Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Giulia Pozzi | + 1948 word(s) | 1948 | 2022-01-26 07:20:05 | | | |
| 2 | Catherine Yang | Meta information modification | 1948 | 2022-02-11 10:51:29 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Pozzi, G. Role of Hydrogen Sulfide. Encyclopedia. Available online: https://encyclopedia.pub/entry/19362 (accessed on 07 February 2026).
Pozzi G. Role of Hydrogen Sulfide. Encyclopedia. Available at: https://encyclopedia.pub/entry/19362. Accessed February 07, 2026.
Pozzi, Giulia. "Role of Hydrogen Sulfide" Encyclopedia, https://encyclopedia.pub/entry/19362 (accessed February 07, 2026).
Pozzi, G. (2022, February 11). Role of Hydrogen Sulfide. In Encyclopedia. https://encyclopedia.pub/entry/19362
Pozzi, Giulia. "Role of Hydrogen Sulfide." Encyclopedia. Web. 11 February, 2022.
Copy Citation
Hydrogen sulfide (H2S) is a gasotransmitter able to induce/inhibit immunological responses, playing a role in inflammatory and autoimmune diseases, neurological disorders, asthma, acute pancreatitis, and sepsis. Both endogenous and exogenous H2S modulate numerous important cell signaling pathways. In monocytes, polymorphonuclear, and T cells H2S impacts on activation, survival, proliferation, polarization, adhesion pathways, and modulates cytokine production and sensitivity to chemokines.
Th17 cells
Tregs
Hydrogen sulfide
1. Introduction
T lymphocytes develop from CD7+CD34+ lymphoid progenitors, generated in the bone marrow and differentiated in the thymus. During thymic selection, they develop the ability to discriminate between self and non-self. T lymphocytes can be grouped into two main categories: helper CD4+ T cells, that regulate the whole immune response, and cytotoxic CD8+ T cells, that actively kill pathogens. Since T cells are essential components of adaptive immune responses, impaired T cell functions ultimately lead to immunodeficiency, promoting pathogen infections as well as various forms of tumors. Autoimmune disorders caused by uncontrolled autoreactive T cells include multiple sclerosis, rheumatoid arthritis, inflammatory bowel disease, diabetes, psoriasis, and autoimmune thyroiditis [1][2][3].
T-helper (Th) cells have key functions in adaptive immunity and are involved in autoimmunity, asthma, allergy reactions, and tumor immunity. During T cell receptor (TCR)-mediated activation in the presence of specific cytokines in the surrounding microenvironment, naïve CD4+ T cells can polarize into one of multiple Th cell lineages, including Th1, Th2, Th17, and regulatory T (Treg) cells (Figure 1). Differentiation of different CD4+ effector/regulatory T-cell subpopulations is predominantly induced by specific sets of cytokines and finely tuned by different signaling pathways and transcription factors [4][5][6][7]. Th1 cells produce interferon-γ (IFN-γ), boosting cell-mediated immunity towards intracellular infections, whereas Th2 cells release interleukin (IL)-4, promoting humoral immunity to parasitic helminths. Th17 cells produce IL-17 and may have adapted to defend humans against microorganisms that Th1 and Th2 responses are not specific for, such as invasive bacteria as well as certain fungi [8][9][10]. The peculiar characteristic of IL-17 is that it has a potent activity on stromal cells in all tissues, leading to the production of inflammatory cytokines and chemiotaxis of leukocytes, particularly neutrophils, thus linking innate to adaptive immunity. Despite their significant role in host defense, Th17 have attracted great interest in recent years for their contribution in the pathogenesis of several autoimmune and inflammatory diseases [11]. Indeed, Th17 are pro-inflammatory T cells, and when in excess they promote autoimmunity and tissue damage. On the other hand, Treg cells, characterized by the expression of forkhead box transcription factor FoxP3, are required for immunological self-tolerance and homeostasis. They inhibit a wide range of immune responses (activated by Th1, Th2, and Th17 cells) as well as undesired immunity against a multitude of antigens, such as self-antigens, bacteria-originated antigens, and exogenous allergens. As a result, a deficiency in Treg cell population can result in acute inflammatory disorders such as autoimmunity, colitis, and allergies [12][13].
