Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ramcés Falfán-Valencia | + 1284 word(s) | 1284 | 2021-08-24 12:02:11 | | | |
| 2 | Rita Xu | Meta information modification | 1284 | 2022-02-11 02:20:40 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Falfán-Valencia, R. Angiotensin-Converting Enzyme 2 (EC 3.4.15.1). Encyclopedia. Available online: https://encyclopedia.pub/entry/19341 (accessed on 07 February 2026).
Falfán-Valencia R. Angiotensin-Converting Enzyme 2 (EC 3.4.15.1). Encyclopedia. Available at: https://encyclopedia.pub/entry/19341. Accessed February 07, 2026.
Falfán-Valencia, Ramcés. "Angiotensin-Converting Enzyme 2 (EC 3.4.15.1)" Encyclopedia, https://encyclopedia.pub/entry/19341 (accessed February 07, 2026).
Falfán-Valencia, R. (2022, February 10). Angiotensin-Converting Enzyme 2 (EC 3.4.15.1). In Encyclopedia. https://encyclopedia.pub/entry/19341
Falfán-Valencia, Ramcés. "Angiotensin-Converting Enzyme 2 (EC 3.4.15.1)." Encyclopedia. Web. 10 February, 2022.
Copy Citation
Angiotensin-Converting Enzyme 2 (ACE2) is an 805 amino acid protein encoded by the ACE2 gene expressed in various human cells, especially in those located in the epithelia.
Angiotensin-Converting Enzyme 2 (ACE2)
severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)
1. Introduction
Angiotensin-Converting Enzyme 2 (ACE2) (EC 3.4.15.1) is an 805 amino acid protein encoded by the ACE2 gene located in cytogenetic band 22.2 on the short arm of the X chromosome. The gene is 41,115 base pairs (bp) long and is organized into nineteen exons and eighteen introns that can give rise to five different transcripts, of which only two are translated into functional proteins (Figure 1) [1]. In addition, there are two paralogs to ACE2, Angiotensin-Converting Enzyme 1 (ACE) and AC113554.1, although no functional protein product has been associated with the latter.
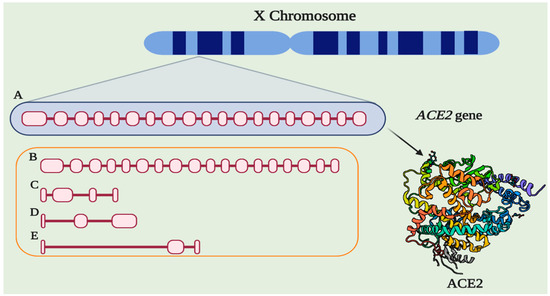
Figure 1. The structure of ACE2. (A) Gene ACE2 conformed by nineteen exons and eighteen introns (3507 bp). (B) Alternative functional transcript without the last exon (3339). (C) Alternative transcript without the protein product (4 exons, 998 bp). (D) Alternative transcript without the protein product (3 exons, 786 bp). (E) Alternative transcript without the protein product (3 exons, 599 bp). Protein structure-based in PDB 1R42.
ACE2 was first described in patients with heart failure [2]; initially, it was suggested that it is expressed in the heart and kidneys. However, recent research has shown that different cells can express ACE2, those located in the epithelium of specific organs, such as the lungs, and fulfill various biological functions related to the immune response and homeostasis [3][4][5].
2. Location and Expression
ACE2 is an enzyme located in the cellular membrane of different human body organs, mainly in the epithelium. Through expression analyses and proteomic databases, as well as in vitro investigations, it has been shown that ACE2 is expressed in the lungs, liver, kidneys, stomach, intestines, arteries and veins, heart, oral mucosa, nasopharynx, colon, thymus, bladder, and central nervous system [6][7][8][9][10]. The primary cells that express this enzyme are stomach epithelial cells, proximal renal tubes, vascular endothelial cells, glia, neurons, spinal cord cells, cholangiocytes, esophageal keratinocytes, ileal and rectal enterocytes, and epithelial oral cavity epithelial cells [7][8][11][12][13]. The lungs are the main organs of expression at the respiratory system level, with type 2 alveolar and pulmonary epithelial cells as the principal cells in ACE2 expression [11]. The expression level depends on the degree of cell differentiation, and mature cells show the highest level of ACE2 expression (Figure 2) [14]. In animal models, it has been demonstrated that ACE2 expression is decreased in males compared to females, although this difference was believed to be due to estrogens [15].
