Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Aref Abbasi Moud | + 6272 word(s) | 6272 | 2022-02-10 07:21:57 | | | |
| 2 | Camila Xu | Meta information modification | 6272 | 2022-02-10 09:29:39 | | | | |
| 3 | Camila Xu | Meta information modification | 6272 | 2022-02-10 10:22:43 | | | | |
| 4 | Camila Xu | Meta information modification | 6272 | 2022-02-10 10:24:27 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Abbasi Moud, A. Cellulose and Microfluidics. Encyclopedia. Available online: https://encyclopedia.pub/entry/19304 (accessed on 07 February 2026).
Abbasi Moud A. Cellulose and Microfluidics. Encyclopedia. Available at: https://encyclopedia.pub/entry/19304. Accessed February 07, 2026.
Abbasi Moud, Aref. "Cellulose and Microfluidics" Encyclopedia, https://encyclopedia.pub/entry/19304 (accessed February 07, 2026).
Abbasi Moud, A. (2022, February 10). Cellulose and Microfluidics. In Encyclopedia. https://encyclopedia.pub/entry/19304
Abbasi Moud, Aref. "Cellulose and Microfluidics." Encyclopedia. Web. 10 February, 2022.
Copy Citation
Cellulose, a linear polysaccharide, is the most common and renewable biopolymer in nature.
microfluidics
CNC
cellulose
paper-based microfluidics
1. Introduction
The most prevalent and renewable biopolymer in nature is cellulose, which is a linear polysaccharide. Cellulose is an organic molecule with a formula comprising a polysaccharide composed of a linear polymer of hundreds or even thousands of connected D-glucose units. Cellulose is a structural component of the major cell wall of plants, many types of algae, and oomycetes [1][2][3]. This natural polymer cannot be melted (heated) or dissolved (in common organic solvents). By derivatized chemical modification or direct dissolving, cellulose can be converted into a processible liquid state [4]. As adsorbents, cellulose and cellulose derivatives have been utilized in the form of hydrogels [5][6], films [7][8], beads [9][10], microfibers [11], and microcrystals [12][13]. In all these applications, cellulose as a solid phase provides a large surface area that may separate chemicals from flowing liquids due to cellulose active functional groups. In chromatography [14], protein purification [15], and drug delivery [16][17][18][19], cellulose beads can be utilized as the stationary phase. Papermaking and the synthesis of micro fibrillated cellulose have both employed partially or considerably fibrillated cellulose. Micro fibrillated cellulose was created from wood using a high-pressure homogenization process [20] and has since been utilized as a filter aid as well as an excellent thickener [21]. In general, considerable energy consumption is unavoidable for the nanoscale fibrillation of wood or other cellulosed source items that need cleaves of interfibrillar hydrogen bonds [22].
Cellulose nanocrystals (CNCs) receive more research attention than their CNC counterparts (in this case micro fibrillated cellulose) [23][24]. The reason for this is because nanoparticles with their nanosized (higher surface area) have superior characteristics. The popularity of nanocellulose materials is continuously increasing. CNCs and nano fibrillated cellulose (CNF) (or alternatively cellulose nanofibrils) can be used in applications ranging from small-scale medical-grade items to larger-scale sorbent products. For instance, CNF shows promise for applications that need flexibility, such as possibly wearable electrochemical applications [25]. CNF-based aerogels are reasonably simple to make using freeze drying or critical point drying and have received a lot of attention [26]. To research material/cell interactions using CNFs, CNF-based nanocomposite hydrogels can be employed as sophisticated origami actuators. Artificial tissue, medical devices, diagnostics, and biosensors have all used these actuators [27]. Because of their ionic connections, CNF and poly ethylene glycol (PEG) can undergo a reversible sol gel transition when subjected to strain or temperature ramping [28].