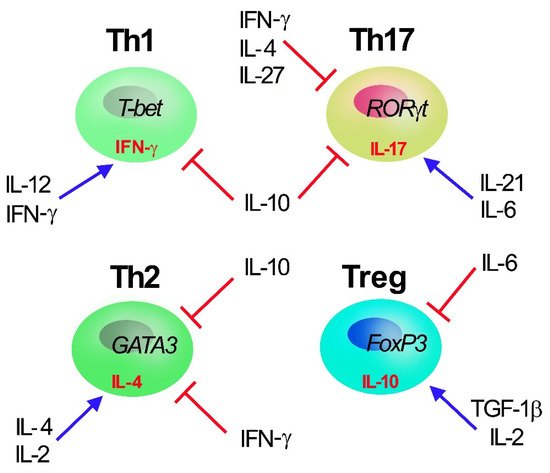
Figure 1. Th1, Th2, Th17, and Treg T CD4+ subset cells. Master transcription factors promoting Th polarization are reported inside cells (T-bet, GATA3, RORγT and Foxp3 for Th1, Th2, Th17, and Treg cell, respectively) together with selective secreted cytokines (γ-IFN, IL-4, IL-17 and IL-10 for Th1, Th2, Th17, and Treg cell, respectively). The main cytokines (IL-2, IL-4, IL-6, IL-12, IL-10, IL-21, IFN-γ, and TGF-1β) regulating Th polarization are reported: IL-10, secreted by Treg, acts as major inhibiting factors of Th polarization and proliferation.
Endogenous hydrogen sulfide (H2S) exerts a variety of physiologically relevant activities. It belongs to the “gasotransmitter” family, along with nitric oxide (NO), carbon monoxide (CO), and sulfur dioxide (SO2). Once considered as poisonous and possibly fatal gases, they are now recognized as crucial intracellular signaling molecules with a wide range of physiological activities, and several H2S-releasing compounds are currently in preclinical and clinical trial, showing promising effects and therapeutic potential [14]. Specifically, the relevance of H2S in immune and inflammatory responses has long been a relevant topic of scientific research. H2S has been shown to modulate several immune cell activities, including monocyte and polymorphonuclear cell apoptosis, leukocyte adhesion and infiltration, T-cell activation, proliferation, and inflammatory cytokine production. Autoimmune disorders, neurodegenerative diseases, asthma, acute pancreatitis, and sepsis have all been related to the impact of H2S in inflammation [15][16][17][18]. Interestingly, H2S has been demonstrated to modulate T-cell lineage polarization, therefore representing a new and potential target to modulate and improve adaptive immunity responses.
2. Role of H2S in Th17 Cells
Th17 cells have been widely investigated in various diseases, including inflammatory bowel disease (IBD), colorectal tumors, autoimmune arthritis, psoriasis, hypoxia-induced pulmonary hypertension, and ischemic brain injury (HBI) [19][20][21][22][23][24]. Altogether these studies demonstrate that Th17 cells exert a role in the pathogenesis of inflammatory diseases, while also having a beneficial role in maintaining health [25].
Physiologically, intestinal bacteria are required to maintain a Th17 response in the mucosa [26][27][28]. However, increased Th17 cells and related cytokines (such as IL-17, IL-21 and IL-22) are linked to inflammatory disease severity, such as in IBD patients [29]. The role of H2S in the context of innate immunity in the mucosa has been explored in a colitis mouse model. Interestingly, it has been demonstrated that sulfate-reducing bacteria (SRB), that produce H2S, potentiate the mucosal Th17 response [30]. Indeed, SRB colonization enhanced the number of CD11b+, B, and T cells and boosted the formation and/or activation of Th17 cells in the mucosal immune system, as confirmed by upregulation of IL-6 and IL-17 by mesenteric lymph node cells in germ-free mice. Accordingly, H2S was demonstrated to influence type 2 immunity being a potent inducer of pro-inflammatory Th17 cells and Tregs in the intestine [31].