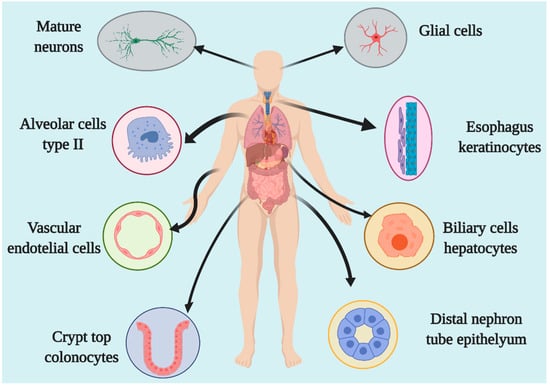
Figure 2. Organs and cells expressing ACE2.
The research about ACE2 and other potential receptors has been studied in different biological samples. The expression of ACE2 is increased in patients infected with SARS-CoV-2 in the airway epithelium and immunological cells but not in other epithelia [6][7][8]. In some studies, the differences are not significant. In an analysis of the patients infected with SARS-CoV-2, including environmental variables like smoking or other lung diseases (asthma and COPD), the expression of ACE2 is increased significantly, and, interestingly, this further increases in severe COVID-19 [7].
3. ACE2/Ang (1–7)/MasR Effector Axis
ACE2 is a member of the counter-regulatory axis of the renin–angiotensin system (RAS), and its leading role is to degrade the pro-hypertrophic and profibrotic peptide angiotensin II (Ang II), limiting the adverse effects of angiotensin II. Additionally, it participates in generating angiotensin (1–7) (Ang (1–7)) (Figure 3) in cooperation with neprilysin, mainly in the liver and kidneys; in the lungs, ACE2 works in conjunction with prolyl oligopeptidase (POP) to generate Ang (1–7) [9]. There are different pathways related to the RAS axis; Ang II is the most-studied in ACE axis, and the angiotensin II type 1 receptor (AT1R), called the classical system, while ACE2, Ang (1–7), and the Mas receptor (MasR) is one of the nonclassical pathways.
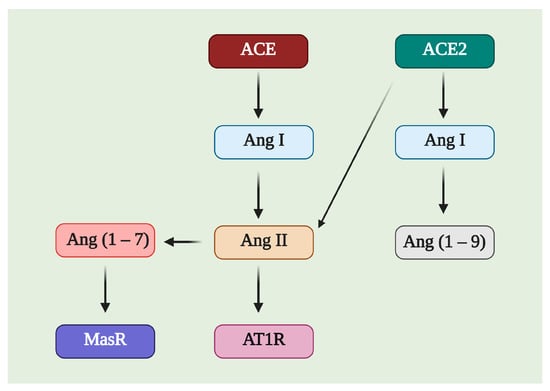
Figure 3. Image of the possible substrates and products of Angiotensin-Converting Enzyme 2 (ACE2). ACE2 can affect Ang I and Ang II, producing Ang (1–9) and Ang (1–7), respectively. Ang (1–7) is a molecule with affinity for the Mas receptor (MasR), while Ang II is related to the angiotensin II type 1 receptor (AT1R).
There is an inverse relationship between the ACE/Ang II/AT1R axis and ACE2/Ang (1–7)/MasR; a worsening of the inflammatory processes occurs when this relationship favors the first set, while it favors an increase in the levels of α-smooth muscle actin (α-SMA) and types I collagen at the lung and hepatic levels in fibrotic models [10]. In cellular assays, the phosphorylation levels of vascular endothelial growth factor receptor 2 (VEGFR2), c-Jun, mitogen-activated protein kinase 1/2 (MEK1/2), and extracellular signal-regulated protein kinase (ERK1/2) have been found to decrease via stimulation of the nonclassical pathway of the RAS axis, which significantly inhibits the vascularization processes of tumor cells and pulmonary and hepatic fibrosis and causes injury in the same organs [11][12]. On the other hand, ACE2 affects some oxidative stress pathways via increasing Ang (1–7); if the levels of this enzyme begin to decline, the expression of NADPH oxidases 2/4 (NOX2/NOX4) increases, along with the levels of the reactive oxygen species (ROS) and loss of the stability of the mitochondrial membrane and B-cell lymphoma 2 protein (Bcl2) [13].
