Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Valentine Comaills | + 9396 word(s) | 9396 | 2021-07-15 04:31:30 | | | |
| 2 | Bruce Ren | -193 word(s) | 9203 | 2022-02-11 02:14:25 | | | | |
| 3 | Bruce Ren | Meta information modification | 9203 | 2022-02-14 07:35:43 | | | | |
| 4 | Valentine Comaills | -1309 word(s) | 7894 | 2022-02-14 07:38:23 | | | | |
| 5 | Bruce Ren | Meta information modification | 7894 | 2022-02-15 01:39:59 | | | | |
| 6 | Bruce Ren | Meta information modification | 7894 | 2022-02-15 02:02:05 | | | | |
| 7 | Bruce Ren | -53 word(s) | 7841 | 2022-02-15 02:03:36 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Comaills, V. Nuclear Envelope Integrity in Health and Disease. Encyclopedia. Available online: https://encyclopedia.pub/entry/19325 (accessed on 07 February 2026).
Comaills V. Nuclear Envelope Integrity in Health and Disease. Encyclopedia. Available at: https://encyclopedia.pub/entry/19325. Accessed February 07, 2026.
Comaills, Valentine. "Nuclear Envelope Integrity in Health and Disease" Encyclopedia, https://encyclopedia.pub/entry/19325 (accessed February 07, 2026).
Comaills, V. (2022, February 10). Nuclear Envelope Integrity in Health and Disease. In Encyclopedia. https://encyclopedia.pub/entry/19325
Comaills, Valentine. "Nuclear Envelope Integrity in Health and Disease." Encyclopedia. Web. 10 February, 2022.
Copy Citation
The dynamic nature of the nuclear envelope (NE) is often underestimated. The NE morphology is characterized by a wide range of diversity and abnormality such as invagination and blebbing, and it is a diagnostic factor for pathologies such as cancer.
nuclear envelope
nuclear envelope disruption
inflammation
cGAS/STING
chromosomal instability
envelopathy
cancer
lipodystrophy
neuropathy
1. Nuclear Envelope Biology
The main role of the nuclear envelope (NE) is to compartmentalize and protect the unfolded genomic DNA from the cytoplasm in eukaryote cells. It is composed of a lipid bilayer reinforced in its inner side with a sheet-like structure of proteins called the nuclear lamina. The outer nuclear membrane (ONM) shares a common border with the endoplasmic reticulum (ER). The nuclear envelope has also many nuclear pores that facilitate the transport of molecules between the cytosol and the nucleus (Figure 1A). The NE is a dynamic organelle that expends, disrupts and reconstitutes during mitosis. In the interphase, it constantly remodels to adapt to nuclear growth.

Figure 1. Nuclear envelope composition and organization. (A). The nucleus is surrounded by the nuclear envelope (NE). The Outer Nuclear Membrane (ONM) is continuous with the endoplasmic reticulum. The nuclear pore complex (NPC) regulates the export and import between the nucleus and the cytoplasm. The genome is organized in different compartments: euchromatin, heterochromatin, and nucleolus. (B). Structure of lamin layers in the Inner Nuclear Membrane (INM). (C). The NE is composed of a lipid bilayer anchored by several proteins forming the lamin-associated protein, the LINC complex, and by the lamins. The NE proteins regulate gene organization with the Lamin-Associated Domain (LAD).
1.1. Nuclear Lamina
The lamina conveys strength, flexibility, and rigidity as a function of its variable composition and ratio among the various lamins [1][2]. In addition to providing mechanical support and being an anchorage platform, the nuclear lamina regulates important cellular events such as DNA replication [3] and cell division [4]. The lamina is primordial for gene regulation [5][6] through the repression of genes in the Lamin-Associated Domain (LAD, Figure C), DNA repair [7], organization of the nucleolus [8], as well as chromosomal positioning [9]. The Lamina contributes to the organization of the genome into its different compartments: (i) the heterochromatin, characterized by repressed DNA that is tethered into the LAD, (ii) the euchromatin, the active compartment with a loose chromatin structure that is active for transcription, and (iii) the nucleolus, the site of ribosome production and assembly (Figure 1A).
The lamina also possesses mechano-responsivity in order to adapt to the cell’s environment. Lamin levels are dynamic, regulated by cell differentiation, and depend on the tissue stiffness [2][10]. Lamins are interconnected to the cytoskeleton by intermediate proteins such as the Linker of the Nucleoskeleton and Cytoskeleton (LINC) complex composed by SUN1/2 and Nesprins proteins (Figure 1C), which allow sensing and rapid cellular response via the complex post-translational control of its proteins [2][11]. The NE can also adapt in response to stiffness [2] or to function. For example, migrasive and invasive cells need to acquire nuclear elasticity and can decrease nuclear envelope proteins in order to compress through narrow space [1][10][12].
The nuclear lamina is composed of lamins and nuclear lamin-associated membrane proteins (Figure 1B,C). Lamins are type V intermediate filaments fibrous proteins that are divided into two major categories, the A and B type. The gene LMNA can be spliced in two predominant isoforms, the longer version encoding the protein Lamin A and the shorter isoform generating the Lamin C protein. The type B lamins are expressed by two different genes: LMNB1 and LMNB2, encoding Lamin B1 and B2, respectively. While Lamin A is expressed in differentiated cells, type B lamins are ubiquitously expressed in all cells. Similar to other intermediate filament proteins, lamins self-assemble into complex structures. Lamins are highly dynamic and regulated proteins that assemble and disassemble pending stimuli [13]. They are organized into distinct networks at the nuclear periphery [14] (Figure 1B). Lamin B1 forms an outer concentric ring, and its localization is curvature-dependent. This suggests a role of Lamin B1 in stabilizing nuclear shape by restraining outward protrusions of the Lamin A/C network [15]. Lamins are subject to numerous post-translational modifications, most prominently phosphorylation, such as Lamin A that harbors more than 70 identified unique phosphorylation sites. Such phosphorylation regulates/coordinates the different structural state of lamins. For example, during interphase, Lamin A phosphorylation on Serine S22 and S390 promotes lamin degradation and nuclear softening in response to low cytoskeleton tension [16][17]. Other post-translational modifications include farnesylation, sumoylation, and acetylation [18]. Of particular interest is the farnesylation of the carboxyl terminal end cysteine (CaaX) that anchors lamins into the lipid layers. Type B lamins are permanently farnesylated (Figure 1B), while type A lamins are only transiently farnesylated before the carboxy-terminal peptide is released by the cleavage of prelamin A by ZMPSTE24 to form the mature Lamin A protein. Lamin C proteins are not farnesylated due to their lack of the CaaX motif. Interestingly, the majority of premature aging diseases such as the Hutchinson–Gilford progeria syndrome aka progeria ensue from single point mutations within the LMNA gene that give rise to a permanently farnesylated mutant Lamin A protein, which is termed progerin.
Other important constituents of the lamina are the lamin-associated proteins that mediate the attachment of lamins to the nuclear envelope. Their role is to assist lamins in regulating the chromatin, as exemplified by Lamin B Receptor (LBR) that interacts with several histone modifiers [19], as well as facilitating mechanotransduction as for Emerin [20] and regulating signaling pathways such as TGFβ by MAN1 [21] (Figure 1C). The diversity of lamin-associated proteins justifies the various roles and functions played by the nuclear lamina.
1.2. Nuclear Pore Complex
The nuclear pore complex (NPC) is one of the largest macromolecular assemblies in cells [22] composed of approximately 1000 protein subunits, which are named nucleoporins (NUP). The main function of the NPC is to control the trafficking in and out of the nucleus by allowing the passage through the lipid layers (Figure 1A,C). Moreover, NPC is known to have important functions in chromosomal organization and gene regulation, as it can interact with the genomic region enhancers and super enhancers [23].
1.3. Nuclear Lipid Bilayer
The nuclear envelope (NE) is composed of two phospholipid bilayers organized in an inner nuclear membrane (INM) and outer nuclear membrane (ONM) separated by a lumenal space (Figure 1B,C). The layers are composed of several kind of lipids with different physical properties such as cylindrical lipids (phosphatidylcholine, PC) and conical lipids (phosphatidylethanolamine, PE, diacylglycerol, DAG). Eukaryotic cells maintain their membrane lipid composition within narrow limits with phosphatidylcholine (PC) being the most abundant phospholipid in their nuclear envelope [24]. Despite this relative stable composition, studies in yeasts have shown that pending the environment, de novo specific lipid synthesis can take place in order to relieve the curvation elastic stress on the nuclear membrane protecting the nucleus from breakdown [24]. Interestingly, PCYT1A, the rate-limiting enzyme of PC synthesis involved in this curvation compliance, is localized at the INM in mammalian cells, suggesting a similar adaptability in response to stress and cellular needs in higher organisms [24]. Interestingly, it has also been shown that yeast cells keep lipid droplets at the INM, highlighting that INM has its own lipid metabolism and striking metabolic adaptability [25]. The formation of those lipid droplets through nucleation is influenced by membrane proteins, lipids, and mechanical properties [26]. Thus, multiple adaptability mechanisms may exist to selectively enrich and regulate specific lipid species at the INM in eukaryote cells [27].
2. Nuclear Envelope Diversity and Abnormalities
Nuclear morphology is a common marker for cell determination and classification [28][29]. The nucleus can transpire defects depending of its cell state, aging, or upon mutations. Instead of the typical circular shape, the nucleus can be enlarged, loose circularity, displaying invaginations and a macronucleolus. It can also suffer from altered heterochromatin and irregular nuclear margins as well as a loss of proper compartmentalization involving NE collapse or leakage (Figure 2).
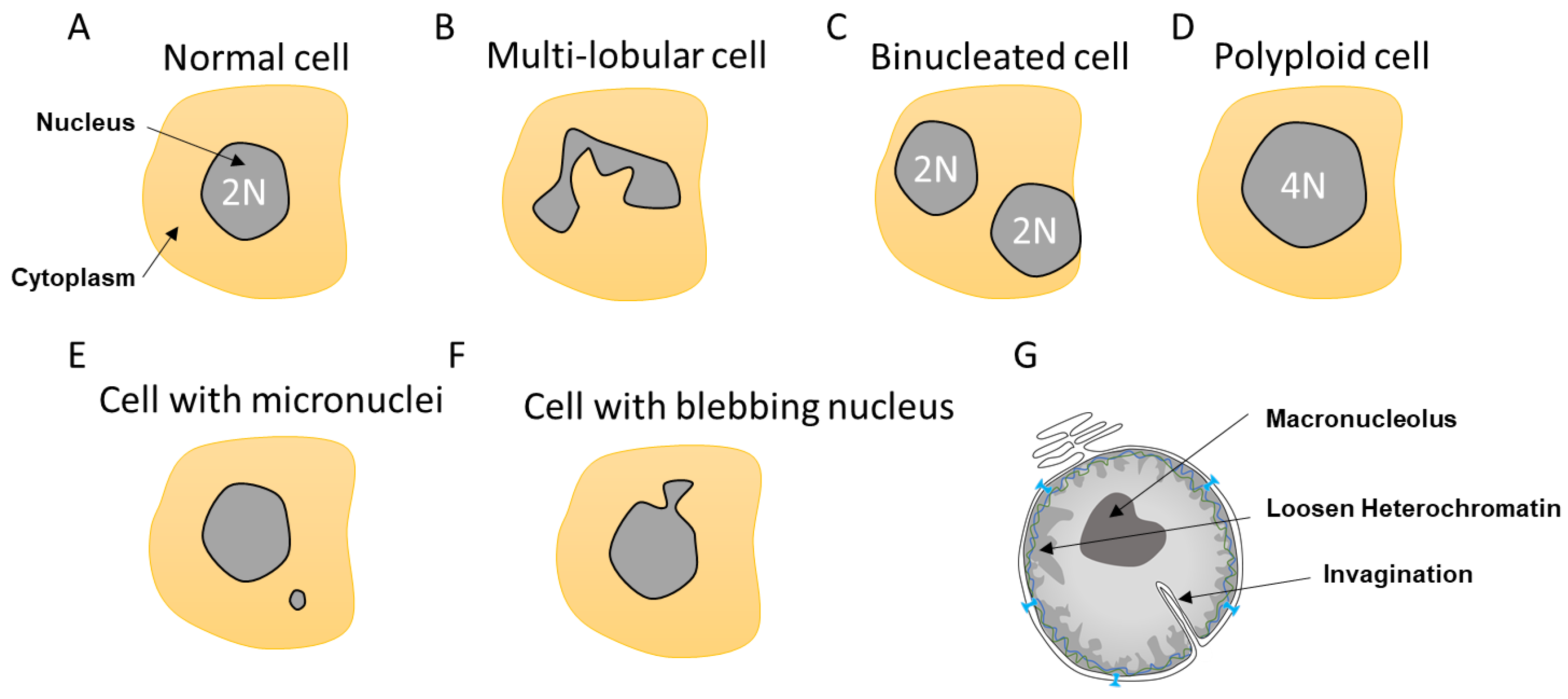
Figure 2. Different nuclear abnormalities. (A) Classical cells have one single round nucleus that contains a diploid genome (2N). (B) Cells such as neutrophils possess multi-lobar nucleus. (C,D) Polyploid cells (>4N) can either have two or more separated nuclei or one enlarged nucleus. (E) Some cells can have a smaller nucleus, which is called a micronucleus, in their cytoplasm. Micronuclei contain a full chromosome or a piece thereof. (F) Nuclei can also display blebbing characterized by an outward extension of the nuclear envelope (NE). (G). In addition, cancer cells can show abnormalities such as NE invagination, loosen heterochromatin as a consequence in changes in DNA compaction as well as a single and bigger nucleolus, which is called a macronucleolus.