The relative numbers of the three lymphocyte subsets Th1, Th2, and Th17 are imbalanced in HBI. Upon HBI T-cell activation shifted to a pro-inflammatory Th1 setting while having no effect on the Th17 response [32]. While it is known that H2S levels and its enzymes are dysregulated following HBI, it was only recently explored the hypothesis that they may influence immune cell functions in neonatal mice, including local microglia and infiltrating peripheral immune cells [33][34][35]. Increase of H2S levels was obtained using L-Cysteine, a common substrate for its production [14][36]. H2S treatment inhibited CD4+T cell infiltration while simultaneously dramatically lowering the fraction of Th1 cells and increasing the Th17/Th2 ratio following HBI. These results suggest that L-Cysteine exerts anti-inflammatory effects by increasing the shift of T cells to Th2 response [35]. It is not clear whether L-Cysteine modulates only the recruitment of Th subpopulations and/or Th polarization in the HBI context.
Th1 and Th17 cells can cooperate and promote the development of autoimmune diseases [37]. Indeed, psoriasis was once thought to be a Th1-mediated skin disorder, but the attention has recently switched to IL-17-producing cells, such as Th17 lymphocytes [38]. Interestingly, patients affected by psoriasis have significantly higher homocysteine (Hcy) level in serum which is responsible for the pathologic stimulation of Th1 and Th17 cells [39]. Under physiological conditions, Hcy is metabolized to cysteine, which then produces H2S. On the contrary, in pathological conditions, high levels of Hcy inhibit CSE activity and reduce endogenous H2S generation. Accordingly, certain H2S donors have been reported to suppress Hcy levels, limiting Th1 and Th17 overactivation in psoriasis [40][41].
Diet is a means to increase H2S bioavailability [42][43]. As an example, the main biologically active molecules of garlic are amino acids, vitamins, micronutrients, and organosulfur compounds (OSCs), the latter being able to raise endogenous H2S [43][44]. It has been shown that pretreatment with a mixture containing dipropyl polysulfides (DPPS), components of garlic [45], significantly mitigated Concanavalin A (ConA)-induced hepatitis in mice. DPPS pretreatment reduced inflammatory cytokines while increasing Treg lymphocytes in the livers of ConA mice. DPPS demonstrated hepatoprotective benefits in ConA-induced hepatitis, as evidenced by reduced inflammation and a shift in the Th17/Treg balance in favor of Treg cells, implying possible applications of DPPS mixtures in inflammatory immune-mediated liver disorders [46]. Furthermore, Diallyl Trisulfide (DATS), an organosulfur molecule isolated from garlic bulbs, reduced inflammatory cytokine production, and controlled immune function in a collagen-induced arthritis mouse model. The suppression of the NF-κB and Wnt signaling pathways restored the equilibrium between Th17 and Treg cells [47]. It is commonly acknowledged that an imbalance in Th17/Treg levels is deleterious to RA. Adjustment of these imbalances may reduce joint inflammation and improve disease prognosis, implying a role for DATS as anti-arthritic drugs.