Additionally, the participation of microRNAs (miRNAs), such as miR-4262, related to the regulation of apoptosis mechanisms, may be involved in regulating the molecular processes related to ACE2 [14]. Through different exogenous blockers, it has been possible to identify two primary receptors for Ang (1–7), called Mas-related G-protein coupled receptor type D (MrgD) and MasR [15]. MasR is described as the central receptor associated with the axis-regulating mechanism that is encoded by the MAS1 oncogene and is characterized as a heptameric G protein-associated receptor, serving as a starting point in various signaling cascades that promote the phosphorylation of second messengers such as phosphoinositol-3-phosphate kinase (PI3K), phospholipase A2 (PLA2), and phospholipase C (PLC), among others. Another result is the decreased phosphorylation of ERK1/2, c-Jun, mitogen-activated protein kinase (MAPK), and the Smad family [16] but increased phosphorylation of endothelial nitric oxide synthase (eNOS) and cyclooxygenase 2 (COX-2), improving the vasodilation and blocking of the transcription of profibrotic and proinflammatory genes [17]. A worsening of the inflammatory processes has been documented in animals deficient in MAS1, as in arteriosclerosis, in which more significant macrophage infiltration and proinflammatory cytokine production are observed [10]. These inflammatory effects can be mitigated through the use of diminazen aceturate (DIZE), which is an ACE2 inducer that decreases the expression of interleukin-1β (IL-1β), interleukin -6 (IL-6), tumor necrosis factor-α (TNF-α), and monocyte chemoattractant protein-1 (MCP-1) [11]. Such a decrease in the inflammatory marker expression is mainly associated with the blockade of the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling pathway, a process described at the liver, lung, and kidney levels in different study models treated with ACE2 inducers and exogenous ACE2/Ang (1–7) therapy (Figure 4) [18].
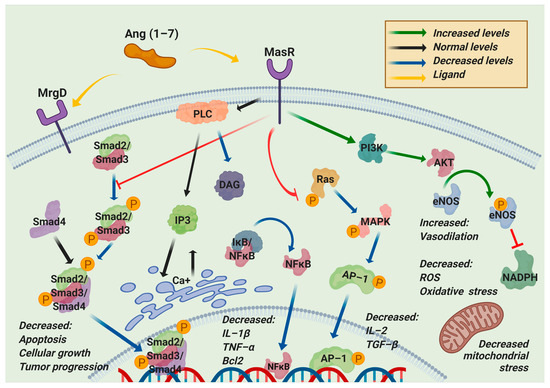
Figure 4. Regulated pathways of the nonclassical RAS axis. Angiotensin (1–7) (Ang (1–7)) can bind to Mas-related G-protein coupled receptor type D (MrgD) or Mas receptor (MasR). This interaction blocks the phosphorylation of the components of the two main pathways, Ras/Mitogen-activated protein kinase (MAPK) and Smad, resulting in the downregulation of apoptosis, cellular growth, tumor progression, inflammation, oxidative stress, reactive oxygen species, and mitochondrial stress. On the other hand, this interaction upregulates phosphatidylinositol-3-kinase (PI3K) to increase endothelial nitric oxide synthase (eNOS) phosphorylation, blocking the nicotinamide adenine dinucleotide phosphate reduced (NADPH) activity, decreasing the oxidative stress, and increasing the vasodilation. PLC: Phospholipase C; DAG: diacylglycerol; IP3: Inositol trisphosphate; IkB: inhibitor of nuclear factor-kappa; NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells; IL-1β: interleukin 1 beta; TNF-α: Tumor necrosis factor-alpha; Bcl2: B-cell lymphoma 2; Ras: Rat sarcoma virus; AP-1: activator protein 1; AKT1: AKT serine/threonine kinase 1; ROS: reactive oxygen species.
Furthermore, increases in aldosterone can block MasR expression but not that of ACE2 or Ang (1–7). Spironolactone, an aldosterone antagonist, has been shown to have effects on this receptor, in addition to promoting a balance towards the ACE2/Ang (1–7) axis [19].
References
- Gene. ACE2 (ENSG00000130234)—Summary—Homo Sapiens—Ensembl Genome Browser 99. Available online: https://www.ensembl.org/Homo_sapiens/Gene/Summary?db=core;g=ENSG00000130234;r=X:15561033-15602148 (accessed on 25 March 2020).
- Zisman, L.S.; Keller, R.S.; Weaver, B.; Lin, Q.; Speth, R.; Bristow, M.R.; Canver, C.C. Increased Angiotensin-(1–7)-Forming Activity in Failing Human Heart Ventricles: Evidence for Upregulation of the Angiotensin-Converting Enzyme Homologue ACE2. Circulation 2003, 108, 1707–1712.
