions; Galanakis, C.M., Ed.; Academic Press/Elsevier: London, UK, 2017; pp. 55–78.
- Friedman, M. Antibacterial, antiviral, and antifungal properties of wines and winery byproducts in relation to their flavonoid content. J. Agric. Food Chem. 2014, 62, 6025–6042.
- Souquet, J.-M.; Labarbe, B.; Le Guernevé, C.; Cheynier, V.; Moutounet, M. Phenolic composition of grape stems. J. Agric. Food Chem. 2000, 48, 1076–1080.
- Blackford, M.; Comby, M.; Zeng, L.; Dienes-Nagy, Á.; Bourdin, G.; Lorenzini, F.; Bach, B. A Review on stems composition and their impact on wine quality. Molecules 2021, 26, 1240.
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614.
- Álvarez-Martínez, F.J.; Barrajón-Catalán, E.; Micol, V. Tackling antibiotic resistance with compounds of natural origin: A comprehensive review. Biomedicines 2020, 8, 405.
- Thomford, N.E.; Senthebane, D.A.; Rowe, A.; Munro, D.; Seele, P.; Maroyi, A.; Dzobo, K. Natural products for drug discovery in the 21st century: Innovations for novel drug discovery. Int. J. Mol. Sci. 2018, 19, 1578.
- Wright, G.D. Unlocking the potential of natural products in drug discovery. Microb. Biotechnol. 2019, 12, 55–57.
- Efenberger-Szmechtyk, M.; Nowak, A.; Czyzowska, A. Plant extracts rich in polyphenols: Antibacterial agents and natural preservatives for meat and meat products. Crit. Rev. Food Sci. Nutr. 2021, 61, 149–178.
- Jara-Palacios, M.J.; Hernanz, D.; Cifuentes-Gomez, T.; Escudero-Gilete, M.L.; Heredia, F.J.; Spencer, J.P.E. Assessment of white grape pomace from winemaking as source of bioactive compounds, and its antiproliferative activity. Food Chem. 2015, 78–82.
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181.
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev. 2019, 18, 241–272.
- Álvarez-Martínez, F.J.; Barrajón-Catalán, E.; Encinar, J.A.; Rodríguez-Díaz, J.C.; Micol, V. Antimicrobial capacity of plant polyphenols against Gram-positive bacteria: A comprehensive review. Curr. Med. Chem. 2020, 27, 2576–2606.
- AlSheikh, H.M.A.; Sultan, I.; Kumar, V.; Rather, I.A.; Al-Sheikh, H.; Tasleem, J.A.; Haq, Q.M.R. Plant-based phytochemicals as possible alternative to antibiotics in combating bacterial drug resistance. Antibiotics 2020, 9, 480.
- Bouarab-Chibane, L.; Forquet, V.; Lantéri, P.; Clément, Y.; Léonard-Akkari, L.; Oulahal, N.; Degraeve, P.; Bordes, C. Antibacterial properties of polyphenols: Characterization and QSAR (Quantitative Structure-Activity Relationship) models. Front. Microbiol. 2019, 10, 829.
- Puupponen-Pimiä, R.; Nohynek, L.; Meier, C.; Kähkönen, M.; Heinonen, M.; Hopia, A.; Oksman-Caldentey, K.M. Antimicrobial properties of phenolic compounds from berries. J. Appl. Microbiol. 2001, 90, 494–507.
- Langeveld, W.T.; Veldhuizen, E.J.; Burt, S.A. Synergy between essential oil components and antibiotics: A review. Crit. Rev. Microbiol. 2014, 40, 76–94.
- Lambert, R.J.; Skandamis, P.N.; Coote, P.J.; Nychas, G.J. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J. Appl. Microbiol. 2001, 91, 453–462.
- Roy, R.; Tiwari, M.; Donelli, G.; Tiwari, V. Strategies for combating bacterial biofilms: A focus on anti-biofilm agents and their mechanisms of action. Virulence 2018, 9, 522–554.
- Slobodníková, L.; Fialová, S.; Rendeková, K.; Kováč, J.; Mučaji, P. Antibiofilm activity of plant polyphenols. Molecules 2016, 21, 1717.
- Gyawali, R.; Ibrahim, S.A. Natural products as antimicrobial agents. Food Control 2014, 46, 412–429.
- Farhadi, F.; Khameneh, B.; Iranshahi, M.; Iranshahy, M. Antibacterial activity of flavonoids and their structure-activity relationship: An update review. Phytother Res. 2019, 33, 13–40.