The key attribute that cellulose-based goods provide to a matrix due to their elongated structure is their capacity to enhance mechanical capabilities [29][30]. For instance, enhancing mechanical properties of polymers [31][32][33][34][35], ceramics [36][37], etc. Aerogels made of chemically cross-linked nanostructured materials based on cellulose can be employed as flexible substrates for a variety of functional nanoparticles, including hydrophobic nutritional supplements and nanoparticles [38][39]. CNC aerogel nanostructures’ porous structure enables rapid water absorption and swelling via macropores and the macropillary action of mesopores; that makes this substrate ideal for separation and extraction [40]. CNCs have been linked with biopolymers using cross-linking chemistries to generate a reinforced hydrogel structure, a process that involves, for instance, borax [41]. Basic fibroblast growth factor was loaded into disposable gelatin microspheres, which were then integrated into porous collagen/CNC scaffolds, according to Li et al. [42]. Cotton nanofibrils on their own are more amenable to hydrogel production than CNCs. Dried CNC films with a helix inner structure are usually formed, for example, by depositing a suspension [43] onto a substrate and then drying it. The drying may be separated into many parts that are governed by geometry, the atmospheric partial pressure of water, and temperature. CNC division into liquid crystalline domains depends on aspect ratio and concentration of CNC based on Onsager theory [44][45]. Having stated that, specific applications based on CNC and CNF literature have been identified; there have previously been reviews on the individual subjects of CNF [46], micro fibrillated cellulose [47][48][49][50], cellulose nanocrystals [46][51][52], and cellulose nanocrystals in polymers [47][53][54] and prospective readers are recommended to study the reviews of these references (refs.).
Cellulose has a wide range of characteristics, including, but not limited to, gas barrier ability [55], as liquid crystal assembled structures [56][57][58][59], hydrogel-based templates [60], aerogels [61][62][63], and inks [64][65][66], and the ability to provide Pickering emulsion capability [67][68][69][70][71][72][73][74][75][76][77][78]. Moreover, additional modification such as the hydrophilization of cellulose-based aerogels has piqued the interest of researchers due to its potential in oil/water separations and organic pollutant entrapment [79]. It should be noted that several of the studies given can be classified as belonging to the same category, for example, inks can be classified as belonging to the hydrogel-based templates category.
Microfluidics is the science and technology of systems that are microscale integrated channels through which small quantities of liquid may flow and during which the flow and the material within can be controlled or altered in tandem [80][81]. The history of microfluidics may be traced back to an attempt to perform miniature biochemical analyses [82]. At the microfluidics scale, because the dimensions are small, the specific effects are augmented, resulting in behaviour that differs from that of macroscopic fluids. This causes viscous to inertial forces to become dominant [83], surface effects to become significant, and mass and heat transfer to become efficient [84]. For instance, the size of the particles being focused, a topic that will be covered later, is impacted heavily by inertial forces [85]. This size dependency can be advantageous for biological sample cleanup since smaller particles are sucked out, enhancing final sample purity, or minimizing bacterial contamination [86].
The use of microfluidics simplifies the existence and varied interaction of several phase fluids in a single “lab on chip” [87]. As a result of the characteristics listed, this intriguing subject has led the way for multidisciplined study in the physical, biological, chemical, and medical disciplines. In the production of nanoparticles, super control over reaction kinetics [88], as well as tuning and modifying thermodynamic parameters, can provide nanoparticles with customizable size and crystal structure.
Microfluidic devices can be used for causing the flow-induced orientation of cellulose, as a mixing zone [89], for emulsification (can come under the category of mixing), as a reactor such as acting as a glucose assay [90], and as an analytical tool, or cellulose itself can be used to make a microfluidic device [91]. The microcapsule emulsification approach includes mechanically shearing the system to generate a polydisperse mixture of droplets from the mixing of oil and water. This droplet creation has received much attention in recent decades since it allows for the generation of microparticles. Water-in-oil droplet microfluidics is used to create consistent spherical CNC droplets in a nontoxic and environmentally friendly manner. Following the evaporation of the water within the droplets, the molecular cross-linking of surface modified CNCs is accelerated. On the other hand, on a microfluidic chip, emulsification can occur through three broad designs of co-flow, fluid-focused flow, or the T- or Y-junction meeting of multiple flows [89][92][93][94][95].
2. Design of Cellulose with Microfluidics
In the literature, microfluidic technology has been employed to enhance the fabrication of cellulose-based parts. Figure 1 shows how microfluidics may be used to generate distinct shapes in cellulose products.