3. Role of H2S in Treg
T regulatory cells, commonly known as Tregs, play an important role in immunological homeostasis and self-tolerance. The presence of CD4, CD25, and FoxP3, a critical transcription factor for Treg polarization, distinguishes naturally occurring Tregs (nTregs). A subgroup of Treg cells exists in parallel to nTregs, named induced Tregs, (iTregs). Both iTregs and nTregs regulate immunological activation in a number of ways, both directly and indirectly. The capacity to direct Treg activities might represent an innovative strategy to prevent/treat autoimmune diseases, improve transplant tolerance, and stimulate immune activity against tumors [48][49][50]. Tregs express high levels of CBS and 3-MST but have a low CSE expression [3][51]. Blocking CBS and CSE function in mice reduces the amount of FoxP3+ Tregs, indicating that these enzymes play a role in the T cell polarization and/or maintenance of Tregs [51]. CBS knockout mice have less Tregs, and the reduction of Tregs cells is linked to immune cell infiltration and higher autoantibody production in different anatomical sites. H2S signaling promotes Treg hypomethylation, a crucial aspect of Treg phenotype, by boosting the production of the ten-eleven translocation (Tet) molecules, which are engaged in functional DNA demethylation. The sulfhydration of NFYB (nuclear transcription factor Y subunit beta) was discovered to be crucial in this context and it occurs probably via CSE-originated H2S or polysulfide compounds [51]. In a mesenchymal stem cell (MSC)/T cell coculture model, the involvement of H2S in driving T cell polarization towards Treg cells and in inhibiting Th17 cell polarization, was also established in in vitro system [52]. MSCs stimulated T cell polarization to Tregs, but this activity was reduced when CBS was knocked down. Pharmacological H2S treatment, by NaHS administration, partially reversed this effect, indicating that H2S was essential to retain immunomodulatory activity of MSC [52]. In an elegant recent study on M. tuberculosis infection (Mtb), it has been reported that in the alveoli of CSE knockout mice the number of Treg cells increased after infection [53]. Specifically, four weeks after infection, Treg cells reached a higher level than wild type mice that, in turn, do not retain increased Treg cells and, as a result, do not show an excessive Treg-mediated immune-regulation. These data obtained in Mtb-infected wild type mice are consistent with previous ones showing that high levels of H2S limit the release of pro-inflammatory molecules, including IL-1, IL-6, TNF-α, NO, and mitochondrial-reactive oxygen intermediates, but promote the secretion of the anti-inflammatory cytokine IL-10 [54][55][56]. Accordingly, in a model of colitis, H2S is produced by SRB, which up-regulate Th17 and Treg cytokine profiles (IL-10 increase, IL-2 decrease) in T cells from the mesenteric lymph nodes [57].
Overall, while the evidence for a H2S role in Treg polarization is limited, it is suggested that this gaseous mediator plays an essential, non-redundant role in the modulation of adaptive immunity by stimulating Treg growth and activity (Figure 2).
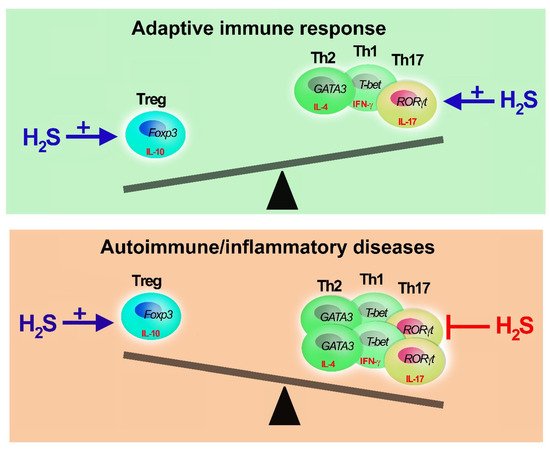
Figure 2. Adaptive immune response, H2S buffering activity. Hydrogen sulfide can restore the equilibrium of Th and Treg cells. H2S is needed to develop appropriate Th-mediated immune response promoting Th and Treg polarization and functions. In case of excessive Th1, Th2 or Th17 activation (unbalanced of immune response), as in immune-mediated diseases, H2S promotes Treg proliferation (+) and inhibits (−) Th activity and expansion. However, when H2S reaches millimolar doses, it has immunosuppressive activities impairing T cell proliferation and cytokine secretion.