- Chen, W.J.; Liu, H.; Wang, Z.H.; Liu, C.; Fan, J.Q.; Wang, Z.L.; Xu, Y.P.; Zhang, B.; Gyawali, L.; Li, Q.; et al. The Impact of Renal Denervation on the Progression of Heart Failure in a Canine Model Induced by Right Ventricular Rapid Pacing. Front. Physiol. 2020, 10, 1625.
- Mukerjee, S.; Gao, H.; Xu, J.; Sato, R.; Zsombok, A.; Lazartigues, E. ACE2 and ADAM17 Interaction Regulates the Activity of Presympathetic Neurons. Hypertension 2019, 74, 1181–1191.
- Sharma, N.; Malek, V.; Mulay, S.R.; Gaikwad, A.B. Angiotensin II Type 2 Receptor and Angiotensin-Converting Enzyme 2 Mediate Ischemic Renal Injury in Diabetic and Non-Diabetic Rats. Life Sci. 2019, 235, 116796.
- Zou, X.; Chen, K.; Zou, J.; Han, P.; Hao, J.; Han, Z. Single-Cell RNA-Seq Data Analysis on the Receptor ACE2 Expression Reveals the Potential Risk of Different Human Organs Vulnerable to 2019-NCoV Infection. Front. Med. 2020, 14, 185–192.
- Baig, A.M.; Khaleeq, A.; Ali, U.; Syeda, H. Evidence of the COVID-19 Virus Targeting the CNS: Tissue Distribution, Host–Virus Interaction, and Proposed Neurotropic Mechanisms. ACS Chem. Neurosci. 2020, 17, 995–998.
- Evans, C.E.; Miners, J.S.; Piva, G.; Willis, C.L.; Heard, D.M.; Kidd, E.J.; Good, M.A.; Kehoe, P.G. ACE2 Activation Protects against Cognitive Decline and Reduces Amyloid Pathology in the Tg2576 Mouse Model of Alzheimer’s Disease. Acta Neuropathol. 2020, 139, 485–502.
- Hamming, I.; Timens, W.; Bulthuis, M.L.C.; Lely, A.T.; Navis, G.J.; van Goor, H. Tissue Distribution of ACE2 Protein, the Functional Receptor for SARS Coronavirus. A First Step in Understanding SARS Pathogenesis. J. Pathol. 2004, 203, 631–637.
- Garabelli, P.J.; Modrall, J.G.; Penninger, J.M.; Ferrario, C.M.; Chappell, M.C. Distinct Roles for Angiotensin-Converting Enzyme 2 and Carboxypeptidase A in the Processing of Angiotensins within the Murine Heart. Exp. Physiol. 2008, 93, 613–621.
- Qi, F.; Qian, S.; Zhang, S.; Zhang, Z. Single Cell RNA Sequencing of 13 Human Tissues Identify Cell Types and Receptors of Human Coronaviruses. Biochem. Biophys. Res. Commun. 2020, 561, 135–140.
- Chappell, M.C. Biochemical Evaluation of the Renin-Angiotensin System: The Good, Bad, and Absolute? Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H137–H152.
- Xu, H.; Zhong, L.; Deng, J.; Peng, J.; Dan, H.; Zeng, X.; Li, T.; Chen, Q. High Expression of ACE2 Receptor of 2019-NCoV on the Epithelial Cells of Oral Mucosa. Int. J. Oral Sci. 2020, 12, 8.
- Lukassen, S.; Lorenz Chua, R.; Trefzer, T.; Kahn, N.C.; Schneider, M.A.; Muley, T.; Winter, H.; Meister, M.; Veith, C.; Boots, A.W.; et al. SARS-CoV-2 Receptor ACE2 and TMPRSS2 Are Primarily Expressed in Bronchial Transient Secretory Cells. EMBO J. 2020, 39, e105114.
- Lee, S.H.; Lee, Y.H.; Jung, S.W.; Kim, D.J.; Park, S.H.; Song, S.J.; Jeong, K.H.; Moon, J.Y.; Ihm, C.-G.; Lee, T.W.; et al. Sex-Related Differences in the Intratubular Renin-Angiotensin System in Two-Kidney, One-Clip Hypertensive Rats. Am. J. Physiol. Ren. Physiol. 2019, 317, F670–F682.
- Li, G.; He, X.; Zhang, L.; Ran, Q.; Wang, J.; Xiong, A.; Wu, D.; Chen, F.; Sun, J.; Chang, C. Assessing ACE2 Expression Patterns in Lung Tissues in the Pathogenesis of COVID-J. J. Autoimmun. 2020, 112, 102463.