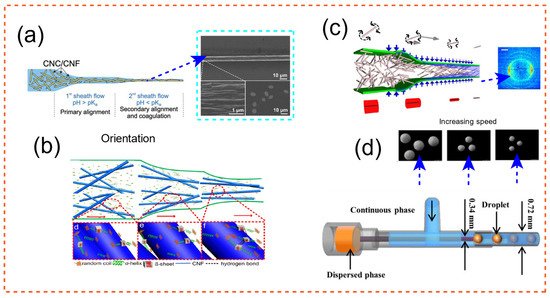
Figure 1. Cellulose shape formation using flow modulation of microfluidics [96][97][98][99]. (a) CNC–CNF joint orientation along channels of a microfluidic chip for particle production with optimized qualities. Adapted with permission from Ref. [99]. Copyright 2018 Wiley-VCH. (b) The assembly process of regenerated silk fibroin (RSF) fibrils suspended in RSF/CNF passing via a microfluidic channel is depicted. RSF and CNF are distributed; the majority of RSF molecules are in random-coil form. Adapted with permission from Ref. [96]. Copyright 2019 American Chemical Society. (c) The nanofibrils are focused in the channels, and a gelation agent (NaCl) is injected along the route to help the cellulose rods stay in place as they exit the channel. Adapted from Ref. [98]. (d) Microfluidics-based microparticle manufacturing with customizable final sizes. The setup for the experiment is shown at the bottom. Adapted from Ref. [97].
To create strong fibres from CNF and CNC, a continuous wet spinning technique based on microfluidic flow focusing has been devised. For the first time, fibres with an average breaking tenacity of 29.5 centi Newton per tex have been recorded. CNCs are an appealing building element for producing lightweight yet robust and flexible textiles due to their high strength and modulus. When CNCs are added to CNFs alone, the concentration of dope can be increased by 4 to 5 times [99] (See Figure 1a).
In Lu et al. [96], cellulose hydrogel was utilized to create a microfluidic device using a 3D printer. Indeed, silk fibroin and CNF hybrid fibres were dry-spun via a microfluidic chip that resembled the structure of a spider’s main ampullate gland in this work [96]. Many researchers have used nano-scale innovations, such as the use of titanium dioxide [100], graphene oxide [101], carbon nanotubes [102], and CNC, to improve the mechanical qualities of artificial silk. The authors’ research in Lu et al. [96] revealed that CNF may easily be used to enhance the mechanical properties of silk fibres. Stress at break of RSF/CNF with 0.1 wt% CNF was determined to be about 485 ± 106 MPa, representing a 58 percent increase over RSF fibres spun from silkworm (maximum recorder was 686 MPa). The method of integration of the two ingredients is depicted in Figure 1b. Spider silks have amazing mechanical qualities; hence, one of the areas of research in the field of biomimetic fibres has been the construction of high-performance artificial silk fibres as waveguides [103]. Strong fibres such as the one introduced here might be useful in biological media, bio-photonics, and central nervous system interfaces [104]. Similarly, in other refs., the direction and alignment of silk-spinning through microfluidic chips have been optimized through flow analysis [105][106][107]. This finding sets the path for further research into the demystification of the enigma of the natural spinning process. It offers a complete and methodical look at the process of creating highly oriented artificial fibres for biological applications [108]. In the development, regeneration and characterization of a blended system combining Bombyx Mori silk fibroin protein and cellulose acetate, a cellulose derivative, silk may be mixed with cellulose derivatives [109]. Many studies on the combination of silk and cellulose acetate for filament/fibre manufacturing may be found in the literature [96][104][108][110][111][112][113][114][115][116][117][118].