References
- Medzhitov, R. Recognition of microorganisms and activation of the immune response. Nature 2007, 449, 819–826.
- Zhu, J.; Yamane, H.; Paul, W.E. Differentiation of effector CD4 T cell populations. Annu. Rev. Immunol. 2010, 28, 445–489.
- Dilek, N.; Papapetropoulos, A.; Toliver-Kinsky, T.; Szabo, C. Hydrogen sulfide: An endogenous regulator of the immune system. Pharmacol. Res. 2020, 161, 105119.
- Dong, C. Cytokine Regulation and Function in T Cells. Annu. Rev. Immunol. 2021, 39, 51–76.
- Martini, S.; Pozzi, G.; Carubbi, C.; Masselli, E.; Galli, D.; Di Nuzzo, S.; Banchini, A.; Gobbi, G.; Vitale, M.; Mirandola, P. PKCε promotes human Th17 differentiation: Implications in the pathophysiology of psoriasis. Eur. J. Immunol. 2018, 48, 644–654.
- Bassini, A.; Zauli, G.; Migliaccio, G.; Migliaccio, A.R.; Pascuccio, M.; Pierpaoli, S.; Guidotti, L.; Capitani, S.; Vitale, M. Lineage-restricted expression of protein kinase C isoforms in hematopoiesis. Blood 1999, 93, 1178–1188.
- Gobbi, G.; Mirandola, P.; Carubbi, C.; Galli, D.; Vitale, M. Protein kinase C ε in hematopoiesis: Conductor or selector? Semin. Thromb. Hemost. 2013, 39, 59–65.
- Mosmann, T.R.; Cherwinski, H.; Bond, M.W.; Giedlin, M.A.; Coffman, R.L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 1986, 136, 2348–2357.
- Harrington, L.E.; Hatton, R.D.; Mangan, P.R.; Turner, H.; Murphy, T.L.; Murphy, K.M.; Weaver, C.T. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 2005, 6, 1123–1132.
- Park, H.; Li, Z.; Yang, X.O.; Chang, S.H.; Nurieva, R.; Wang, Y.H.; Wang, Y.; Hood, L.; Zhu, Z.; Tian, Q.; et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 2005, 6, 1133–1141.
- Tesmer, L.A.; Lundy, S.K.; Sarkar, S.; Fox, D.A. Th17 cells in human disease. Immunol. Rev. 2008, 223, 87–113.
- Sakaguchi, S.; Yamaguchi, T.; Nomura, T.; Ono, M. Regulatory T cells and immune tolerance. Cell 2008, 133, 775–787.
- Ohkura, N.; Hamaguchi, M.; Morikawa, H.; Sugimura, K.; Tanaka, A.; Ito, Y.; Osaki, M.; Tanaka, Y.; Yamashita, R.; Nakano, N.; et al. T cell receptor stimulation-induced epigenetic changes and Foxp3 expression are independent and complementary events required for Treg cell development. Immunity 2012, 37, 785–799.
- Huang, Y.Q.; Jin, H.F.; Zhang, H.; Tang, C.S.; Du, J.B. Interaction among Hydrogen Sulfide and Other Gasotransmitters in Mammalian Physiology and Pathophysiology. Adv. Exp. Med. Biol. 2021, 1315, 205–236.
- Predmore, B.L.; Lefer, D.J.; Gojon, G. Hydrogen sulfide in biochemistry and medicine. Antioxid. Redox Signal. 2012, 17, 119–140.
- Wang, R. Physiological implications of hydrogen sulfide: A whiff exploration that blossomed. Physiol. Rev. 2012, 92, 791–896.
- Xu, M.; Zhang, L.; Song, S.; Pan, L.; Arslan, M.I.; Chen, Y.; Yang, S. Hydrogen sulfide: Recent progress and perspectives for the treatment of dermatological diseases. J. Adv. Res. 2020, 27, 11–17.