2.1. Multi-Lobular Nucleus, Micronuclei, Macronucleoli, and Invagination
Most cells contain a single circular nucleus with one copy of the genome (Figure 2A). However, depending on the nuclear envelope flexibility and abnormalities, the nuclear shape can vary. Some cells have a multi-lobular nucleus such as the neutrophils that required special flexibility needs due to their invasion properties (Figure 2B). Other cells can be polyploid due to an extra copy of the genome sequestered either in a separated nucleus, as in the case of binucleated cells, or contained within the same nucleus, generating an enlarged nucleus (Figure 2C,D). These polyploid cells can arise by a variety of mechanisms, including mitotic slippage, cytokinesis failure, endoreplication, and viral-induced cell fusion [30][31]. Polyploid cells can be found in the pancreas, placenta, muscle, lactating breast, liver, or heart tissues to either improve cell function or support tissue repair and regeneration [31]. Such normal polyploid cells are strictly controlled and not proliferative and are likely senescent. However, it was described in the case of liver that senescence could be reversible and hectaploid hepatocytes may re-enter mitosis, giving rise to tetraploid and diploid cells, which is a dynamic process referred to as a ‘ploidy conveyor’, highlighting the mitosis plasticity of such cells [31][32].
Other nuclear abnormalities include the formation of small nuclei in close proximity to the nucleus (Figure 2E). These small nuclei, referred to as micronuclei (MN), contain either an intact chromosome or pieces thereof and stem from inappropriate chromosome segregation during the anaphase. This DNA, which is excluded from the main nucleus, will attract NE components to form an MN. These extra nuclei bodies have in recent years come into the limelight, as they are a source of substantial DNA damage and can generate diverse complex chromosome rearrangements that are observed in several diseases such as cancer [33].
Nuclear morphology can also be severely altered as a result of impaired nuclear rigidity precipitated by either disruption of the lamina content and chromatin compaction [34] or to increased cytoskeleton forces during attraction and invasion [10][35][36] causing NE blebbing (Figure 2F). This lack of NE strength can also lead to the formation of invaginations, which can affect both the inner and outer nuclear membrane [37]. Invaginations can enclose cytoskeletal elements, both actin and cytokeratin intermediate filaments [38] (Figure 2G). In view of the intimate connection between epigenetic modifiers and the lamina, it is not surprising that both blebbing and MN provoke alterations in the epigenetic landscape [17].
2.2. Apoptosis, Senescence, and Aging
The nuclear envelope integrity can also be jeopardized by various cellular processes. During apoptosis, the lamina and nuclear pore complex are targeted by the apoptotic machinery, disturbing the permeability and subsequent breakdown of the NE resulting in destruction of the nucleus [39] and cell death. A peculiar mode of apoptosis is NETosis in which neutrophils voluntarily disrupt their nuclear envelope and plasmatic membrane in response to an infection to release DNA that forms an extracellular mesh with the goal to trap pathogens such as bacteria and signal the immune system to activate the innate immune response [40].
Aging and senescence also cause a broad range of abnormalities in the nuclear morphology that include enlarged nuclei, loss of circularity, appearance of a multi-lobular nucleus, presence of nuclear envelope invaginations, changes in nucleolus, and relaxed heterochromatin [41]. Such abnormalities induced during senescence have been linked to a decrease of Lamin B levels through enhanced autophagy and decrease in RNA stability [42][43][44], compromising NE rigidity. In contrast, aging cells exhibit changes in Lamin A/C localization, with decreased expression in the nucleoplasm and a concomitant accumulation at the nuclear rim, leading to a reduction in heterochromatin-specific tri-methylation of Lys9 on histone H3 (Tri-Me-K9H3) [45]. These changes in heterochromatin might compromise the homeostasis forces applied to the NE and therefore disrupt the normal shape. The NPC is also affected in aging cells resulting in compromised nuclear permeability barrier and an accumulation of cytoplasmic tubulin, which is a finding consistent with ‘leakiness’: a loss of nucleo-cytoplasmic compartmentalization [46]. Lamins are also important regulators for telomere maintenance, and telomere shortening during aging could affect lamin organization and composition [47].
2.3. Cancer
Morphological irregularities of the nucleus are not only a characteristic of aging and senescent cells but also a diagnostic factor for tumor cells (Figure 3A) [37]. Nuclear morphology allows the classification of cell states by pathologists [28][29], such as in the case of cervical cancer and the examination of cervical cells using the Papanicolaou smear test. In this test, progression toward cancer due to the infection with the Papilloma virus is characterized by a strident and folded nuclear envelope as well as the presence of micronuclei and multi-lobular nuclei [28][48]. Another example is the pancreatic ductal adenocarcinoma where during progression toward cancer, the nuclear membrane acquires an irregular shape, resulting in an enlarged nucleus and increased nucleus/cytoplasm ratio as well as the presence of macronucleoli [49][50].
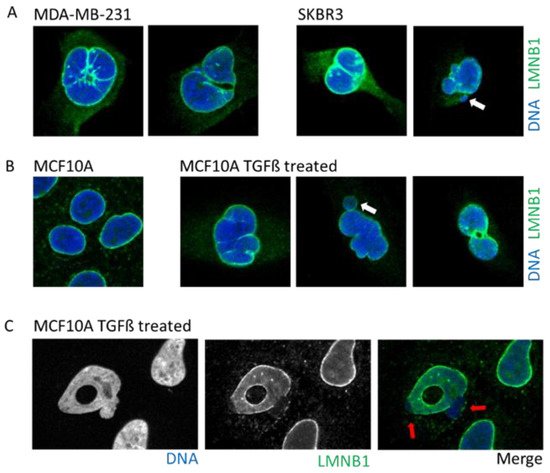
Figure 3. Example of cells with abnormal nuclear envelope. (A). MDA-MB-231 and SKBR3 breast cancer cells presenting invaginations, twisted nuclei, and micronuclei (white arrow). (B). The breast epithelial cell line MCF10A enters in Epithelial to Mesenchymal Transition upon treatment with TGFß. Upon transformation, cells present nuclear invagination, the presence of micronuclei (white arrow), or donut-shaped nuclei. (C). Confocal image of TGFß-treated MCF10A cells highlight nuclear blebbing (red arrow) with a rupture in the Lamin B1 network. Cells are stained for Lamin B1 (LMNB1-green) and DNA (dapi-blue). Adapted from Comaills et al. [10].
Tumors cells can display a myriad of nuclear aberrations such as a loosened NE, twisted nuclei, and invagination, as observed in breast tumor MDA-MB-231 and SKBR3 cells (Figure 3A). Such NE abnormalities can stem from an increase in DNA content, gain in chromosomes and/or ploidy, changes in epigenetic state, and modification in the compaction of chromatin, all increasing the nucleus size. Genetic instability is one of the hallmarks of cancer and is frequently associated with the presence of micronuclei. In some instances, cancer cells may express higher levels of progerin, the immature and farnesylated form of Lamin A, favoring NE defects [51].
2.4. Cellular Plasticity and Its Effect on Nuclear Envelope
Cells are known to be subject to cellular plasticity in response to the microenvironment signaling upon injuries and inflammation [52][53]. Those transient changes in cellular identity can have a profound effect. Cellular plasticity entails rapid epigenetic changes with remodeling of the chromatin, as a consequence of repression and/or activation of genomic regions through the modulation of the Lamin-Associated Domains (LAD) among other regions. One outstanding question is whether such alterations modifies the NE composition to adapt to this new cellular state.
One of the most studied cellular plasticity is the transient and reversible epigenetic reprogramming of Epithelial-to-Mesenchymal Transition (EMT) that allows cells to gain migration and invasiveness properties. EMT drives important aspects of embryologic development such as gastrulation, neural crest, and mesectoderm or heart development [54]. Furthermore, EMT seems to be a major player during the metastasis process [54][55] to allow primary tumor cells to invade new environments. EMT can be induced by TGFß, a cytokine detected under an inflamed microenvironment, or release by platelets in the bloodstream. The EMT induces a rapid opening of the chromatin to increase the accessibility of repressed genomic regions as well as enhancers [56]. Interestingly, a study using breast epithelial cell lines revealed that activation of the EMT program decreased protein levels, but not those of RNA, of several nuclear envelope proteins such as lamins, NPC, and nucleolus proteins [10]. An induction of EMT by either TGFß treatment or overexpression of the transcription factor SNAIL prompts profound alterations in the NE morphology that includes blebbing, twisting, invagination, and donut shape nuclei, which is a phenotype associated with decreased farnesylation of Lamin B1 [57] (Figure 3B).
Importantly, a decrease in nuclear envelope proteins leads to failed mitosis and the formation of lagging chromosomes during the anaphase, resulting in the formation of either MN or binucleation in up to 10% of cells [10]. Correlation between the presence of MN and binucleated cells and mesenchymal state was also observed in circulating tumor cells isolated from estrogen receptor-positive breast cancer patients [10][58].
2.5. Envelopathies
Envelopathies are rare diseases stemming from mutations in nuclear envelope encoding genes including lamin (i.e., laminopathies), proteins from the inner nuclear membrane, such as emerin or SUN proteins, outer nuclear membranes, such as nesprins and proteins involved in the regulation of NE proteins such as ZMPSTE24 [59]. Envelopathies display a large variety of clinical symptoms including metabolic syndrome, muscular dystrophy, lipodystrophy, neuropathy, and progeria (premature aging), among many others. Lipodystrophies result in an array of metabolic complications as insulin resistance, type 2 diabetes, hypertriglyceridemia, and hepatic steatosis [59][60][61][62][63]. Interestingly, patients with metabolic syndrome also exhibit an unusual high prevalence of laminopathies [64]. To date, nearly 500 mutations have been identified in LMNA, which cause a plethora of diseases such as Emery–Dreifuss Muscular Dystrophy (EDMD), dilated cardiomyopathy (DCM), Hutchinson–Gilford Progeria Syndrome (HGPS), Lipodystrophy syndrome, and peripheral neuropathy. The latter highlights the complex genotype–phenotype associations and clinical heterogeneity. Thus, the same variant can lead to different phenotypes, and a similar phenotype can arise from different variants.
Envelopathies clinical heterogeneity is also expressed in overlapping syndromes such as lipodystrophy with myopathy, neuropathy, and/or premature aging stigmata, giving rise to the concept of a multisystem dystrophy syndrome [65].
Similar to the heterogeneity of clinical phenotypes, mutations in different NE protein can also differentially impact NE integrity. For example, cells bearing mutations within the Lamin A gene that leads to premature aging HGPS, display an armada of nuclear alterations such as nuclear blebbing, micronuclei, and a honeycomb pattern [66], as well weakened adaptability to external mechanical stress [67]. Importantly, mutations in the LMNA gene can result in the loss of proper compartmentalization, leading to transient nuclear envelope disruption (NED) during interphase [68].
3. Nuclear Envelope Disruption
The fundamental role of the nuclear envelope is to protect the genome from the damaging effects of the cytoplasm. However, when challenged by either nuclear envelope abnormality or variations in the mechanical force homeostasis, the NE can collapse, impairing proper compartmentalization and exposure to the cytoplasm. Such an event, especially during interphase, when the genome is unfolded and unprotected, can have profound and long-term effects on the genome, including massive genomic instability and the induction of a pro-inflammatory immune response.
3.1. Nuclear Envelope Disruption and Repair
The development of high-resolution microscopy tools for the observation of living cells has allowed the detection of NED events during interphase. Using time lapse imaging in cells with a stable expression of fluorescent markers linked to a nuclear localization signal (NLS), it has been possible to observe the proper nuclear compartmentalization [10][35][36][69][70][71] (Figure 4) as well as to quantify the time of NED rupture and repair. The collapse of NE can vary from seconds up to several hours [10][69][72] (Figure 4A,B). Resealing of the nuclear envelope is driven by the Endosomal Sorting Complexes Required for Transport III (ESCRT III) machinery [35][36]. Upon NE rupture, CHMP7–LEMD2 complexes accumulate to the site of rupture and recruit the soluble monomeric ESCRT-III subunits that will polymerize into filaments at the site of rupture to drive membrane sealing [73]. However, in the case of micronuclei, the repair of the NE is impaired [73], and the rupture is sustained until the next mitosis cycle [71].