- Papuc, C.; Goran, G.V.; Predescu, C.N.; Nicorescu, V.; Stefan, G. Plant polyphenols as antioxidant and antibacterial agents for shelf-life extension of meat and meat Products: Classification, structures, sources, and action mechanisms. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1243–1268.
- Xie, Y.; Yang, W.; Tang, F.; Chen, X.; Ren, L. Antibacterial activities of flavonoids: Structure-activity relationship and mechanism. Curr. Med. Chem. 2015, 22, 132–149.
- Sánchez-Maldonado, A.F.; Schieber, A.; Gänzle, M.G. Structure-function relationships of the antibacterial activity of phenolic acids and their metabolism by lactic acid bacteria. J. Appl. Microbiol. 2011, 111, 1176–1184.
- Cueva, C.; Moreno-Arribas, M.V.; Martín-Alvarez, P.J.; Bills, G.; Vicente, M.F.; Basilio, A.; Rivas, C.L.; Requena, T.; Rodríguez, J.M.; Bartolomé, B. Antimicrobial activity of phenolic acids against commensal, probiotic and pathogenic bacteria. Res. Microbiol. 2010, 161, 372–382.
- Tesaki, S.; Tanabe, S.; Moriyama, M.; Fukushi, E.; Kawabata, J.; Watanabe, M. Isolation and identification of an antibacterial compound from grape and its application to foods. Health Environ. Res. Online 1999, 73, 125–128.
- Baydar, N.G.; Göktürk, G.; Sağdiç, O. Total phenolic contents and antibacterial activities of grape (Vitis Vinifera L.) extracts. Food Control 2004, 15, 0956–7135.
- Baydar, N.G.; Sagdic, O.; Ozkan, G.; Cetin, S. Determination of antibacterial effects and total phenolic contents of grape (Vitis vinifera L.) seed extracts. Int. J. Food Sci. Technol. 2006, 41, 799–804.
- Cheng, V.J.; El-Din, A.; Bekhit, A.; McConnell, M.; Mros, S.; Zhao, J. Effect of extraction solvent, waste fraction and grape variety on the antimicrobial and antioxidant activities of extracts from wine residue from cool climate. Food Chem. 2012, 474–482.
- Jayaprakasha, G.K.; Selvi, T.; Sakariah, K.K. Antibacterial and antioxidant activities of grape (Vitis vinifera) seed extracts. Food Res. Int. 2003, 117–122.
- Kačániová, M.; Terentjeva, M.; Kántor, A.; Felsöciová, S.; Puchalski, C.; Kunová, S.; Lopašovský, L.; Žiarovská, J. Antimicrobial activity of Vitis vinifera L. pomace extract. Anim. Sci. Biotechnol. 2018, 51, 124–129.
- Silva, V.; Igrejas, G.; Falco, V.; Santos, T.P.; Torres, C.; Oliveira, A.M.P.; Pereira, J.E.; Amaral, J.S.; Poeta, P. Chemical composition, antioxidant and antimicrobial activity of phenolic compounds extracted from wine industry by-products. Food Control 2018, 92, 516–522.
- Xu, C.; Yagiz, Y.; Hsu, W.Y.; Simonne, A.; Lu, J.; Marshall, M.R. Antioxidant, antibacterial, and antibiofilm properties of polyphenols from muscadine grape (Vitis rotundifolia Michx.) pomace against selected foodborne pathogens. J. Agric. Food Chem. 2014, 62, 6640–6649.
- Xu, Y.; Burton, S.; Kim, C.; Sismour, E. Phenolic compounds, antioxidant, and antibacterial properties of pomace extracts from four Virginia-grown grape varieties. Food Sci. Nutr. 2015, 4, 125–133.
- Katalinić, V.; Možina, S.M.; Skroza, D.; Generalić, I.; Abramovič, H.; Miloš, M.; Ljubenkov, I.; Piskernik, S.; Pezo, I.; Terpinc, P.; et al. Polyphenolic profile, antioxidant properties and antimicrobial activity of grape skin extracts of 14 Vitis vinifera varieties grown in Dalmatia (Croatia). Food Chem. 2010, 119, 715–723.