CNF, which has a lot of potential as a building component for biobased products, might need to have hydrodynamic alignment (alignment due to fluid-induced orientation) and a dispersion–gel transition involved in its process. Gelation can occur due to the introduction of NaCl, a coagulant that acts as a charge screener [2]. Knowing these two concepts, alignment and gradual gelation, led the author to design the microfluidic channel in Figure 1c. Based on mechanical examination, the filaments generated were shown to be more durable and stiffer than the precursor material, CNF, and equivalent CNF-based polymer nanocomposites in the literature [2]. The generated fibres are equally as tough and strong as cellulose pulp fibres when equal fibril orientation is used. Figure 1c depicts the assembly process for the design of this durable fiber. The cross section of the fibres is also represented as a diffractogram. The orientation of fibres as a function of residence time and shearing in the microfluidic channel was employed in all three studies listed above. It would have been ideal to assess the level of orientation using the plot introduced by Pignon et al. [119]; small-angle light scattering and small-angle X-ray scattering were used in this experiment. Cluster breakup may also be studied using a confocal setup because gelation is involved. Furthermore, utilizing rheology and theoretical models, the Folgar–Tucker orientation of fibres along the boundaries of the microfluidic setup and at the centre may be determined [31].
By merging microfluidic and flash-freezing methods [97], porous cellulose acetate microspheres with variable particle sizes and pore characteristics were effectively manufactured. These particles exhibited a large specific surface area and good adsorption properties. The diameter of the microspheres may be precisely adjusted by modifying the microfluidic settings. For oil, the developed porous structures were able to adsorb up to 30 times their weight, while for Congo red, they were able to adsorb up to 23.9 mg·g−1. A pictograph of the procedure is shown in Figure 1d. The setup for the experiment is also shown at the bottom. Staying on the subject of using the microsphere as a way of extraction/separation, paclitaxel, one of the natural anticancer drugs that can be isolated from the bark of pacific yew tree, was recognized, and separated in Wu et al. [120] using a sophisticated design of microspheres [120]. These examples demonstrate how microfluidics may be used to design structures that are entirely adjustable and suited for specific applications such as separation.
The highlights of recent research utilizing microfluidics in the development of cellulose-based goods are shown in Table 1.
Table 1. Presentation of research involving microfluidics in the creation of cellulose-based goods, as well as their highlights.
| Study | Application | Highlight |
|---|---|---|
| Baek and Park [89] | Creation of uniformly sized porous cellulose beads | The creation of the cell/N-methyl morpholine N-oxide droplet in the ethylene glycol solution in the T-junction microfluidic chip could not be observed in situ using an optical microscope. As a model study, the form of a cellulose bead after coagulation was explored. |
| Pepicelli et al. [121] | Creation of cellulose-based biodegradable microcapsules. | Gluconacetobacter xylinus may live and flourish in a variety of environments. Cellulose is a major constituent of these self-secreted protective coatings (made with Gluconacetobacter xylinus). The results achieved mark the first step toward the fabrication of self-assembled degradable cellulose capsule. |
| Duong et al. [122] | Cellulose fiber membrane was sandwiched between two silicone elastomer poly(dimethylsiloxane) (PDMS) layers to mimic BBB | In vitro, a microfluidic system was created to replicate the human blood–brain barrier (BBB). BBB formation was assessed using cell survival, actin filament (F-actin) formation, and transepithelial electrical resistance (TEER). Overall, the model showed a simple to duplicate and low-cost framework for in vitro drug test. |
| Jayapiriya and Goel [123] | Creation of paper-based energy harvesting device | Using E. coli as the biocatalyst, a paper fuel cell can generate 11.8 W·cm−2 of electricity. Fuel cell construction that is both cost-effective and thrifty can be utilized to power a wide range of low-power point-of-care devices. |
| Sharratt et al. [124] | Creation of hydrogel microparticles | Hydrogel microparticles (HMPs) have a wide range of practical uses, from medication delivery to tissue development. The kinetics of gelation fronts are initially determined using 1D microfluidic studies. The effective diffusive coefficients rise with Fe3+ content and drop with NaCMC concentration. |