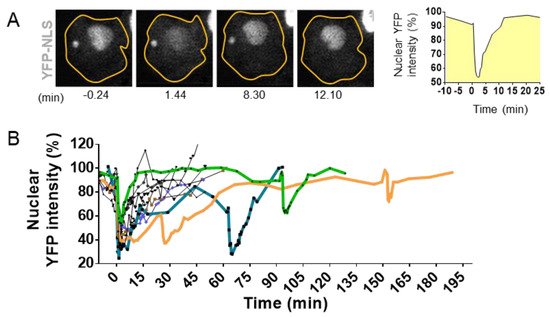
Figure 4. Visualization and quantification of nuclear envelope disruption (NED). (A). Time lapse imaging of migrating MCF10A cells treated with TGFß and stably expressing the fluorescent markers YFP linked to the Nuclear Localization Signal (YFP-NLS) [74]. Confocal imaging in YFP channel and bright field allows the visualization of sudden leakage of YFP-NLs into the cytoplasm, showing a loss of proper compartmentalization of the nucleus during 8.30 mn and the loss of compartmentalization of the micronuclei at 12.10 mn of time. (B). Graph shows nuclear fluorescence intensity changes upon time in 10 cells with events of interphase NED/nuclear envelope repair observed in TGFß-treated MCF10A cells. Bold colored lines highlight repetitive NED events from the same cell. Adapted from Comaills et al. [10].
3.2. Causes of Nuclear Envelope Disruption
The NE homeostasis is achieved through a balance between different mechanical forces from within and from outside the nuclei (Figure 5A). From within, forces are determined by the chromatin rigidity that vary upon the chromatin state and compaction of the heterochromatin. Drastic changes in heterochromatin density can occur during cellular plasticity such as during EMT [56][75] or during aging [45]. Interestingly, the chromatin is also mechanoresponsive, and chromatin can alter its own mechanical state to maintain genome integrity in response to deformation [76].

Figure 5. Nuclear envelope (NE) equilibrium and cases of nuclear envelope disruption (NED). (A). NE homeostasis between the chromatin rigidity forces (black arrow), cytoskeleton forces (green arrow), and the effect of the environment stiffness (blue arrow). Mechanosensitivity of the NE is done through the connection of the cytoskeleton by the LINC complex (blue ovals). (B). Example of nucleus with several NE abnormalities leading to extreme NE curvature at invagination, blebbing, or on the NE of the micronuclei that might be involved in NE fragility (highlighted in red). (C). Cells under migration have strong cytoskeleton attraction that can lead to local tension and the formation of nuclear envelope disruption. (D). Cells in migration through tiny constrictions also endure new stiffness forces and will deform NE locally to ensure the nuclear passage and create new NE tension and extreme NE bending, leading to NED. (E). Two daughter cells experiencing telomere fusion have their nucleus connected by a chromatin bridge and sharing the same NE. The need of extra NE to cover the extended NE surface leads to NE weakness and break.
From the outside, the nucleus possesses mechanosensitive properties via its connection with the cytoskeleton. As a result, gene regulation can drastically change depending on the stiffness of the environment and can result in cellular differentiation [2]. The Linker of Nucleoskeleton and Cytoskeleton (LINC) complex bridges the nucleus to the cytoskeleton (Figure 1C and Figure 5A) and is the major force-transmitting sensor. LINC serves as a mechanosensor, translating mechanical cues, which include physical forces compression of the actin cap [77], shear stress, and alterations in extracellular matrix stiffness, into biochemical signals, thus allowing cells to adapt to their physical environment [78]. Cells also monitor their own shape and develop an active contractile response when the nucleus deformed below a specific threshold. Transition in the mechanical state of the NE induces calcium release, activating the calcium-dependent phospholipase cPLA2 and downstream myosin II, causing cells to move with the goal to rescue nuclei from a constraint area [79].
Maintenance of the NE equilibrium is conveyed by a balance between the various forces imposed upon it, which can be disrupted under certain conditions leading to transient NED. For example, cancer leads to improper NE regulation characterized by NE blebbing, micronuclei, and invagination. Such formation involves an extreme bending of the NE where the mechanical properties of the membranes may be important, weakening the integrity of the NE and leading to the plausible cause of NE collapse (Figure 5B).
3.2.1. Alterations in Expression of Lamins
NED can be provoked as a result of decreased lamin expression as observed during epigenetic reconversion of EMT where all the lamins are downregulated [10], or by experimentally targeting type B lamins using shRNA constructs [69][72]. Alterations in lamin expression affect the rigidity and flexibility of the nucleus and disrupt the NE force equilibrium that can end up in transient collapse. As such, in laminopathy, cells with mutations in the LMNA gene have a weaker nuclear envelope and are more susceptible to ruptures [80]. Indeed, 29% of fibroblasts derived from progeria patients display nuclear envelope collapse [68].
3.2.2. Migration and Invasion
During migration, the cytoskeleton imposes considerable traction forces, resulting in strenuous nucleus deformation. The swift reorganization of the cytoskeleton in response to cues may surpass the capacity of the NE to adapt, resulting in the formation of blebbing that can ultimately lead to NED, as observed both in vitro and in vivo during migration [10][35][36] (Figure 5C). Passage through restrained spaces such as during extravasation and passage through capillary increases the local environmental stiffness force, leading to nucleus compression and to extreme NE deformation and curvation that may result in NED [35][36][81]. The ratio of Lamin A/B determines NE flexibility and rigidity, and thus, its capacity to proceed, or not, through restrained spaces [1]. For example, NE blebbing is characterized by reduced Lamin B1 levels, suggesting that Lamin B filaments are overly stiff and unable to bend on high-curvature nuclear membranes, which is a phenomenon that can be explained by Lamin B high affinity for the NE lipid layer due to their farnesylation and attachment to Lamin B Receptor [81] (Figure 3C).
3.2.3. Micronuclei: NE Extreme Curvation and Improper NE Composition
MN are small nuclei containing either a full chromosome that lagged during mitosis or a fragment thereof missing a proper centromere. Several studies have shown that the NE of MN are susceptible to rupture without repair capability [71] (Figure 4A). MN disruption could be due to issues in assembly and composition, as essential NE proteins are lacking [71][82], and 40% of MNs fail to import NLS tagged protein [82]. Interestingly, MN are characterized by lower levels of Lamin B, and the presence of functional Lamin B partly correlates with micronuclei size, suggesting that the right assembly of NE might be sensitive to membrane curvature [83]. A recent study [73] also shows that MN lacks the capacity to repair the NE after a collapse due to the lack in restricting CHMP7–LEMD2 complexes to the site of rupture, resulting in an unrestrained activation of ESCRT-III across the surface of their inner membrane. Rather than repairing the ruptured micronuclei, the hyperaccumulation of ESCRT-III drives dramatic membrane distortion and causes DNA torsional stress, the formation of single-stranded DNA, and chromosome damage [73].
3.2.4. Telomere Fusion
Telomere fusion is characterized by the fusion of two telomeric ends between two chromosomes, called end-to-end fusion, which is a phenomenon often observed in cancer and aging [84]. This telomere crisis leads to dicentric chromosomes that invariably persist through mitosis and form long chromatin bridges that connect daughter cells well into the next G1 phase [85] (Figure 5E and Figure 7). These chromatin bridges are surrounded by a contiguous nuclear envelope that increase the overall NE surface. Stretching of the NE is often associated with rupture of the nuclear envelope of the connected nuclei [70] (Figure 5E). This NED is transient and last around 2 min [70]. Interestingly, the NE protein composition in this chromatin bridge appears to have the same defects than some MN that were trapped in the spindle during the NE formation. By being trapped in the middle of the spindle, the assembly of the NE seems compromised and appears to lack several essential NE proteins such as NPC, affecting the NE maintenance [82].
3.2.5. ATR, RB, and P53 Loss
The modulation of proteins levels involved in genomic stability can lead to NE deformation and rupture. ATR is a serine/threonine protein kinase that activates checkpoint signaling upon genotoxic or replication stresses, thereby acting as a DNA damage sensor. Activated ATR performs multiple cellular functions to maintain genomic integrity and the prevention of replication and mitotic catastrophe [86]. However, ATR is also known to have other functions such as maintaining NE integrity in response to mechanical stress [87]. Mutations in the ATR gene as well as shRNA-mediated silencing compromise NE integrity and are associated with NE blebbing [87] and invaginations as well as transient NED [88]. The NE lipid composition is altered in ATR-silenced cells [87], substantiating the roles of ATR in the NE adaptability and regulation, as well as in heterochromatin compaction, affecting the nuclear stiffness. In vitro studies have also shown that cells depleted for either retinoblastoma protein (Rb) or the tumor suppressor P53 exhibit increased incidence of NE rupture [89]. Such a phenotype is not associated with greater mobility nor changes in NE protein composition, but it seems to be linked with genome reorganization and increased nuclei size [89]. These examples demonstrate the complex relationship between genome organization, genome maintenance, and NE integrity.
3.2.6. Heterochromatin Modulation
Chromatin compaction profoundly affects nucleus stiffness (Figure 5A). Certain cellular states or transient epigenetic transformation can induce a complete chromatin landscape reorganization such as during EMT [56][75] or during aging [45]. These changes modify homeostasis, which can weaken the NE and develop NED [10]. Another evidence of the role of chromatin compaction on NE homeostasis is the modulation of the nucleosome binding protein HMGN5. Study in vitro and in vivo have shown that the overexpression of HMGN5 leads to enlarged nuclei and NED [90]. The effect of HMGM5 overexpression in the NE integrity was more obvious in contractile tissues due to extensive cytoskeleton forces. This study confirms that heterochromatin provides mechanical stability to the nucleus. Then cellular plasticity might have more profound effects than expected and could drive NE fragility, deformation and eventual collapse.
3.2.7. Virus Infection
Many DNA as well as several RNA viruses hijack the host replicative system in order to propagate and as such have developed various mechanisms to shuttle in and out of the nucleus, either by usurping cellular transport pathways through the nuclear pore complex or translocating directly through the NE [91][92]. Interestingly, some viruses use non-invasive mechanisms in order to secure proper and efficiency replication of the particle while escaping the activation of cytoplasmic DNA sensor pathways. In contrast, other viruses induce the nuclear and membrane breakdown later in infection in order to release mature viral particles, as in the case of adenoviruses, papillomaviruses, and polyomaviruses [91]. As a result of their smaller size, non-enveloped viruses can provoke transient NED as observed during early infection of the mouse parvovirus minute virus (MVM) [92][93][94]. The early infection of human papillomavirus type 16 requires breakdown of the NE to access the nucleus, and it is believed they enter during the mitotic NED. However, it is possible they use other mechanisms such as transient NED. It is also important to note that many viruses destabilize the cell cycle [95], causing mitotic defect that can result indirectly in NED. For example, Hepatitis B virus X protein affects S phase progression, leading to chromosome segregation defects [96]. Lagging chromosomes will generate MN and subsequently give rise to NED and ultimately genomic instability and cancer development. Such a process could potentially be implicated in the early stages of liver cancer.
4. Cytoplasmic DNA Induces Inflammation by cGAS/STING Pathway
Any DNA within the cytoplasmic compartment is sensed as foreign DNA, mimicking a viral or bacterial infection, resulting in the activation of DNA recognition pathways by the cell in order to neutralize the invader. In mammalian cells, the three major DNA-sensing receptors that drive immune responses to foreign DNA are the Toll-like receptor 9 (TLR9), the absent in melanoma 2 (AIM2), and the cyclic GMP–AMP synthase (cGAS) [97]. These DNA-sensing receptors are mostly expressed in blood and immune cells with the exception of the cGAS pathway that can be induced in several cell types. cGAS is an intracellular enzyme that binds to double-stranded DNA (dsDNA) and initiates a tightly regulated signaling cascade to induce the expression of inflammatory genes. The classical role of cGAS is to detect DNA from pathogens, such as bacteria and virus, and to activate the innate immune pathway (Figure 6A). However, the origin of DNA in the cytoplasm can be diverse, and cGAS can be activated by DNA from its own cell. The outcome of genomic instability is ultimately linked to the release of DNA in the cytoplasm such as during DNA damage or NED from MN and nucleus. Mitochondria can also release part of the genome during mitochondrial stress or during apoptosis [98]. DNA produced during the resolution of replication stress or retroelements such as retrotransposons are also a source of aberrant cytoplasmic DNA (Figure 6A). This DNA is usually degraded by the TREX1 protein, which is a DNA exonuclease that clears normal endogenous cytosolic DNA to prevent aberrant stimulation of the cGAS pathway [99].