- Szabo, C. Hydrogen sulfide, an endogenous stimulator of mitochondrial function in cancer cells. Cells 2021, 10, 220.
- Liu, Z.; Fu, Q.; Tang, S.; Xie, Y.; Meng, Q.; Tang, X.; Zhang, S.; Zhang, H.; Schroyen, M. Proteomics analysis of lung reveals inflammation and cell death induced by atmospheric H2S exposure in pig. Environ. Res. 2020, 191, 110204.
- Ueno, A.; Jeffery, L.; Kobayashi, T.; Hibi, T.; Ghosh, S.; Jijon, H. Th17 plasticity and its relevance to inflammatory bowel disease. J. Autoimmun. 2018, 87, 38–49.
- Hurtado, C.G.; Wan, F.; Housseau, F.; Sears, C.L. Roles for interleukin 17 and adaptive immunity in pathogenesis of colorectal cancer. Gastroenterology 2018, 155, 1706–1715.
- Lubberts, E. The IL-23-IL-17 axis in inflammatory arthritis. Nat. Rev. Rheumatol. 2015, 7, 415–429.
- Lin, H.; Tong, Z.; Xu, Q.; Wu, X.; Wang, X.; Jin, X.; Ma, W.; Cheng, X.; Zhou, Q.; Shi, H. Interplay of Th1 and Th17 cells in murine models of malignant pleural effusion. Am. J. Respir. Crit. Care Med. 2014, 189, 697–706.
- Wang, L.; Liu, J.; Wang, W.; Qi, X.; Wang, Y.; Tian, B.; Dai, H.; Wang, J.; Ning, W.; Yang, T.; et al. Targeting IL-17 attenuates hypoxia-induced pulmonary hypertension through downregulation of β-catenin. Thorax 2019, 74, 564–578.
- Stockinger, B.; Omenetti, S. The dichotomous nature of T helper 17 cells. Nat. Rev. Immunol. 2017, 17, 535–544.
- Farkas, A.M.; Panea, C.; Goto, Y.; Nakato, G.; Galan-Diez, M.; Narushima, S.; Honda, K.; Ivanov, I.I. Induction of Th17 cells by segmented filamentous bacteria in the murine intestine. J. Immunol. Methods 2015, 421, 104–111.
- Atarashi, K.; Tanoue, T.; Umesaki, Y.; Honda, K. Regulation of Th17 cell differentiation by intestinal commensal bacteria. Benef. Microbes 2010, 1, 327–334.
- Atarashi, K.; Tanoue, T.; Ando, M.; Kamada, N.; Nagano, Y.; Narushima, S.; Suda, W.; Imaoka, A.; Setoyama, H.; Nagamori, T.; et al. Th17 cell induction by adhesion of microbes to intestinal epithelial cells. Cell 2015, 163, 367–380.
- Jiang, W.; Su, J.; Zhang, X.; Cheng, X.; Zhou, J.; Shi, R.; Zhang, H. Elevated levels of Th17 cells and Th17-related cytokines are associated with disease activity in patients with inflammatory bowel disease. Inflamm. Res. 2014, 63, 943–950.
- Figliuolo, V.R.; Dos Santos, L.M.; Abalo, A.; Nanini, H.; Santos, A.; Brittes, N.M.; Bernardazzi, C.; de Souza, H.S.P.; Vieira, L.Q.; Coutinho-Silva, R.; et al. Sulfate-reducing bacteria stimulate gut immune responses and contribute to inflammation in experimental colitis. Life Sci. 2017, 189, 29–38.
- Ohnmacht, C.; Park, J.H.; Cording, S.; Wing, J.B.; Atarashi, K.; Obata, Y.; Gaboriau-Routhiau, V.; Marques, R.; Dulauroy, S.; Fedoseeva, M.; et al. Mucosal Immunology. The microbiota regulates type 2 immunity through RORgammat+ T cells. Science 2015, 349, 989–993.