| Chen et al. [125] | Creation of core–shell microparticles | Polysaccharides have been shown to be useful in medication encapsulation and delivery. Authors offered a multicompartment polysaccharide core–shell microparticle that may be used to build a long-lasting dual-release system of active molecules for wound healing. Microparticles reduced inflammation while also promoting granulation tissue development, collagen deposition, and angiogenesis. |
| Liu et al. [126] | Creation of monodisperse ethyl cellulose (EC) hollow microcapsules | A simple and new approach is used to effectively create monodisperse ethyl cellulose hollow microcapsules. Microfluidic double emulsification and solvent diffusion are used in this method. Microcapsules manufactured in an iso-osmotic environment have a flawless spherical form and no collapse. |
| Li et al. [127] | Using bacterial cellulose for wound healing | Bacterial cellulose is a type of nano-biomaterial that may be used in tissue engineering. It is unknown how bacterial cellulose’s nanoscale structure impacts skin wound healing. The lower portion of bacterial cellulose film can encourage cell migration to aid in wound healing. |
| Zhao et al. [128] | Creation of cellulose-based flexible electronics | Cellulose is a natural biopolymer with several benefits such as low cost, ease of processing, and degradability. It is extensively used in flexible electronics as a substrate, dielectric material, gel electrolyte, and derived carbon-made material. |
| Mahapatra et al. [129] | Creation of cellulose-based sensing devices | For its unique features, including biocompatibility, cellulose has the potential to be used in the creation of cytosensors, and organisms in a variety of materials. |
| Del Giudice et al. [130] | Assessing morphological structure of hydroxyethyl cellulose with microfluidics | Non-modified hydroxyethyl cellulose acts as a linear uncharged polymer when dissolved in water, with an entangled mass concentration of 0.3 wt%. For the first time, authors presented the concentrations scaling for hydroxy ethyl cellulose solutions with the longest relaxation period. |
| Zeng et al. [131] | CNFs produced by microfluidic homogenization | The purpose of this research was to investigate and compare the shape and rheology of cellulose nanofibrils derived from bleached softwood kraft pulp. CNFs had the greatest viscous, bulk modulus, and loss modulus, as well as the largest aspect ratio. |
| Wang et al. [132] | Creation of uniform size CNCs via microfluidic technology | CNC is a novel form of molecular substance derived from biomass. CNCs with a good dividend and consistency were achieved by hydrolysis process in a microfluidic system using a 60% sulfuric acid solution at 35 °C for 40 min. |
| Lari et al. [93] | Creation of poly(ε-caprolactone) and cellulose acetate nanoparticles | The purpose of this study was to compare two types of microfluidic-assisted nanoparticles (NPs) based on poly(-caprolactone) (PCL) and cellulose acetate (CA). It was discovered that CA NPs had a smaller average diameter (37 nm) and a lower polydispersity index (PDI) (0.035) than PCL NPs. |
| Carrick et al. [133] | Creation of cellulose capsules | For medication delivery or controlled release capsules, cellulose capsules with a limited size distribution might be advantageous. Capsules were carboxymethylated to make them pH responsive and to expand roughly 10% when the pH was changed from 3 to 10. |
| Pei et al. [134] | Cross-linked cellulose hydrogel was used for making a chip | To create cellulose–collagen hybrid hydrogels, collagen, a critical extracellular component for cell culture, was cross-linked in the cellulose hydrogel. Researchers revealed that they have excellent structural reproduction ability, physical qualities, and cell culture cytocompatibility. |
| Zhang et al. [135] | Creation of a technology for adsorption and isolation of nucleic acids on cellulose magnetic beads | The use of a 3D-printed microfluidic chip enables the extraction of nucleic acids without the need of vortexes or centrifuges. Magnetic, interfacial, and viscous drag forces are described inside the chip’s microgeometries. Across a variety of HPV plasmid levels, an overall extraction efficiency of 61% is reported. |
| Wenzlik et al. [136] | In a microfluidic setting, cholesteric particles were made from cellulose derivatives | Co-flowing injection of drops of liquid crystalline mixes of cellulose derivatives into microspheres on the micrometre scale is used in the process. |
| Miyashita et al. [137] | The diamagnetic director for microfluidic systems is made up of microcrystal-like cellulose fibrils | Cellulose is a potential material for the development of biogenic optical systems that imitate the unique optical capabilities of living creatures. In a microfluidic laboratory, magnetic orientation tests on microcrystalline cellulose were performed. During the dispersed light intensity process, light intensity altered depending on the direction of the magnetic field. |