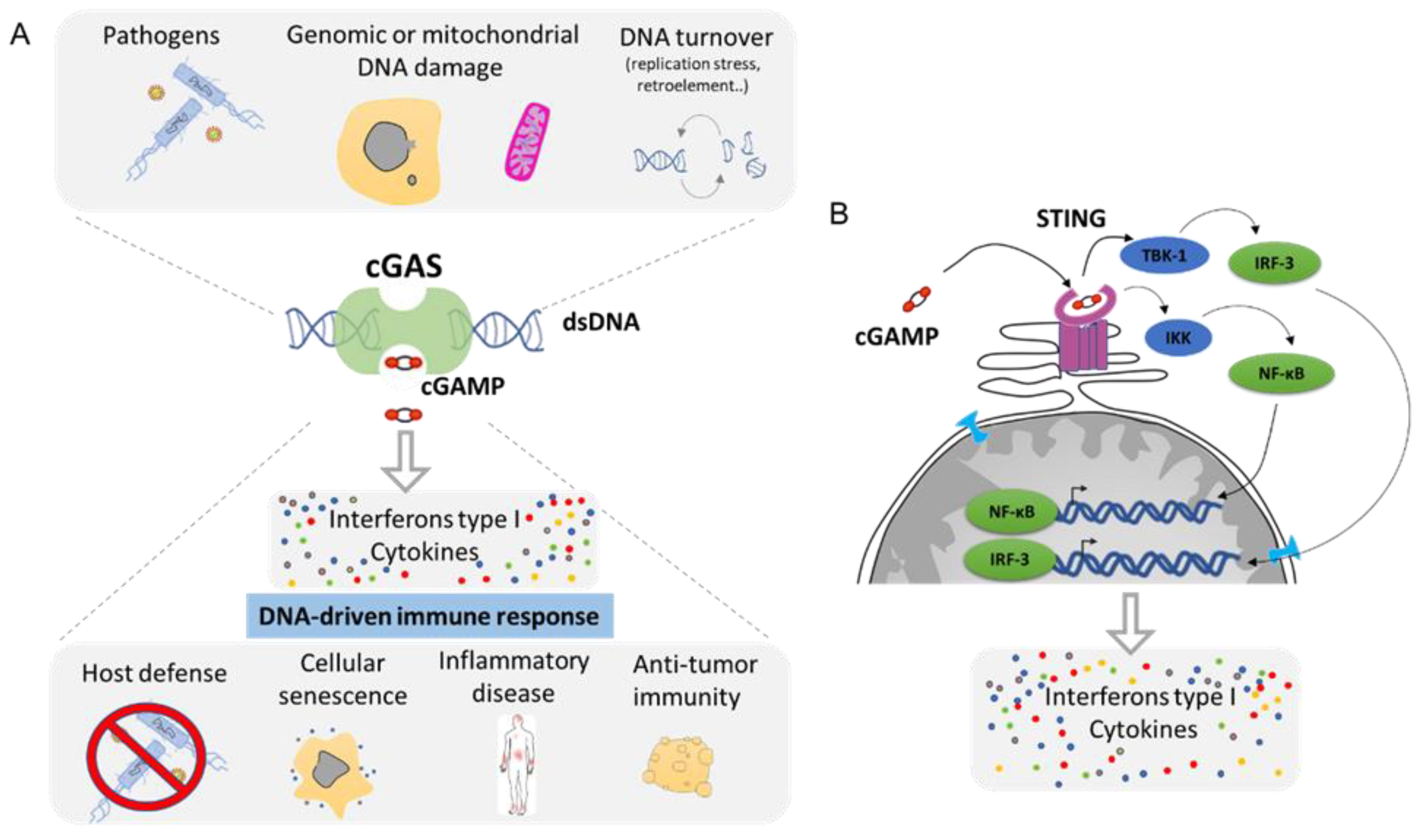
Figure 6. The cGAS–cGAMP–STING signaling pathway: a universal sensor for double-strand DNA (dsDNA). (A). cGAS function is to sense DNA from the cytoplasm as result of infection, genomic, or mitochondrial instability. cGAS activation through the generation of cGAMP will drive the activation of the innate immune response and leads to the secretion of Interferon type 1 as well as a cocktail of cytokines. (B). Molecular mechanism of cGAS/STING pathway. cGAMP is the ligand for the STING receptor (shown in pink) and results in the activation of the transcription regulator factors NF-κβ and IRF-3 that drive the secretion of several pro-inflammatory molecules (Adapted from Ablasser et al. [100] and Motwani et al. [97]).
Mechanistically, cGAS activation by cytosolic DNA leads to the generation of cyclic GMP–AMP (cGAMP), which binds to the receptor STImulator of INterferon Genes (STING). Activation of STING results in the activation of TANK-binding kinase 1 (TBK1) and in the dimerization and nuclear translocation of the interferon regulatory transcription factor 3 (IRF3), resulting in the transcription of genes encoding type I interferons (reviewed in [100][101]) (Figure 6B). DNA sensing through the cGAS–STING pathway also results in the activation of the nuclear factor NF-κB through IKK and leads to the transcription of pro-inflammatory cytokines such as IL-6 and tumor necrosis factor (TNF) [102]. This cocktail of signal attracts, alerts, and activates the immune system of a potential danger. Then, the cGAS pathway is involved in several functions such as host defense in response to bacterial or viral infection. It supports the natural antitumor activity by facilitating the recognition of cellular damage and in promoting cellular senescence [100][103][104] (Figure 6A). However, the cGAS/STING pathway plays also a crucial role in many inflammation-related diseases such as cardiovascular disease, neurodegenerative disease, inflammatory bowel disease, metabolic syndrome such as diabetes, fibrosis, lupus, arthritis, and psoriasis [100][105][106] (Figure 6A).
5. Clinical Consequences of Nuclear Envelope Rupture
The release of genomic DNA into the cytoplasm leads to major consequences with diverse clinical outcomes: the creation of chromosomal instability and the induction of pro-inflammatory pathways. Recent technological advances in sequencing tools as well as imaging have shifted interest in the role of NE fragility as the origin of some congenital diseases, in the creation of cancer, and to certain inflammatory syndromes. The role of NED and activation of the innate immune response through cGAS could be in fact linked to much more diseases than expected.
5.1. Envelopathies and Inflammation
Nuclear envelope abnormalities due to mutation in NE proteins are well described and are associated to a myriad of clinical phenotypes, ranging from cardiac and skeletal myopathies to partial lipodystrophy, peripheral neuropathy, and premature aging [125]. The recent discovery of NED during interphase observed with the use of time lapse imaging has allowed a better understanding of the role of certain mutations on NE integrity, highlighting the implication of NED, genomic instability and activation of the innate immune response in the establishment of tissue degeneration. Most of envelopathies are tissue-specific, affecting the skeletal muscle, heart, peripheral nerves, bone, or adipose tissue, and they are caused by mutations in ubiquitously expressed proteins, as are the proteins from the NE. Then, the disruption of their function may be limited by epigenetic and specific chromatin compaction, or in response to specific stiffness of their environment, such muscle cells that are constantly confronted to mechanostress. However, the link between NED and local inflammation could explain the diversity of pathology, and the role of cGAS/STING pathway activity during NED could be in fact largely underestimated.
5.1.1. Emery–Dreifuss Muscular Dystrophy: Cell Death Due to Increase DNA Damage
The Emery–Dreifuss muscular dystrophy (EDMD) is a rare disease caused by mutations in nuclear envelope proteins such as Lamin A, Emerin, or Nesprin 1 and 2. The clinical symptoms are skeletal muscle wasting, joint contractures, cardiomyopathy, and congenital muscular dystrophy. Using several mice models and patients’ samples with mutated Lamin A [80] or in SYNE-1 [126] (coding for the Nesprin 1 protein) genes, researchers observed the presence of NED during muscular contraction. Muscle cells bearing one of the EDMD mutations display increased DNA damage and increased apoptosis. Importantly, NED also led to activation of the cGAS pathway [80], which might play a major role in the development of the disease.
5.1.2. Progeria: DNA Damage, NED, and cGAS Activation
Hutchinson–Gilford progeria (HGPS) arises from single point mutations within the Lamin A gene, leading to its permanent farnesylation, also called progerin, and it leads to premature aging, among other symptoms. Patients die from complications of atherosclerosis, such as heart attack or stroke in their early teens. Atherosclerosis is a chronic inflammatory disease of the vessel wall that is largely driven by an innate immune response [127], and it seems to be driven by the cGAS/STING pathway [128]. A study of cells from progeria patients has shown NE fragility that tends to disrupt [68]. In addition, progerin expression renders smooth muscle cells more susceptible to cell death in response to mechanical stress, which is likely due to nuclear envelope disruption [129]. Other studies have shown that progerin expression activates the cGAS/STING/IFN pathway [130][131][132] and suggest that this activation is due to either replication stress or to oxidative stress. However, the role of NED in this pathway activation cannot be excluded.
5.1.3. Metabolic Syndrome: A Common Symptom in Envelopathies
Some common clinical symptoms of envelopathies patients are the lipodystrophy and lipoatrophic diabetes (Table 1). However, the link between metabolic syndrome and nuclear envelope dysfunction is not yet understood. Lipodystrophies are characterized by near-total loss of body fat. In view to compensate for the lack of adipocyte tissues, the fat is stocked in ectopic fat stores, particularly certain subcutaneous depots but also within and around the skeletal muscle, heart, liver, pancreas, and kidneys, leading to deregulation in metabolic homeostasis [133]. The syndrome can result in an array of metabolic complications such as insulin resistance, type 2 diabetes, hypertriglyceridemia, and hepatic steatosis. Interestingly, lipodystrophy is often associated with autoimmune disorders including lupus, dermatomyositis, Celiac disease, pernicious anemia, and vasculitis [133][134], highlighting the role of immunity in this disease. Whether NED-mediated cGAS activation is a major factor in the development of this clinical symptom is an open question.
Table 1. Syndromes associated with lipodystrophy, which is a common pathology in envelopathy patients. Online Mendelian Inheritance in Man (OMIM) ID is given to refer to the catalogue of genetic disorder.
In favor of such a premise, mutations in lamin and lamin-associated genes are known to induce seven syndromes associated with lipodystrophy and lipoatrophic diabetes (Table 1). Lamins are known to regulate gene expression at the epigenetic level through their Lamina-Associated Domains (LADs). Consequently, mutations in lamin genes may impact the regulation of metabolic genes in a tissue-specific manner as each tissue possesses a distinct epigenetic statue. Nonetheless, a general hallmark of dystrophy is the degeneration and death of tissues, suggesting that additional and more mechanisms are also involved. The link between NED, cGAS activation, and lipodystrophy is not yet known, but in the case of some Lamin A mutations such as HGPS and EDMD, it was demonstrated that the NE can collapse [68][80], highlighting a probable role of NE fragility and plausible NED and cGAS activation in the development of such clinical outcomes. Interestingly, laminopathies seem to be under-diagnosed, as a study analyzing metabolic syndrome reported that 10% of patients presented abnormal nuclear envelope and 3.6% possessed a mutation in the Lamin A (LMNA) gene [64].
Interestingly, BSCL2 and AGPAT2 gene mutations induce Berardinelli–Seip congenital lipodystrophy type 2, which is the most severe form of human lipodystrophy. BSCL2 is a reticulum endoplasmic protein involved in the regulation of lipid droplets by facilitating continuous triglyceride transfer [135]. BSCL2 is also a key factor for nuclear lipid droplet generation and lipid homeostasis [136]. Indeed, BSCL2 connects the lipid layer from the inner NE and the nuclear lipid droplet, and it might affect the inner nuclear membrane maintenance [136]. AGPAT2 converts lysophosphatidic acid to phosphatidic acid (PA), which is the second step in de novo phospholipid biosynthesis. Nevertheless, PA are substrates for enzymes producing lipids that are involved in fission or fusion, contributing to membrane rearrangements by generating negative membrane curvature [137]. Then, both proteins are important players in the maintenance of the lipid bilayer that might be involved in NE adaptability to external forces and could present NE fragility in certain context as in adipocytes.
5.1.4. Neuropathies, Nuclear Envelope, and Inflammation
Mutation in lamin genes can also cause neuropathies [138][139][140][141]. Other genetic diseases that causes neurological lesion are the tauopathies, which are neurodegenerative disorders characterized by the deposition of abnormal tau protein in the brain. Tau is a microtubule-associated protein expressed in neurons that are involved in neurodegenerative diseases, including Alzheimer disease (AD), frontotemporal dementia with parkinsonism-17, Pick disease, progressive supranuclear palsy, and corticobasal degeneration [142]. These diseases indirectly involve the NE dysfunction, as the abnormal localization of Tau generates the production of nuclear envelope invaginations and is associated with lamin dysfunction [143]. Whether such NE deformations are linked with NED is still not fully known, but it was recently described that the nucleocytoplasmic transport that is compromised is a model of frontotemporal dementia that could be in fact the result of NED [144].