- Radzikowska, U.; Ding, M.; Tan, G.; Zhakparov, D.; Peng, Y.; Wawrzyniak, P.; Wang, M.; Li, S.; Morita, H.; Altunbulakli, C.; et al. Distribution of ACE2, CD147, CD26, and Other SARS-CoV-2 Associated Molecules in Tissues and Immune Cells in Health and in Asthma, COPD, Obesity, Hypertension, and COVID-19 Risk Factors. Allergy 2020, 75, 2829–2845.
- Lange, C.; Wolf, J.; Auw-Haedrich, C.; Schlecht, A.; Boneva, S.; Lapp, T.; Horres, R.; Agostini, H.; Martin, G.; Reinhard, T.; et al. Expression of the COVID-19 Receptor ACE2 in the Human Conjunctiva. J. Med. Virol. 2020, 92, 2081–2086.
- Serfozo, P.; Wysocki, J.; Gulua, G.; Schulze, A.; Ye, M.; Liu, P.; Jin, J.; Bader, M.; Myöhänen, T.; García-Horsman, J.A.; et al. Ang II (Angiotensin II) Conversion to Angiotensin-(1–7) in the Circulation Is POP (Prolyloligopeptidase)-Dependent and ACE2 (Angiotensin-Converting Enzyme 2)-Independent. Hypertension 2020, 75, 173–182.
- Tao, M.-X.; Xue, X.; Gao, L.; Lu, J.-L.; Zhou, J.-S.; Jiang, T.; Zhang, Y.-D. Involvement of Angiotensin-(1–7) in the Neuroprotection of Captopril against Focal Cerebral Ischemia. Neurosci. Lett. 2018, 687, 16–21.
- Zhang, Q.; Lu, S.; Li, T.; Yu, L.; Zhang, Y.; Zeng, H.; Qian, X.; Bi, J.; Lin, Y. ACE2 Inhibits Breast Cancer Angiogenesis via Suppressing the VEGFa/VEGFR2/ERK Pathway. J. Exp. Clin. Cancer Res. 2019, 38, 173.
- Wang, J.; Chen, S.; Bihl, J. Exosome-Mediated Transfer of ACE2 (Angiotensin-Converting Enzyme 2) from Endothelial Progenitor Cells Promotes Survival and Function of Endothelial Cell. Oxid. Med. Cell. Longev. 2020, 2020, 4213541.
- Bao, H.; Gao, F.; Xie, G.; Liu, Z. Angiotensin-Converting Enzyme 2 Inhibits Apoptosis of Pulmonary Endothelial Cells during Acute Lung Injury Through Suppressing MiR-4262. Cell. Physiol. Biochem. 2015, 37, 759–767.
- Gunarathne, L.S.; Angus, P.W.; Herath, C.B. Blockade of Mas Receptor or Mas-Related G-Protein Coupled Receptor Type D Reduces Portal Pressure in Cirrhotic but Not in Non-Cirrhotic Portal Hypertensive Rats. Front. Physiol. 2019, 10, 1169.
- Choi, H.S.; Kim, I.J.; Kim, C.S.; Ma, S.K.; Scholey, J.W.; Kim, S.W.; Bae, E.H. Angiotensin- Attenuates Kidney Injury in Experimental Alport Syndrome. Sci. Rep. 2020, 10, 4225.
- Hekmat, A.S.; Zare, N.; Moravej, A.; Meshkibaf, M.H.; Javanmardi, K. Effect of Prolonged Infusion of Alamandine on Cardiovascular Parameters and Cardiac ACE2 Expression in a Rat Model of Renovascular Hypertension. Biol. Pharm. Bull. 2019, 42, 960–967.
- Ali, R.M.; Al-Shorbagy, M.Y.; Helmy, M.W.; El-Abhar, H.S. Role of Wnt4/β-Catenin, Ang II/TGFβ, ACE2, NF-κB, and IL-18 in Attenuating Renal Ischemia/Reperfusion-Induced Injury in Rats Treated with Vit D and Pioglitazone. Eur. J. Pharmacol. 2018, 831, 68–76.
- Stoll, D.; Yokota, R.; Sanches Aragão, D.; Casarini, D.E. Both Aldosterone and Spironolactone Can Modulate the Intracellular ACE/ANG II/AT1 and ACE2/ANG (1–7)/MAS Receptor Axes in Human Mesangial Cells. Physiol. Rep. 2019, 7, e14105.
More
Information
Subjects:
Respiratory System
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.0K
Revisions:
2 times
(View History)
Update Date:
11 Feb 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