| Chen et al. [138] | A multilayer microfluidic device with a PDMS–cellulose composite film was developed | This paper describes an integrated multilayer microfluidic system that can pre-treat raw samples and detect them using immunoassays. Using the crossflow concept, a polydimethylsiloxane (PDMS)-cellulose composite film was employed to extract plasma from raw samples. |
| Włodarczyk and Zarzycki [139] | On silica and cellulose micro-TLC plates, the chromatographic behaviour of chosen colours was studied | The chromatographic behaviour of 18 colourants, including amaranth, black PN, bromophenol blue, and bromocresol green, was investigated. Data were gathered using silica and cellulose-coated microplates under thermostatic settings (303 K). Dyes are frequently utilized as colourants in food and industry, as well as sensing compounds in analytical and medicinal purposes. |
| Ghorbani et al. [140] | Creation of CNF- stabilized perfluoro droplets | In a variety of applications, hydrodynamic cavitation on microchips has been emphasised. Cavitating flow patterns may be used to promote a wide range of industrial and technical applications. Inside microfluidic devices, a novel technique involving cellulose nanocomposites perfluoro droplets was tested. |
| Park et al. [141] | Wet-spun microcomposite filaments were made with cellulose | To make microfilaments, cellulose nanocrystals were wet spun in a coagulation bath. The influence of sodium alginate on the characteristics of the micro composite filament was studied. The higher spinning rate of sodium alginate generated a rise in the alignment index of CNCs, leading to an improvement in the material’s tensile characteristics. |
| Grate et al. [142] | Creation of Alexa Fluor-labeled fluorescent CNCs | A group of researchers discovered a mechanism to attach Alexa Fluor dyes to cellulose nanocrystals while preserving the nanocrystal’s overall structure. Bioimaging tests revealed that the spatial positioning of solid cellulose deposits could be detected and their elimination over time under the action of Celluclast® enzymes or microorganisms could be monitored. |
| Ke et al. [143] | Microgels made from carboxymethyl cellulose for cell encapsulation | Carboxy methyl cellulose was modified with 4-hydroxybenzylamine (CMC-Ph) to create carboxy methyl cellulose-based microgels for use in scaffolds. The ATDC5 chondrocytic cell line was grown for up to 40 days after being encased in carboxy methyl cellulose microgels. |
| Rao et al. [144] | Creation of microfluidic paper fuel cell | MMPFCs (Membraneless Microfluidic Paper Fuel Cells) are promising technologies for harvesting energy for a variety of portable applications. Because of the built-in co-laminar flow and integrated capillary, the devices remove the need for membranes and additional pumps. |
| Shen et al. [145] | Creation of paper-based microfluidic fuel cells | Microfluidic fuel cells made of paper are emerging as possible renewable energy sources for small-scale electronic systems. The textural qualities of the paper channels have a considerable impact on the performance of paper fuel cells. The use of paper with a bigger mean pore width may result in a greater peak power density and open circuit voltage. |
| Shefa et al. [146] | A method of incorporation of curcumin (Cur) into a hydrogel system based on cellulose was developed | A freeze–thaw technique was used to create a Cur including physically crosslinked TEMPO-oxidized CNC–polyvinyl–alcohol curcumin– hydrogel, that produced curcumin to speed wound healing. L929 fibroblast cells incorporated curcumin within 4 h of incubation, according to in vitro experiments. |
| Chen et al. [147] | Separation of glycoproteins was achieved using bacterial cellulose microfluidic column | A simple technique was used to produce a regenerated bacterial cellulose column containing concanavalin A (Con A) lectin immobilised in a microfluidic device to evaluate and separate glycoproteins. Schiff-base formation was used to covalently link lectin Con A to the RBC matrix surface. |
Table 1 displays a presentation of research involving microfluidics in the creation of cellulose-based goods, as well as their highlights. As a recap of Table 1, a study of silk-spinning through microfluidic chips has uncovered the secrets of the natural spinning process [104], a study easily extendable to cellulose. Paper as a substrate aids in reducing existing stiff wastes and inevitable pollution [90]. Polysaccharides have been shown to be useful in medication encapsulation and delivery. Using E. coli as a biocatalyst, a paper fuel cell can generate 11.8 W·cm−2 of electricity using paper cells [123]. Membrane-less Microfluidic Paper Fuel Cells are promising technologies for harvesting energy. H2O2 is used as both fuel and oxidant in a paper-based microfluidic fuel cell for portable electronics. The fuel cell does not require precious-metal catalysts, and the fuel utilized is carbon free and environmentally friendly [148], with a peak energy capacity of 0.88 mW·cm−2.