Impairment of nucleocytoplasmic transport has also recently emerged as a central disease mechanism in amyotrophic lateral sclerosis and frontotemporal dementia due to hexanucleotide expansions in the C9ORF72 gene [145] and in Huntington’s disease [146][147]. Expression of those repeats leads to morphological abnormalities in the architecture of the nuclear envelope. It is not clear if the impairment of nucleocytoplasmic transport is due to disruption of nuclear import or due to NE collapse.
Interestingly, chronic activation of an innate immune response in the central nervous system is frequently associated with neuronal damage [148]. Recently, cGAS/STING activation [149][150][151] was discovered to be involved in neurological diseases as multiple sclerosis [152] or Huntington’s disease [153]. The release of genomic DNA into the cytoplasm due to NE weakness and subsequent activation of the cGAS/STING pathway could be then a major driver in the development of neuroinflammation, leading to degeneration of neurons.
5.1.5. Congenital Disease Due to Complex Chromosomal Rearrangement
Most human embryos are aneuploid and do not develop to term, making aneuploidy in embryos a leading cause of miscarriages and infertility [154]. Aneuploidy frequently arises during the early mitotic divisions of the embryo. During the fecundation, the parental genomes fuse and cluster to form the embryo. However, clustering often fails, leading to chromosome segregation errors and micronuclei, which are incompatible with healthy embryo development [155]. Recent advances in sequencing methodology have allowed the detection and the description of complex structural variations inside the genome of patients with developmental disease, as well as in phenotypically normal individuals [156]. Complex chromosomal rearrangements as chromothripsis are detected in developmentally delayed children but also in mothers suffering spontaneous abortions [156][157]. Carriers of chromosomes with chromosomal complex structural reorganization as chromothripsis cannot pair their chromosome with their partners, leading to infertility. The MN formed during the failed clustering of parental genome during the fecundation is hypothetically the origin of those atopic genome.
6.1.6. Cancer: Origin, Evolution, and Survival
The genomic instability linked with NED can have profound effects on cell evolution. These genomic insults not only increase the pool of genetic diverse cells but also events that can progress into cancers. Recent advances in sequencing have allowed the observation that chromothripsis and other complex chromosomal rearrangements are early events leading to tumorigenesis in pancreatic adenocarcinoma [158], multiple myeloma [159], or breast cancers [160], among many others [161][162]. Genetic insults associated with NED contribute to all steps in cancer, from the origin to the progression and establishment of drug resistance. They are indispensable in the generation of genomic heterogeneity.
Chromothripsis accounts for a substantial proportion of human cancers, with a general prevalence of 49% and up to 80% in breast cancers [162]. Forty percent of tumors with chromothripsis harbor only one chromosome but might have more complex patterns with at least five chromosomes affected in 61% of osteosarcomas [161]. Importantly, polyploidy tumors have 1.5 times more probability to generate an event of chromothripsis [161], which is in line with the genetic instability of whole genome doubled cells and increase of MN formation. Circular extrachromosomal DNA or ecDNA, associated with events of chromothripsis, are found in 40% of cancer cells [116] and are primordial in tumor evolution.
The activation of the cGAS/STING pathway in response to NED is an important anti-tumorigenic mechanism via activating the immune surveillance to mediate tumor clearance (Figure 6). It is likely that during the first step of tumorigenesis, the cGAS pathway plays a primordial role in inducing an immune attack or by promoting intrinsic senescence [163]. Nevertheless, mounting evidence suggests that depending on the context, the cGAS/STING pathway can have tumor and metastasis-promoting functions [163]. It was shown recently that metastatic cells harboring high chromosome instability could engage the STING-dependent non-canonical NF-κB pathway as well as suppress anti-viral type 1 IFN production, thereby activating inflammatory responses that favor invasion and metastasis [164]. Metastatic cells can also communicate with their microenvironment through cGAMP signaling. In metastatic human breast tumors, brain metastatic cancer cells converse with adjacent astrocytes through cGAMP generated by the tumor. cGAMP is exported to astrocytes via gap junctions, which in turn activate the STING pathway and initiate the release of inflammatory cytokines, favoring the brain metastases survival [165]. Moreover, STING chronic activation could induce paradoxically an immune-suppressive environment [163]. Cancer cells treated with STING agonist markedly increased PD-L1 expression and pro-inflammatory cytokines [166]. Cancer cells expressing PD-L1 support the evasion of T cell immune surveillance by blocking T cell killing [163]. Although the precise mechanism remains to be elucidated, it is tempting to speculate that RelA/NF-κB signaling stabilizing the PD-L1 protein could be involved in this paradox [167].
6. Concluding Remarks
Over the past 10 years, the research on NE biology has revealed unexpected consequences of its deregulation. The discovery of NED and its role in activation of the cGAS/STING pathway as well as its role in creating chromosomal instability has stirred this field. The NE composition is highly malleable and adapts according to epigenetic changes and mechano-stress. However, under certain conditions and due to some genetic predispositions, the NE can collapse. Naked DNA is sensed as a danger by intra-cellular DNA recognition pathways, and in particular, the cGAS/STING pathway as well as by TREX1 or APOBEC. Cells activate the immune response and attack their own DNA, creating massive DNA damage that could generate complex chromosomal rearrangement as chromothripsis, ecDNA, or Kataegis, leading to tumorigenesis. This defense mechanism has major impact leading to tissue degeneration, as in the case of some laminopathy and in neurodegenerative diseases as well as to induce local inflammation that can drive to autoimmune disease. The role of cGAS/STING signaling in immune and autoimmune diseases seems to be a major driver, and there is an increased interest in targeting this pathway. Several molecules and strategies to disrupt this pathway are being delineated and evaluated in preclinical models [105][168] and will soon enter clinical trials. Such discoveries could be translated to several NE associated disease such as laminopathy and also be used in the cancer field in an attempt to block the growth of metastasis.
To conclude, the understanding of NE dynamic is indispensable in order to understand a myriad of diseases with a broad range of clinical symptoms. The interplay between inflammation, genomic instability, and NE fragility is fascinating, and harnessing its secrets may open new avenues in the development of innovative therapeutic strategies.
References
- Shin, J.W.; Spinler, K.R.; Swift, J.; Chasis, J.A.; Mohandas, N.; Discher, D.E. Lamins regulate cell trafficking and lineage maturation of adult human hematopoietic cells. Proc. Natl. Acad. Sci. USA 2013, 110, 18892–18897.
- Swift, J.; Ivanovska, I.L.; Buxboim, A.; Harada, T.; Dingal, P.C.; Pinter, J.; Pajerowski, J.D.; Spinler, K.R.; Shin, J.W.; Tewari, M.; et al. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science 2013, 341, 1240104.
- Spann, T.P.; Moir, R.D.; Goldman, A.E.; Stick, R.; Goldman, R.D. Disruption of nuclear lamin organization alters the distribution of replication factors and inhibits DNA synthesis. J. Cell Biol. 1997, 136, 1201–1212.
- Tsai, M.Y.; Wang, S.; Heidinger, J.M.; Shumaker, D.K.; Adam, S.A.; Goldman, R.D.; Zheng, Y. A mitotic lamin B matrix induced by RanGTP required for spindle assembly. Science 2006, 311, 1887–1893.
- Gonzalez-Sandoval, A.; Gasser, S.M. On TADs and LADs: Spatial Control over Gene Expression. Trends Genet. 2016, 32, 485–495.
- Briand, N.; Collas, P. Lamina-associated domains: Peripheral matters and internal affairs. Genome Biol. 2020, 21, 85.
- Lambert, M.W. The functional importance of lamins, actin, myosin, spectrin and the LINC complex in DNA repair. Exp. Biol. Med. (Maywood) 2019, 244, 1382–1406.
- Martin, C.; Chen, S.; Maya-Mendoza, A.; Lovric, J.; Sims, P.F.; Jackson, D.A. Lamin B1 maintains the functional plasticity of nucleoli. J. Cell Sci. 2009, 122, 1551–1562.
- Bera, M.; Sengupta, K. Nuclear filaments: Role in chromosomal positioning and gene expression. Nucleus 2020, 11, 99–110.
- Comaills, V.; Kabeche, L.; Morris, R.; Buisson, R.; Yu, M.; Madden, M.W.; LiCausi, J.A.; Boukhali, M.; Tajima, K.; Pan, S.; et al. Genomic Instability Is Induced by Persistent Proliferation of Cells Undergoing Epithelial-to-Mesenchymal Transition. Cell Rep. 2016, 17, 2632–2647.
- Liu, S.Y.; Ikegami, K. Nuclear lamin phosphorylation: An emerging role in gene regulation and pathogenesis of laminopathies. Nucleus 2020, 11, 299–314.
- Alcorta-Sevillano, N.; Macias, I.; Rodriguez, C.I.; Infante, A. Crucial Role of Lamin A/C in the Migration and Differentiation of MSCs in Bone. Cells 2020, 9, 1330.
- Donnaloja, F.; Carnevali, F.; Jacchetti, E.; Raimondi, M.T. Lamin A/C Mechanotransduction in Laminopathies. Cells 2020, 9, 1306.
- Xie, W.; Chojnowski, A.; Boudier, T.; Lim, J.S.; Ahmed, S.; Ser, Z.; Stewart, C.; Burke, B. A-type Lamins Form Distinct Filamentous Networks with Differential Nuclear Pore Complex Associations. Curr. Biol. 2016, 26, 2651–2658.
- Nmezi, B.; Xu, J.; Fu, R.; Armiger, T.J.; Rodriguez-Bey, G.; Powell, J.S.; Ma, H.; Sullivan, M.; Tu, Y.; Chen, N.Y.; et al. Concentric organization of A- and B-type lamins predicts their distinct roles in the spatial organization and stability of the nuclear lamina. Proc. Natl. Acad. Sci. USA 2019, 116, 4307–4315.
- Cho, S.; Vashisth, M.; Abbas, A.; Majkut, S.; Vogel, K.; Xia, Y.; Ivanovska, I.L.; Irianto, J.; Tewari, M.; Zhu, K.; et al. Mechanosensing by the Lamina Protects against Nuclear Rupture, DNA Damage, and Cell-Cycle Arrest. Dev. Cell 2019, 49, 920–935.e925.
- Karoutas, A.; Akhtar, A. Functional mechanisms and abnormalities of the nuclear lamina. Nat. Cell Biol. 2021, 23, 116–126.
- Torvaldson, E.; Kochin, V.; Eriksson, J.E. Phosphorylation of lamins determine their structural properties and signaling functions. Nucleus 2015, 6, 166–171.
- Shevelyov, Y.Y.; Ulianov, S.V. The Nuclear Lamina as an Organizer of Chromosome Architecture. Cells 2019, 8, 136.
- Lammerding, J.; Hsiao, J.; Schulze, P.C.; Kozlov, S.; Stewart, C.L.; Lee, R.T. Abnormal nuclear shape and impaired mechanotransduction in emerin-deficient cells. J. Cell Biol. 2005, 170, 781–791.
- Pan, D.; Estevez-Salmeron, L.D.; Stroschein, S.L.; Zhu, X.; He, J.; Zhou, S.; Luo, K. The integral inner nuclear membrane protein MAN1 physically interacts with the R-Smad proteins to repress signaling by the transforming growth factor- superfamily of cytokines. J. Biol. Chem. 2005, 280, 15992–16001.
- Lin, D.H.; Hoelz, A. The Structure of the Nuclear Pore Complex (An Update). Annu. Rev. Biochem. 2019, 88, 725–783.
- Pascual-Garcia, P.; Capelson, M. Nuclear pores in genome architecture and enhancer function. Curr. Opin. Cell Biol. 2019, 58, 126–133.
- Haider, A.; Wei, Y.C.; Lim, K.; Barbosa, A.D.; Liu, C.H.; Weber, U.; Mlodzik, M.; Oras, K.; Collier, S.; Hussain, M.M.; et al. PCYT1A Regulates Phosphatidylcholine Homeostasis from the Inner Nuclear Membrane in Response to Membrane Stored Curvature Elastic Stress. Dev. Cell 2018, 45, 481–495.e488.
- Romanauska, A.; Kohler, A. The Inner Nuclear Membrane Is a Metabolically Active Territory that Generates Nuclear Lipid Droplets. Cell 2018, 174, 700–715.e718.