- McDonald, C.A.; Penny, T.R.; Paton, M.C.B.; Sutherland, A.E.; Nekkanti, L.; Yawno, T.; Castillo-Melendez, M.; Fahey, M.C.; Jones, N.M.; Jenkin, G.; et al. Effects of umbilical cord blood cells, and subtypes, to reduce neuroinflammation following perinatal hypoxic-ischemic brain injury. J. Neuroinflammation 2018, 15, 47.
- Liu, S.; Xin, D.; Wang, L.; Zhang, T.; Bai, X.; Li, T.; Xie, Y.; Xue, H.; Bo, S.; Liu, D.; et al. Therapeutic effects of L-Cysteine in newborn mice subjected to hypoxia-ischemia brain injury via the CBS/H2S system: Role of oxidative stress and endoplasmic reticulum stress. Redox Biol. 2017, 13, 528–540.
- Xin, D.; Chu, X.; Bai, X.; Ma, W.; Yuan, H.; Qiu, J.; Liu, C.; Li, T.; Zhou, X.; Chen, W.; et al. l-Cysteine suppresses hypoxia-ischemia injury in neonatal mice by reducing glial activation, promoting autophagic flux and mediating synaptic modification via H2S formation. Brain Behav. Immun. 2018, 73, 222–234.
- Li, T.; Chu, X.; Xin, D.; Ke, H.; Wang, S.; Liu, D.; Chen, W.; Wang, Z. H2S prevents peripheral immune cell invasion, increasing i and excessive phagocytosis following hypoxia-ischemia injury in neonatal mice. Biomed. Pharmacother. 2021, 135, 111207.
- Kimura, H. Hydrogen sulfide (H2S) and polysulfide (H2Sn) signaling: The first 25 years. Biomolecules 2021, 11, 896.
- Cai, Y.; Fleming, C.; Yan, J. New insights of T cells in the pathogenesis of psoriasis. Cell. Mol. Immunol. 2012, 9, 302–309.
- Diani, M.; Altomare, G.; Reali, E. T Helper cell subsets in clinical manifestations of psoriasis. J. Immunol. Res. 2016, 2016, 7692024.
- Tsai, T.Y.; Yen, H.; Huang, Y.C. Serum homocysteine, folate and vitamin B12 levels in patients with psoriasis: A systematic review and meta-analysis. Br. J. Dermatol. 2019, 180, 382–389.
- Yakovleva, O.V.; Ziganshina, A.R.; Dmitrieva, S.A.; Arslanova, A.N.; Yakovlev, A.V.; Minibayeva, F.V.; Khaertdinov, N.N.; Ziyatdinova, G.K.; Giniatullin, R.A.; Sitdikova, G.F. Hydrogen sulfide ameliorates developmental impairments of rat offspring with prenatal hyperhomocysteinemia. Oxidative Med. Cell. Longev. 2018, 2018, 2746873.
- Lin, X.; Meng, X.; Song, Z. Homocysteine and psoriasis. Biosci. Rep. 2019, 39, BSR20190867.
- Rose, P.; Moore, P.K.; Whiteman, M.; Kirk, C.; Zhu, Y.Z. Diet and hydrogen sulfide production in mammals. Antioxid. Redox Signal. 2021, 34, 1378–1393.
- Rodrigues, C.; Percival, S.S. Immunomodulatory effects of glutathione, garlic derivatives, and hydrogen sulfide. Nutrients 2019, 11, 295.
- Rose, P.; Moore, P.K.; Zhu, Y.Z. Garlic and gaseous mediators. Trends Pharmacol. Sci. 2018, 39, 624–634.
- Münchberg, U.; Anwar, A.; Mecklenburg, S.; Jacob, C. Polysulfides as biologically active ingredients of garlic. Org. Biomol. Chem. 2007, 5, 1505–1518.