A 3D-printed microfluidic chip allows for nucleic acid extraction without the need of vortexes or centrifuges [149]. Inside the chip’s microgeometries, magnetic, interfacial, and viscous drag forces are defined. Cavitating flow patterns have the potential to be utilized to promote a wide range of industrial and technological applications. In a coagulation bath, cellulose nanocrystals were wet spun [141]. The effect of sodium alginate on the properties of the micro composite filament was investigated. Bioimaging experiments demonstrated that solid cellulose deposits may be recognized in their spatial location [142].
3. Cellulose as a Microfluidic Building Block
We offered a generalization on the issue of microfluidics and cellulose in the preceding section. The use of cellulose as a microfluidics building component will be discussed here. Paper, elastomer, thermosets, silicon/glass, thermoplastics, and hydrogels are some of the materials that may be used to make microfluidics chips [150]. Here, we focus on paper-based microfluidics.
Paper-based microfluidics, often known as “lab on paper,” is a revolutionary fluid management and analysis technology. The system is said to be low-cost, simple to use, disposable, and requires no equipment. Indeed, paper is an appealing substrate for these devices since it is omnipresent, ubiquitous, and incredibly inexpensive. As a result, the material is also compatible with a wide range of additional chemical, biomedical, biomedical, biochemical, and medicinal applications. It transfers liquid through capillary forces without the help of any external forces. Microfluidic paper-based analytical devices, for example, may be utilized to measure the concentration of various analytes in a solution while also serving as an excellent platform for point-of-care diagnostics (dubbed as POC). Furthermore, it has found use in water quality analysis, as water pollution is harmful to human health. In Chen et al. [151], a layered multilayer electrostatic printing approach for manufacturing nanofiber-based microfluidic chips for water quality analysis was created. Devices provide easy fabrication techniques, flexible prototyping, mass production possibilities, and multi-material integration.
As stated earlier, cellulose is a plentiful natural solid carbohydrate biopolymer that is vital to the biosphere and plays an important role in the global carbon budget [152]. The use of cellulose-derived nanoparticles for cell imaging, material science, sensors, and other medical applications is gaining popularity [152]. One application for cellulose is as a component in the manufacture of microfluidic chips. Overall, few procedures for developing microfluidic devices, photolithography [153][154][155], plotting using a plotter [156], etching [157][158][159], plasma [160], cutting [161][162] and wax printing [163][164][165], flexography printing [166], screen printing [167], and laser treatment [168] have been documented. These approaches can be utilized to make microfluidic devices; to classify them, photolithography, etching, spraying, screen printing, and dipping wax are examples of indirect patterning processes, whereas wax priming, plotting, flexography, writing, stamping, and inkjet printing are examples of direct patterning methods.
Figure 2 shows the technology involved in patterning a PAD, including 3D printing, wax printing, flexography printing, cutting, photolithography, and plotting.
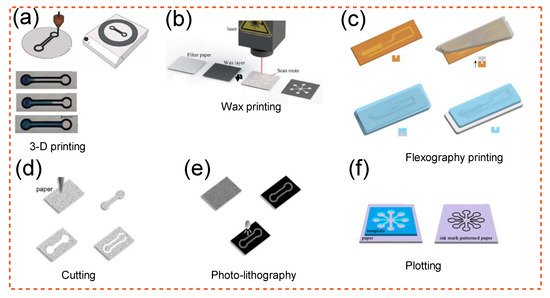
Figure 2. Methods of patterning a PAD [169][170][171][172][173]: (a) 3D printing; Adapted from Ref. [169]. (b) wax printing; Adapted from Ref. [170]. (c) flexography printing; Adapted with permission from Ref. [172]. Copyright 2019 Elsevier. (d) cutting; Adapted with permission from Ref. [171]. Copyright 2015 Wiley-VCH. (e) photo-lithography; Adapted with permission from Ref. [171]. Copyright 2015 Wiley-VCH. (f) plotting; Adapted with permission from Ref. [173]. Copyright 2016 Springer-Verlag. These strategies are described in detail in the main text.