- Thiam, A.R.; Ikonen, E. Lipid Droplet Nucleation. Trends Cell Biol. 2021, 31, 108–118.
- Bahmanyar, S.; Schlieker, C. Lipid and protein dynamics that shape nuclear envelope identity. Mol. Biol. Cell 2020, 31, 1315–1323.
- Fischer, E.G. Nuclear Morphology and the Biology of Cancer Cells. Acta Cytol. 2020, 64, 511–519.
- Skinner, B.M.; Johnson, E.E. Nuclear morphologies: Their diversity and functional relevance. Chromosoma 2017, 126, 195–212.
- Ganem, N.J.; Storchova, Z.; Pellman, D. Tetraploidy, aneuploidy and cancer. Curr. Opin. Genet. Dev. 2007, 17, 157–162.
- Ovrebo, J.I.; Edgar, B.A. Polyploidy in tissue homeostasis and regeneration. Development 2018, 145, dev156034.
- Duncan, A.W.; Taylor, M.H.; Hickey, R.D.; Hanlon Newell, A.E.; Lenzi, M.L.; Olson, S.B.; Finegold, M.J.; Grompe, M. The ploidy conveyor of mature hepatocytes as a source of genetic variation. Nature 2010, 467, 707–710.
- Maciejowski, J.; Hatch, E.M. Nuclear Membrane Rupture and Its Consequences. Annu. Rev. Cell Dev. Biol. 2020, 36, 85–114.
- Stephens, A.D.; Liu, P.Z.; Banigan, E.J.; Almassalha, L.M.; Backman, V.; Adam, S.A.; Goldman, R.D.; Marko, J.F. Chromatin histone modifications and rigidity affect nuclear morphology independent of lamins. Mol. Biol. Cell 2018, 29, 220–233.
- Denais, C.M.; Gilbert, R.M.; Isermann, P.; McGregor, A.L.; te Lindert, M.; Weigelin, B.; Davidson, P.M.; Friedl, P.; Wolf, K.; Lammerding, J. Nuclear envelope rupture and repair during cancer cell migration. Science 2016, 352, 353–358.
- Raab, M.; Gentili, M.; de Belly, H.; Thiam, H.R.; Vargas, P.; Jimenez, A.J.; Lautenschlaeger, F.; Voituriez, R.; Lennon-Dumenil, A.M.; Manel, N.; et al. ESCRT III repairs nuclear envelope ruptures during cell migration to limit DNA damage and cell death. Science 2016, 352, 359–362.
- Malhas, A.N.; Vaux, D.J. Nuclear envelope invaginations and cancer. Adv. Exp. Med. Biol. 2014, 773, 523–535.
- Jorgens, D.M.; Inman, J.L.; Wojcik, M.; Robertson, C.; Palsdottir, H.; Tsai, W.T.; Huang, H.; Bruni-Cardoso, A.; Lopez, C.S.; Bissell, M.J.; et al. Deep nuclear invaginations are linked to cytoskeletal filaments—Integrated bioimaging of epithelial cells in 3D culture. J. Cell Sci. 2017, 130, 177–189.
- Lindenboim, L.; Zohar, H.; Worman, H.J.; Stein, R. The nuclear envelope: Target and mediator of the apoptotic process. Cell Death Discov. 2020, 6, 29.
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535.
- Pathak, R.U.; Soujanya, M.; Mishra, R.K. Deterioration of nuclear morphology and architecture: A hallmark of senescence and aging. Ageing Res. Rev. 2021, 67, 101264.
- Dreesen, O.; Chojnowski, A.; Ong, P.F.; Zhao, T.Y.; Common, J.E.; Lunny, D.; Lane, E.B.; Lee, S.J.; Vardy, L.A.; Stewart, C.L.; et al. Lamin B1 fluctuations have differential effects on cellular proliferation and senescence. J. Cell Biol. 2013, 200, 605–617.
- Martins, F.; Sousa, J.; Pereira, C.D.; da Cruz, E.S.O.A.B.; Rebelo, S. Nuclear envelope dysfunction and its contribution to the aging process. Aging Cell 2020, 19, e13143.
- Dou, Z.; Xu, C.; Donahue, G.; Shimi, T.; Pan, J.A.; Zhu, J.; Ivanov, A.; Capell, B.C.; Drake, A.M.; Shah, P.P.; et al. Autophagy mediates degradation of nuclear lamina. Nature 2015, 527, 105–109.
- Scaffidi, P.; Misteli, T. Lamin A-dependent nuclear defects in human aging. Science 2006, 312, 1059–1063.
- D’Angelo, M.A.; Raices, M.; Panowski, S.H.; Hetzer, M.W. Age-dependent deterioration of nuclear pore complexes causes a loss of nuclear integrity in postmitotic cells. Cell 2009, 136, 284–295.
- Burla, R.; La Torre, M.; Saggio, I. Mammalian telomeres and their partnership with lamins. Nucleus 2016, 7, 187–202.
- Smith, E.R.; George, S.H.; Kobetz, E.; Xu, X.X. New biological research and understanding of Papanicolaou’s test. Diagn. Cytopathol. 2018, 46, 507–515.
- Ren, B.; Liu, X.; Suriawinata, A.A. Pancreatic Ductal Adenocarcinoma and Its Precursor Lesions: Histopathology, Cytopathology, and Molecular Pathology. Am. J. Pathol. 2019, 189, 9–21.
- Hruban, R.H.; Maitra, A.; Goggins, M. Update on pancreatic intraepithelial neoplasia. Int. J. Clin. Exp. Pathol. 2008, 1, 306–316.
- Tang, Y.; Chen, Y.; Jiang, H.; Nie, D. Promotion of tumor development in prostate cancer by progerin. Cancer Cell Int. 2010, 10, 47.
- Yuan, S.; Norgard, R.J.; Stanger, B.Z. Cellular Plasticity in Cancer. Cancer Discov. 2019, 9, 837–851.
- Merrell, A.J.; Stanger, B.Z. Adult cell plasticity in vivo: De-differentiation and transdifferentiation are back in style. Nat. Rev. Mol. Cell Biol. 2016, 17, 413–425.
- Nieto, M.A.; Huang, R.Y.; Jackson, R.A.; Thiery, J.P. Emt: 2016. Cell 2016, 166, 21–45.
- Brabletz, T.; Kalluri, R.; Nieto, M.A.; Weinberg, R.A. EMT in cancer. Nat. Rev. Cancer 2018, 18, 128–134.
- Guerrero-Martinez, J.A.; Ceballos-Chavez, M.; Koehler, F.; Peiro, S.; Reyes, J.C. TGFbeta promotes widespread enhancer chromatin opening and operates on genomic regulatory domains. Nat. Commun. 2020, 11, 6196.
- Verstraeten, V.L.; Peckham, L.A.; Olive, M.; Capell, B.C.; Collins, F.S.; Nabel, E.G.; Young, S.G.; Fong, L.G.; Lammerding, J. Protein farnesylation inhibitors cause donut-shaped cell nuclei attributable to a centrosome separation defect. Proc. Natl. Acad. Sci. USA 2011, 108, 4997–5002.
- Yu, M.; Bardia, A.; Aceto, N.; Bersani, F.; Madden, M.W.; Donaldson, M.C.; Desai, R.; Zhu, H.; Comaills, V.; Zheng, Z.; et al. Cancer therapy. Ex vivo culture of circulating breast tumor cells for individualized testing of drug susceptibility. Science 2014, 345, 216–220.
- Janin, A.; Bauer, D.; Ratti, F.; Millat, G.; Mejat, A. Nuclear envelopathies: A complex LINC between nuclear envelope and pathology. Orphanet J. Rare Dis. 2017, 12, 147.
- Galant, D.; Gaborit, B.; Desgrouas, C.; Abdesselam, I.; Bernard, M.; Levy, N.; Merono, F.; Coirault, C.; Roll, P.; Lagarde, A.; et al. A Heterozygous ZMPSTE24 Mutation Associated with Severe Metabolic Syndrome, Ectopic Fat Accumulation, and Dilated Cardiomyopathy. Cells 2016, 5, 21.
- Kreienkamp, R.; Gonzalo, S. Metabolic Dysfunction in Hutchinson-Gilford Progeria Syndrome. Cells 2020, 9, 395.
- Desgrouas, C.; Varlet, A.A.; Dutour, A.; Galant, D.; Merono, F.; Bonello-Palot, N.; Bourgeois, P.; Lasbleiz, A.; Petitjean, C.; Ancel, P.; et al. Unraveling LMNA Mutations in Metabolic Syndrome: Cellular Phenotype and Clinical Pitfalls. Cells 2020, 9, 310.
- Charar, C.; Gruenbaum, Y. Lamins and metabolism. Clin. Sci. (Lond.) 2017, 131, 105–111.
- Dutour, A.; Roll, P.; Gaborit, B.; Courrier, S.; Alessi, M.C.; Tregouet, D.A.; Angelis, F.; Robaglia-Schlupp, A.; Lesavre, N.; Cau, P.; et al. High prevalence of laminopathies among patients with metabolic syndrome. Hum. Mol. Genet. 2011, 20, 3779–3786.
- Guillin-Amarelle, C.; Fernandez-Pombo, A.; Sanchez-Iglesias, S.; Araujo-Vilar, D. Lipodystrophic laminopathies: Diagnostic clues. Nucleus 2018, 9, 249–260.
- van Tienen, F.H.J.; Lindsey, P.J.; Kamps, M.A.F.; Krapels, I.P.; Ramaekers, F.C.S.; Brunner, H.G.; van den Wijngaard, A.; Broers, J.L.V. Assessment of fibroblast nuclear morphology aids interpretation of LMNA variants. Eur. J. Hum. Genet. 2019, 27, 389–399.
- Dahl, K.N.; Scaffidi, P.; Islam, M.F.; Yodh, A.G.; Wilson, K.L.; Misteli, T. Distinct structural and mechanical properties of the nuclear lamina in Hutchinson-Gilford progeria syndrome. Proc. Natl. Acad. Sci. USA 2006, 103, 10271–10276.
- De Vos, W.H.; Houben, F.; Kamps, M.; Malhas, A.; Verheyen, F.; Cox, J.; Manders, E.M.; Verstraeten, V.L.; van Steensel, M.A.; Marcelis, C.L.; et al. Repetitive disruptions of the nuclear envelope invoke temporary loss of cellular compartmentalization in laminopathies. Hum. Mol. Genet. 2011, 20, 4175–4186.
- Vargas, J.D.; Hatch, E.M.; Anderson, D.J.; Hetzer, M.W. Transient nuclear envelope rupturing during interphase in human cancer cells. Nucleus 2012, 3, 88–100.
- Maciejowski, J.; Li, Y.; Bosco, N.; Campbell, P.J.; de Lange, T. Chromothripsis and Kataegis Induced by Telomere Crisis. Cell 2015, 163, 1641–1654.
- Hatch, E.M.; Fischer, A.H.; Deerinck, T.J.; Hetzer, M.W. Catastrophic nuclear envelope collapse in cancer cell micronuclei. Cell 2013, 154, 47–60.
- Chen, N.Y.; Yang, Y.; Weston, T.A.; Belling, J.N.; Heizer, P.; Tu, Y.; Kim, P.; Edillo, L.; Jonas, S.J.; Weiss, P.S.; et al. An absence of lamin B1 in migrating neurons causes nuclear membrane ruptures and cell death. Proc. Natl. Acad. Sci. USA 2019, 116, 25870–25879.
- Vietri, M.; Schultz, S.W.; Bellanger, A.; Jones, C.M.; Petersen, L.I.; Raiborg, C.; Skarpen, E.; Pedurupillay, C.R.J.; Kjos, I.; Kip, E.; et al. Unrestrained ESCRT-III drives micronuclear catastrophe and chromosome fragmentation. Nat. Cell Biol. 2020, 22, 856–867.
- Beier, K.T.; Samson, M.E.; Matsuda, T.; Cepko, C.L. Conditional expression of the TVA receptor allows clonal analysis of descendents from Cre-expressing progenitor cells. Dev. Biol. 2011, 353, 309–320.
- McDonald, O.G.; Wu, H.; Timp, W.; Doi, A.; Feinberg, A.P. Genome-scale epigenetic reprogramming during epithelial-to-mesenchymal transition. Nat. Struct. Mol. Biol. 2011, 18, 867–874.
- Nava, M.M.; Miroshnikova, Y.A.; Biggs, L.C.; Whitefield, D.B.; Metge, F.; Boucas, J.; Vihinen, H.; Jokitalo, E.; Li, X.; Garcia Arcos, J.M.; et al. Heterochromatin-Driven Nuclear Softening Protects the Genome against Mechanical Stress-Induced Damage. Cell 2020, 181, 800–817.e822.