- Arsenijevic, D.; Stojanovic, B.; Milovanovic, J.; Arsenijevic, A.; Simic, M.; Pergal, M.; Kodranov, I.; Cvetkovic, O.; Vojvodic, D.; Ristanovic, E.; et al. Hepatoprotective Effect of Mixture of Dipropyl Polysulfides in Concanavalin A-Induced Hepatitis. Nutrients 2021, 13, 1022.
- Liang, J.J.; Li, H.R.; Chen, Y.; Zhang, C.; Chen, D.G.; Liang, Z.C.; Shi, Y.Q.; Zhang, L.L.; Xin, L.; Zhao, D.B. Diallyl Trisulfide can induce fibroblast-like synovial apoptosis and has a therapeutic effect on collagen-induced arthritis in mice via blocking NF-kappaB and Wnt pathways. Int. Immunopharmacol. 2019, 71, 132–138.
- Whiteside, T.L.; Schuler, P.; Schilling, B. Induced and natural regulatory T cells in human cancer. Expert Opin. Biol. Ther. 2012, 12, 1383–1397.
- Zhang, Y.; Zhang, Y.; Gu, W.; He, L.; Sun, B. Th1/Th2 cell’s function in immune system. Adv. Exp. Med. Biol. 2014, 841, 45–65.
- Karimi, S.; Chattopadhyay, S.; Chakraborty, N.G. Manipulation of regulatory T cells and antigen-specific cytotoxic T lymphocyte-based tumour immunotherapy. Immunology 2015, 144, 186–196.
- Yang, R.; Qu, C.; Zhou, Y.; Konkel, J.E.; Shi, S.; Liu, Y.; Chen, C.; Liu, S.; Liu, D.; Chen, Y.; et al. Hydrogen sulfide promotes Tet1- and Tet2-mediated Foxp3 demethylation to drive regulatory T cell differentiation and maintain immune homeostasis. Immunity 2015, 43, 251–263.
- Yang, R.; Yu, T.; Liu, D.; Shi, S.; Zhou, Y. Hydrogen sulfide promotes immunomodulation of gingiva-derived mesenchymal stem cells via the Fas/FasL coupling pathway. Stem Cell Res. Ther. 2018, 9, 62.
- Rahman, M.A.; Cumming, B.M.; Addicott, K.W.; Pacl, H.T.; Russell, S.L.; Nargan, K.; Naidoo, T.; Ramdial, P.K.; Adamson, J.H.; Wang, R.; et al. Hydrogen sulfide dysregulates the immune response by suppressing central carbon metabolism to promote tuberculosis. Proc. Natl. Acad. Sci. USA 2020, 117, 6663–6674.
- Whiteman, M.; Li, L.; Rose, P.; Tan, C.H.; Parkinson, D.B.; Moore, P.K. The effect of hydrogen sulfide donors on lipopolysaccharide-induced formation of inflammatory mediators in macrophages. Antioxid. Redox Signal. 2010, 12, 1147–1154.
- Castelblanco, M.; Lugrin, J.; Ehirchiou, D.; Nasi, S.; Ishii, I.; So, A.; Martinon, F.; Busso, N. Hydrogen sulfide inhibits NLRP3 inflammasome activation and reduces cytokine production both in vitro and in a mouse model of inflammation. J. Biol. Chem. 2018, 293, 2546–2557.
- Liu, F.; Liu, G.J.; Liu, N.; Zhang, G.; Zhang, J.X.; Li, L.F. Effect of hydrogen sulfide on inflammatory cytokines in acute myocardial ischemia injury in rats. Exp. Ther. Med. 2015, 9, 1068–1074.
- Hatfield, P.; Merrick, A.E.; West, E.; O’Donnell, D.; Selby, P.; Vile, R.; Melcher, A.A. Optimization of dendritic cell loading with tumor cell lysates for cancer immunotherapy. J. Immunother. 2008, 31, 620–632.
More
Information
Subjects:
Biology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.5K
Revisions:
2 times
(View History)
Update Date:
11 Feb 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