- Serra, A.T.; Matias, A.A.; Nunes, A.V.M.; Leitão, M.C.; Brito, D.; Bronze, R.; Silva, S.; Pires, A.; Crespo, M.T.; San Romão, M.V.; et al. In vitro evaluation of olive- and grape-based natural extracts as potential preservatives for food. Innov. Food Sci. Emerg. Technol. 2008, 9, 311–319.
- Ali, N.; Afrasiab, H.; Anwar, S. Antibacterial activity of leaf extracts of seven grape cultivars against six strains of bacteria. Adv. Life Sci. 2019, 6, 139–146.
- Oliveira, D.A.; Salvador, A.A.; Smânia, A., Jr.; Smânia, E.F.; Maraschin, M.; Ferreira, S.R. Antimicrobial activity and composition profile of grape (Vitis vinifera) pomace extracts obtained by supercritical fluids. J. Biotechnol. 2013, 164, 423–432.
- Özkan, G.; Sagdiç, O.; Göktürk Baydar, N.; Kurumahmutoglu, Z. Antibacterial activities and total phenolic contents of grape pomace extracts. J. Sci. Food Agric. 2004, 84, 1807–1811.
- Gómez-Mejía, E.; Roriz, C.L.; Heleno, S.A.; Calhelha, R.; Dias, M.I.; Pinela, J.; Rosales-Conrado, N.; León-González, M.E.; Ferreira, I.C.F.R.; Barros, L. Valorisation of black mulberry and grape seeds: Chemical characterization and bioactive potential. Food Chem. 2021, 337, 127998.
- Felhi, S.; Baccouch, N.; Ben Salah, H.; Smaoui, S.; Allouche, N.; Gharsallah, N.; Kadri, A. Nutritional constituents, phytochemical profiles, in vitro antioxidant and antimicrobial properties, and gas chromatography-mass spectrometry analysis of various solvent extracts from grape seeds (Vitis vinifera L.). Food Sci. Biotechnol. 2016, 25, 1537–1544.
- Radulescu, C.; Buruleanu, L.C.; Nicolescu, C.M.; Olteanu, R.L.; Bumbac, M.; Holban, G.C.; Simal-Gandara, J. Phytochemical profiles, antioxidant and antibacterial activities of grape (Vitis vinifera L.) seeds and skin from organic and conventional vineyards. Plants 2020, 9, 1470.
- Stefanović, O.D. Synergistic Activity of Antibiotics and Bioactive Plant Extracts: A Study against Gram-Positive and Gram-Negative Bacteria. In Bacterial Pathogenesis and Antibacterial Control; IntechOpen: London, UK, 2018.
- Kawabata, K.; Yoshioka, Y.; Terao, J. Role of intestinal microbiota in the bioavailability and physiological functions of dietary polyphenols. Molecules 2019, 24, 370.
- Costa, J.R.; Xavier, M.; Amado, I.R.; Gonçalves, C.; Castro, P.M.; Tonon, R.V.; Cabral, L.M.C.; Pastrana, L.; Pintado, M.E. Polymeric nanoparticles as oral delivery systems for a grape pomace extract towards the improvement of biological activities. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 119, 111551.
- Aiyegoro, O.A.; Okoh, A.I. Use of bioactive plant products in combination with standard antibiotics: Implications in antimicrobial chemotherapy. J. Med. Plants Res. 2009, 3, 1147–1152.
- Siriwong, S.; Teethaisong, Y.; Thumanu, K.; Dunkhunthod, B.; Eumkeb, G. The synergy and mode of action of quercetin plus amoxicillin against amoxicillin-resistant Staphylococcus epidermidis. BMC Pharm. Toxicol. 2016, 17, 39.
- Sanhueza, L.; Melo, R.; Montero, R.; Maisey, K.; Mendoza, L.; Wilkens, M. Synergistic interactions between phenolic compounds identified in grape pomace extract with antibiotics of different classes against Staphylococcus aureus and Escherichia coli. PLoS ONE 2017, 12, e0172273.
- Mattos, G.N.; Tonon, R.V.; Furtado, A.A.L.; Cabral, L.M.C. Grape by-product extracts against microbial proliferation and lipid oxidation: A review. J. Sci. Food Agric. 2017, 97, 1055–1064.
- Cushnie, T.P.; Lamb, A.J. Recent advances in understanding the antibacterial properties of flavonoids. Int. J. Antimicrob. Agents 2011, 38, 99–107.
- Savoia, D. Plant-derived antimicrobial compounds: Alternatives to antibiotics. Future Microbiol. 2012, 7, 979–990.