- Khatau, S.B.; Kim, D.H.; Hale, C.M.; Bloom, R.J.; Wirtz, D. The perinuclear actin cap in health and disease. Nucleus 2010, 1, 337–342.
- Stroud, M.J.; Banerjee, I.; Veevers, J.; Chen, J. Linker of nucleoskeleton and cytoskeleton complex proteins in cardiac structure, function, and disease. Circ. Res. 2014, 114, 538–548.
- Lomakin, A.J.; Cattin, C.J.; Cuvelier, D.; Alraies, Z.; Molina, M.; Nader, G.P.F.; Srivastava, N.; Saez, P.J.; Garcia-Arcos, J.M.; Zhitnyak, I.Y.; et al. The nucleus acts as a ruler tailoring cell responses to spatial constraints. Science 2020, 370, eaba2894.
- Earle, A.J.; Kirby, T.J.; Fedorchak, G.R.; Isermann, P.; Patel, J.; Iruvanti, S.; Moore, S.A.; Bonne, G.; Wallrath, L.L.; Lammerding, J. Mutant lamins cause nuclear envelope rupture and DNA damage in skeletal muscle cells. Nat. Mater. 2020, 19, 464–473.
- Xia, Y.; Pfeifer, C.R.; Zhu, K.; Irianto, J.; Liu, D.; Pannell, K.; Chen, E.J.; Dooling, L.J.; Tobin, M.P.; Wang, M.; et al. Rescue of DNA damage after constricted migration reveals a mechano-regulated threshold for cell cycle. J. Cell Biol. 2019, 218, 2545–2563.
- Liu, S.; Kwon, M.; Mannino, M.; Yang, N.; Renda, F.; Khodjakov, A.; Pellman, D. Nuclear envelope assembly defects link mitotic errors to chromothripsis. Nature 2018, 561, 551–555.
- Kneissig, M.; Keuper, K.; de Pagter, M.S.; van Roosmalen, M.J.; Martin, J.; Otto, H.; Passerini, V.; Campos Sparr, A.; Renkens, I.; Kropveld, F.; et al. Micronuclei-based model system reveals functional consequences of chromothripsis in human cells. Elife 2019, 8.
- Chakravarti, D.; LaBella, K.A.; DePinho, R.A. Telomeres: History, health, and hallmarks of aging. Cell 2021, 184, 306–322.
- Maciejowski, J.; de Lange, T. Telomeres in cancer: Tumour suppression and genome instability. Nat. Rev. Mol. Cell Biol. 2017, 18, 175–186.
- Yazinski, S.A.; Zou, L. Functions, Regulation, and Therapeutic Implications of the ATR Checkpoint Pathway. Annu. Rev. Genet. 2016, 50, 155–173.
- Kumar, A.; Mazzanti, M.; Mistrik, M.; Kosar, M.; Beznoussenko, G.V.; Mironov, A.A.; Garre, M.; Parazzoli, D.; Shivashankar, G.V.; Scita, G.; et al. ATR mediates a checkpoint at the nuclear envelope in response to mechanical stress. Cell 2014, 158, 633–646.
- Kidiyoor, G.R.; Li, Q.; Bastianello, G.; Bruhn, C.; Giovannetti, I.; Mohamood, A.; Beznoussenko, G.V.; Mironov, A.; Raab, M.; Piel, M.; et al. ATR is essential for preservation of cell mechanics and nuclear integrity during interstitial migration. Nat. Commun. 2020, 11, 4828.
- Yang, Z.; Maciejowski, J.; de Lange, T. Nuclear Envelope Rupture Is Enhanced by Loss of p53 or Rb. Mol. Cancer Res. 2017, 15, 1579–1586.
- Furusawa, T.; Rochman, M.; Taher, L.; Dimitriadis, E.K.; Nagashima, K.; Anderson, S.; Bustin, M. Chromatin decompaction by the nucleosomal binding protein HMGN5 impairs nuclear sturdiness. Nat. Commun. 2015, 6, 6138.
- Mettenleiter, T.C. Breaching the Barrier-The Nuclear Envelope in Virus Infection. J. Mol. Biol. 2016, 428, 1949–1961.
- Fay, N.; Pante, N. Nuclear entry of DNA viruses. Front. Microbiol. 2015, 6, 467.
- Cohen, S.; Marr, A.K.; Garcin, P.; Pante, N. Nuclear envelope disruption involving host caspases plays a role in the parvovirus replication cycle. J. Virol. 2011, 85, 4863–4874.
- Cohen, S.; Behzad, A.R.; Carroll, J.B.; Pante, N. Parvoviral nuclear import: Bypassing the host nuclear-transport machinery. J. Gen. Virol. 2006, 87, 3209–3213.
- Fan, Y.; Sanyal, S.; Bruzzone, R. Breaking Bad: How Viruses Subvert the Cell Cycle. Front. Cell Infect. Microbiol. 2018, 8, 396.
- Martin-Lluesma, S.; Schaeffer, C.; Robert, E.I.; van Breugel, P.C.; Leupin, O.; Hantz, O.; Strubin, M. Hepatitis B virus X protein affects S phase progression leading to chromosome segregation defects by binding to damaged DNA binding protein 1. Hepatology 2008, 48, 1467–1476.
- Motwani, M.; Pesiridis, S.; Fitzgerald, K.A. DNA sensing by the cGAS-STING pathway in health and disease. Nat. Rev. Genet. 2019, 20, 657–674.
- Riley, J.S.; Tait, S.W. Mitochondrial DNA in inflammation and immunity. EMBO Rep. 2020, 21, e49799.
- Simpson, S.R.; Hemphill, W.O.; Hudson, T.; Perrino, F.W. TREX1—Apex predator of cytosolic DNA metabolism. DNA Repair (Amst.) 2020, 94, 102894.
- Ablasser, A.; Chen, Z.J. cGAS in action: Expanding roles in immunity and inflammation. Science 2019, 363.
- Wu, J.; Sun, L.; Chen, X.; Du, F.; Shi, H.; Chen, C.; Chen, Z.J. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science 2013, 339, 826–830.
- Ishikawa, H.; Barber, G.N. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature 2008, 455, 674–678.
- Gluck, S.; Guey, B.; Gulen, M.F.; Wolter, K.; Kang, T.W.; Schmacke, N.A.; Bridgeman, A.; Rehwinkel, J.; Zender, L.; Ablasser, A. Innate immune sensing of cytosolic chromatin fragments through cGAS promotes senescence. Nat. Cell Biol. 2017, 19, 1061–1070.
- Yang, H.; Wang, H.; Ren, J.; Chen, Q.; Chen, Z.J. cGAS is essential for cellular senescence. Proc. Natl. Acad. Sci. USA 2017, 114, E4612–E4620.
- Decout, A.; Katz, J.D.; Venkatraman, S.; Ablasser, A. The cGAS-STING pathway as a therapeutic target in inflammatory diseases. Nat. Rev. Immunol. 2021, 1–22.
- Chen, Q.; Sun, L.; Chen, Z.J. Regulation and function of the cGAS-STING pathway of cytosolic DNA sensing. Nat. Immunol. 2016, 17, 1142–1149.
- Zhang, C.Z.; Spektor, A.; Cornils, H.; Francis, J.M.; Jackson, E.K.; Liu, S.; Meyerson, M.; Pellman, D. Chromothripsis from DNA damage in micronuclei. Nature 2015, 522, 179–184.
- Ly, P.; Brunner, S.F.; Shoshani, O.; Kim, D.H.; Lan, W.; Pyntikova, T.; Flanagan, A.M.; Behjati, S.; Page, D.C.; Campbell, P.J.; et al. Chromosome segregation errors generate a diverse spectrum of simple and complex genomic rearrangements. Nat. Genet. 2019, 51, 705–715.
- Crasta, K.; Ganem, N.J.; Dagher, R.; Lantermann, A.B.; Ivanova, E.V.; Pan, Y.; Nezi, L.; Protopopov, A.; Chowdhury, D.; Pellman, D. DNA breaks and chromosome pulverization from errors in mitosis. Nature 2012, 482, 53–58.
- Umbreit, N.T.; Zhang, C.Z.; Lynch, L.D.; Blaine, L.J.; Cheng, A.M.; Tourdot, R.; Sun, L.; Almubarak, H.F.; Judge, K.; Mitchell, T.J.; et al. Mechanisms generating cancer genome complexity from a single cell division error. Science 2020, 368, eaba0712.
- Kato, H.; Sandberg, A.A. Chromosome pulverization in human cells with micronuclei. J. Natl. Cancer Inst. 1968, 40, 165–179.
- Kwon, M.; Leibowitz, M.L.; Lee, J.H. Small but mighty: The causes and consequences of micronucleus rupture. Exp. Mol. Med. 2020, 52, 1777–1786.
- Ly, P.; Teitz, L.S.; Kim, D.H.; Shoshani, O.; Skaletsky, H.; Fachinetti, D.; Page, D.C.; Cleveland, D.W. Selective Y centromere inactivation triggers chromosome shattering in micronuclei and repair by non-homologous end joining. Nat. Cell Biol. 2017, 19, 68–75.
- Leibowitz, M.L.; Papathanasiou, S.; Doerfler, P.A.; Blaine, L.J.; Sun, L.; Yao, Y.; Zhang, C.Z.; Weiss, M.J.; Pellman, D. Chromothripsis as an on-target consequence of CRISPR-Cas9 genome editing. Nat. Genet. 2021, 53, 895–905.
- Shoshani, O.; Brunner, S.F.; Yaeger, R.; Ly, P.; Nechemia-Arbely, Y.; Kim, D.H.; Fang, R.; Castillon, G.A.; Yu, M.; Li, J.S.Z.; et al. Chromothripsis drives the evolution of gene amplification in cancer. Nature 2021, 591, 137–141.
- Turner, K.M.; Deshpande, V.; Beyter, D.; Koga, T.; Rusert, J.; Lee, C.; Li, B.; Arden, K.; Ren, B.; Nathanson, D.A.; et al. Extrachromosomal oncogene amplification drives tumour evolution and genetic heterogeneity. Nature 2017, 543, 122–125.
- Maciejowski, J.; Chatzipli, A.; Dananberg, A.; Chu, K.; Toufektchan, E.; Klimczak, L.J.; Gordenin, D.A.; Campbell, P.J.; de Lange, T. APOBEC3-dependent kataegis and TREX1-driven chromothripsis during telomere crisis. Nat. Genet. 2020, 52, 884–890.
- Zou, J.; Wang, C.; Ma, X.; Wang, E.; Peng, G. APOBEC3B, a molecular driver of mutagenesis in human cancers. Cell Biosci. 2017, 7, 29.
- Dahiya, R.; Hu, Q.; Ly, P. Mechanistic origins of diverse genome rearrangements in cancer. Semin. Cell Dev. Biol. 2021.
- Pfeifer, C.R.; Xia, Y.; Zhu, K.; Liu, D.; Irianto, J.; Garcia, V.M.M.; Millan, L.M.S.; Niese, B.; Harding, S.; Deviri, D.; et al. Constricted migration increases DNA damage and independently represses cell cycle. Mol. Biol. Cell 2018, 29, 1948–1962.
- Eldarov, C.M.; Vangely, I.M.; Vays, V.B.; Sheval, E.V.; Holtze, S.; Hildebrandt, T.B.; Kolosova, N.G.; Popkov, V.A.; Plotnikov, E.Y.; Zorov, D.B.; et al. Mitochondria in the Nuclei of Rat Myocardial Cells. Cells 2020, 9, 712.
- Sur, I.; Neumann, S.; Noegel, A.A. Nesprin-1 role in DNA damage response. Nucleus 2014, 5, 173–191.
- Lei, K.; Zhu, X.; Xu, R.; Shao, C.; Xu, T.; Zhuang, Y.; Han, M. Inner nuclear envelope proteins SUN1 and SUN2 play a prominent role in the DNA damage response. Curr. Biol. 2012, 22, 1609–1615.
- Pereira, C.D.; Martins, F.; Santos, M.; Mueller, T.; da Cruz, E.S.O.A.B.; Rebelo, S. Nuclear Accumulation of LAP1:TRF2 Complex during DNA Damage Response Uncovers a Novel Role for LAP1. Cells 2020, 9, 1804.
- Worman, H.J.; Bonne, G. “Laminopathies”: A wide spectrum of human diseases. Exp. Cell Res. 2007, 313, 2121–2133.
- Zhou, C.; Li, C.; Zhou, B.; Sun, H.; Koullourou, V.; Holt, I.; Puckelwartz, M.J.; Warren, D.T.; Hayward, R.; Lin, Z.; et al. Novel nesprin-1 mutations associated with dilated cardiomyopathy cause nuclear envelope disruption and defects in myogenesis. Hum. Mol. Genet. 2017, 26, 2258–2276.
- Wolf, D.; Ley, K. Immunity and Inflammation in Atherosclerosis. Circ. Res. 2019, 124, 315–327.
- Lu, G.F.; Chen, S.C.; Xia, Y.P.; Ye, Z.M.; Cao, F.; Hu, B. Synergistic inflammatory signaling by cGAS may be involved in the development of atherosclerosis. Aging (Albany NY) 2021, 13, 5650–5673.
- Kim, P.H.; Luu, J.; Heizer, P.; Tu, Y.; Weston, T.A.; Chen, N.; Lim, C.; Li, R.L.; Lin, P.Y.; Dunn, J.C.Y.; et al. Disrupting the LINC complex in smooth muscle cells reduces aortic disease in a mouse model of Hutchinson-Gilford progeria syndrome. Sci. Transl. Med. 2018, 10, eaat7163.
- Graziano, S.; Kreienkamp, R.; Coll-Bonfill, N.; Gonzalo, S. Causes and consequences of genomic instability in laminopathies: Replication stress and interferon response. Nucleus 2018, 9, 258–275.
- Kreienkamp, R.; Graziano, S.; Coll-Bonfill, N.; Bedia-Diaz, G.; Cybulla, E.; Vindigni, A.; Dorsett, D.; Kubben, N.; Batista, L.F.Z.; Gonzalo, S. A Cell-Intrinsic Interferon-like Response Links Replication Stress to Cellular Aging Caused by Progerin. Cell Rep. 2018, 22, 2006–2015.
- Bidault, G.; Garcia, M.; Capeau, J.; Morichon, R.; Vigouroux, C.; Bereziat, V. Progerin Expression Induces Inflammation, Oxidative Stress and Senescence in Human Coronary Endothelial Cells. Cells 2020, 9, 1201.
- Lanktree, M.B.; Johansen, C.T.; Joy, T.R.; Hegele, R.A. A translational view of the genetics of lipodystrophy and ectopic fat deposition. Prog. Mol. Biol. Transl. Sci. 2010, 94, 159–196.
- Misra, A.; Peethambaram, A.; Garg, A. Clinical features and metabolic and autoimmune derangements in acquired partial lipodystrophy: Report of 35 cases and review of the literature. Medicine (Baltimore) 2004, 83, 18–34.
- Salo, V.T.; Li, S.; Vihinen, H.; Holtta-Vuori, M.; Szkalisity, A.; Horvath, P.; Belevich, I.; Peranen, J.; Thiele, C.; Somerharju, P.; et al. Seipin Facilitates Triglyceride Flow to Lipid Droplet and Counteracts Droplet Ripening via Endoplasmic Reticulum Contact. Dev. Cell 2019, 50, 478–493.e479.
- Jin, Y.; Tan, Y.; Zhao, P.; Ren, Z. SEIPIN: A Key Factor for Nuclear Lipid Droplet Generation and Lipid Homeostasis. Int. J. Mol. Sci. 2020, 21, 8208.
- Zhukovsky, M.A.; Filograna, A.; Luini, A.; Corda, D.; Valente, C. Phosphatidic acid in membrane rearrangements. FEBS Lett. 2019, 593, 2428–2451.
- Padiath, Q.S.; Saigoh, K.; Schiffmann, R.; Asahara, H.; Yamada, T.; Koeppen, A.; Hogan, K.; Ptacek, L.J.; Fu, Y.H. Lamin B1 duplications cause autosomal dominant leukodystrophy. Nat. Genet. 2006, 38, 1114–1123.
- De Sandre-Giovannoli, A.; Chaouch, M.; Kozlov, S.; Vallat, J.M.; Tazir, M.; Kassouri, N.; Szepetowski, P.; Hammadouche, T.; Vandenberghe, A.; Stewart, C.L.; et al. Homozygous defects in LMNA, encoding lamin A/C nuclear-envelope proteins, cause autosomal recessive axonal neuropathy in human (Charcot-Marie-Tooth disorder type 2) and mouse. Am. J. Hum. Genet. 2002, 70, 726–736.
- Parry, D.A.; Martin, C.A.; Greene, P.; Marsh, J.A.; Genomics England Research, C.; Blyth, M.; Cox, H.; Donnelly, D.; Greenhalgh, L.; Greville-Heygate, S.; et al. Heterozygous lamin B1 and lamin B2 variants cause primary microcephaly and define a novel laminopathy. Genet. Med. 2021, 23, 408–414.
- Cristofoli, F.; Moss, T.; Moore, H.W.; Devriendt, K.; Flanagan-Steet, H.; May, M.; Jones, J.; Roelens, F.; Fons, C.; Fernandez, A.; et al. De Novo Variants in LMNB1 Cause Pronounced Syndromic Microcephaly and Disruption of Nuclear Envelope Integrity. Am. J. Hum. Genet. 2020, 107, 753–762.
- Gao, Y.L.; Wang, N.; Sun, F.R.; Cao, X.P.; Zhang, W.; Yu, J.T. Tau in neurodegenerative disease. Ann. Transl. Med. 2018, 6, 175.
- Frost, B.; Bardai, F.H.; Feany, M.B. Lamin Dysfunction Mediates Neurodegeneration in Tauopathies. Curr. Biol. 2016, 26, 129–136.
- Paonessa, F.; Evans, L.D.; Solanki, R.; Larrieu, D.; Wray, S.; Hardy, J.; Jackson, S.P.; Livesey, F.J. Microtubules Deform the Nuclear Membrane and Disrupt Nucleocytoplasmic Transport in Tau-Mediated Frontotemporal Dementia. Cell Rep. 2019, 26, 582–593.e585.
- Freibaum, B.D.; Lu, Y.; Lopez-Gonzalez, R.; Kim, N.C.; Almeida, S.; Lee, K.H.; Badders, N.; Valentine, M.; Miller, B.L.; Wong, P.C.; et al. GGGGCC repeat expansion in C9orf72 compromises nucleocytoplasmic transport. Nature 2015, 525, 129–133.
- Li, N.; Lagier-Tourenne, C. Nuclear pores: The gate to neurodegeneration. Nat. Neurosci. 2018, 21, 156–158.
- Gasset-Rosa, F.; Chillon-Marinas, C.; Goginashvili, A.; Atwal, R.S.; Artates, J.W.; Tabet, R.; Wheeler, V.C.; Bang, A.G.; Cleveland, D.W.; Lagier-Tourenne, C. Polyglutamine-Expanded Huntingtin Exacerbates Age-Related Disruption of Nuclear Integrity and Nucleocytoplasmic Transport. Neuron 2017, 94, 48–57.e44.
- Stephenson, J.; Nutma, E.; van der Valk, P.; Amor, S. Inflammation in CNS neurodegenerative diseases. Immunology 2018, 154, 204–219.
- Chin, A.C. Neuroinflammation and the cGAS-STING pathway. J. Neurophysiol. 2019, 121, 1087–1091.
- Fryer, A.L.; Abdullah, A.; Taylor, J.M.; Crack, P.J. The Complexity of the cGAS-STING Pathway in CNS Pathologies. Front. Neurosci. 2021, 15, 621501.
- Paul, B.D.; Snyder, S.H.; Bohr, V.A. Signaling by cGAS-STING in Neurodegeneration, Neuroinflammation, and Aging. Trends Neurosci. 2021, 44, 83–96.
- Mathur, V.; Burai, R.; Vest, R.T.; Bonanno, L.N.; Lehallier, B.; Zardeneta, M.E.; Mistry, K.N.; Do, D.; Marsh, S.E.; Abud, E.M.; et al. Activation of the STING-Dependent Type I Interferon Response Reduces Microglial Reactivity and Neuroinflammation. Neuron 2017, 96, 1290–1302.e1296.
- Sharma, M.; Rajendrarao, S.; Shahani, N.; Ramirez-Jarquin, U.N.; Subramaniam, S. Cyclic GMP-AMP synthase promotes the inflammatory and autophagy responses in Huntington disease. Proc. Natl. Acad. Sci. USA 2020, 117, 15989–15999.
- Benkhalifa, M.; Kasakyan, S.; Clement, P.; Baldi, M.; Tachdjian, G.; Demirol, A.; Gurgan, T.; Fiorentino, F.; Mohammed, M.; Qumsiyeh, M.B. Array comparative genomic hybridization profiling of first-trimester spontaneous abortions that fail to grow in vitro. Prenat. Diagn. 2005, 25, 894–900.
- Cavazza, T.; Takeda, Y.; Politi, A.Z.; Aushev, M.; Aldag, P.; Baker, C.; Choudhary, M.; Bucevicius, J.; Lukinavicius, G.; Elder, K.; et al. Parental genome unification is highly error-prone in mammalian embryos. Cell 2021, 184, 2860–2877.e2822.
- Zepeda-Mendoza, C.J.; Morton, C.C. The Iceberg under Water: Unexplored Complexity of Chromoanagenesis in Congenital Disorders. Am. J. Hum. Genet. 2019, 104, 565–577.
- Bertelsen, B.; Nazaryan-Petersen, L.; Sun, W.; Mehrjouy, M.M.; Xie, G.; Chen, W.; Hjermind, L.E.; Taschner, P.E.; Tumer, Z. A germline chromothripsis event stably segregating in 11 individuals through three generations. Genet. Med. 2016, 18, 494–500.
- Notta, F.; Chan-Seng-Yue, M.; Lemire, M.; Li, Y.; Wilson, G.W.; Connor, A.A.; Denroche, R.E.; Liang, S.B.; Brown, A.M.; Kim, J.C.; et al. A renewed model of pancreatic cancer evolution based on genomic rearrangement patterns. Nature 2016, 538, 378–382.
- Maura, F.; Bolli, N.; Angelopoulos, N.; Dawson, K.J.; Leongamornlert, D.; Martincorena, I.; Mitchell, T.J.; Fullam, A.; Gonzalez, S.; Szalat, R.; et al. Genomic landscape and chronological reconstruction of driver events in multiple myeloma. Nat. Commun. 2019, 10, 3835.
- Bolkestein, M.; Wong, J.K.L.; Thewes, V.; Korber, V.; Hlevnjak, M.; Elgaafary, S.; Schulze, M.; Kommoss, F.K.F.; Sinn, H.P.; Anzeneder, T.; et al. Chromothripsis in Human Breast Cancer. Cancer Res. 2020, 80, 4918–4931.
- Cortes-Ciriano, I.; Lee, J.J.; Xi, R.; Jain, D.; Jung, Y.L.; Yang, L.; Gordenin, D.; Klimczak, L.J.; Zhang, C.Z.; Pellman, D.S.; et al. Comprehensive analysis of chromothripsis in 2,658 human cancers using whole-genome sequencing. Nat. Genet. 2020, 52, 331–341.
- Voronina, N.; Wong, J.K.L.; Hubschmann, D.; Hlevnjak, M.; Uhrig, S.; Heilig, C.E.; Horak, P.; Kreutzfeldt, S.; Mock, A.; Stenzinger, A.; et al. The landscape of chromothripsis across adult cancer types. Nat. Commun. 2020, 11, 2320.
- Kwon, J.; Bakhoum, S.F. The Cytosolic DNA-Sensing cGAS-STING Pathway in Cancer. Cancer Discov. 2020, 10, 26–39.
- Bakhoum, S.F.; Ngo, B.; Laughney, A.M.; Cavallo, J.A.; Murphy, C.J.; Ly, P.; Shah, P.; Sriram, R.K.; Watkins, T.B.K.; Taunk, N.K.; et al. Chromosomal instability drives metastasis through a cytosolic DNA response. Nature 2018, 553, 467–472.
- Chen, Q.; Boire, A.; Jin, X.; Valiente, M.; Er, E.E.; Lopez-Soto, A.; Jacob, L.; Patwa, R.; Shah, H.; Xu, K.; et al. Carcinoma-astrocyte gap junctions promote brain metastasis by cGAMP transfer. Nature 2016, 533, 493–498.
- Fu, J.; Kanne, D.B.; Leong, M.; Glickman, L.H.; McWhirter, S.M.; Lemmens, E.; Mechette, K.; Leong, J.J.; Lauer, P.; Liu, W.; et al. STING agonist formulated cancer vaccines can cure established tumors resistant to PD-1 blockade. Sci. Transl. Med. 2015, 7, 283ra252.
- Lim, S.O.; Li, C.W.; Xia, W.; Cha, J.H.; Chan, L.C.; Wu, Y.; Chang, S.S.; Lin, W.C.; Hsu, J.M.; Hsu, Y.H.; et al. Deubiquitination and Stabilization of PD-L1 by CSN5. Cancer Cell 2016, 30, 925–939.
- Sheridan, C. Drug developers switch gears to inhibit STING. Nat. Biotechnol. 2019, 37, 199–201.
More
Information
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Revisions:
7 times
(View History)
Update Date:
15 Feb 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