To explain some of the methods briefly: Wax printing uses a basic printer to design wax on paper, after which the wax is melted to produce channels. This process is quick but offers limited resolution due to the isotropy of melted wax [164]. A wax layer creates the hydrophobic boundaries that are needed to guide the flow of a hydrophilic liquid. Inkjet printing involves coating paper with a hydrophobic polymer and then applying an ink that selectively etches the polymer to allow the paper to emerge [171]. Photolithography is comparable to inkjet printing in that etching is accomplished using a photomask and a photoresist polymer [155]. Using a hydrophilizing agents such as fluorocarbon plasma polymerization, the paper first becomes hydrophobic, and then oxygen plasma etching is used to form hydrophilic patterns onto the paper [174]. In flexographic printing, the process involves the usage of conventional graphic printing, functional inks, and a substrate such as paper. Flexography, inkjet printing, wax printing, and 3D printing all have striking parallels in this regard. Filling the vacuum with a hydrophobic substance, such as a solid melted at a certain temperature or a hydrophobic polymer immersed in an organic solvent, is another approach for creating hydrophilic structures on paper. These materials may easily penetrate the porous network in their liquid state and form a barrier once solidified. For applications that demand portable yet small fluid handling, microfluidics parts made by 3D printing with paper as part of the operation is of great use. A 3D printer can also be used to produce hybrid channels. This technology is inexpensive and suited for household usage because it offers accurate fluid handling abilities, functionality (versatile), and user-friendliness [169]. A depiction of 3D-printed microfluidics is shown in Figure 2a. Some examples of other methods mentioned earlier are also depicted in Figure 2b–f.
Aside from selecting a good technique for microfluidic paper-based manufacturing, it is also critical to pick a material that can go through the process. Cellulose and cellulose derivatives are suitable materials for 3D printing; nevertheless, finding strong cellulose solvents is crucial for their efficient use because cellulose cannot be melted (processed). However, due to strong hydrogen bonding, cellulose is also insoluble in water and other organic solvents. Only a few effective solvent systems capable of dissolving cellulose have been discovered thus far. As a result, researchers discovered functionalization processes such as xanthation [175], esterification [176], and etherification [177] on the cellulose hydroxyl group as a method of disrupting hydrogen bonds and breaking cellulose’s tenacity to dissolve. However, non-derivatizing solvents such as ionic liquid [178] can also dissolve cellulose without requiring chemical changes, which is advantageous in many instances [179].
The most significant cellulose derivatives are cellulose ethers and esters [177]. These are found in a variety of goods, including thickeners, binders [180], emulsifiers, coatings, and membranes. The esterification of cellulose allows for the transformation of cellulose into different forms [181]. Cellulose ethers are plentiful, low-cost, environmentally friendly compounds with exceptional characteristics. They have several uses in food, medicines, cosmetics, and other commercial items. They are also commonly employed in 3D printing, where they serve several purposes [182][183]. The properties of ink are vital in 3D printing; specifically, 3D printing ink requires a well-regulated viscoelastic response (such as high viscosity and shear thinning behaviour) [184]. The shear thinning properties of polymer solutions are frequently used to achieve this objective [185][186]. These expected rheological behaviours can be obtained using cellulose ethers. Cellulose ether has been used to change the viscosity of a variety of industrial products [187]. However, when an external force is applied, the mixing energy will break the hydrogen bonds between the cellulose chains, causing the chain to align in the low direction, as seen by the shear thinning of the pseudoplastic behaviour [188]. The qualities of cellulose ether solution are thus sought since they are low at greater shear rates and high when the flow is halted. Furthermore, these materials are thixotropic [182], which is advantageous for becoming an ink since it necessitates the rehabilitation of the structure following fracture via the nozzle. Table 2 contains a substantial amount of the literature devoted to the development of cellulose-based microfluidic devices that can showcase the objective behind developing such systems.
Table 2. Presentation of research in which cellulose was employed as a chip-building material.
| Study | Highlights |
|---|---|
| Lin et al. [189] | Three-dimensional microfluidic paper-based analytical devices (3D-µPADs) are a potential platform technology that enables for complicated fluid manipulation, parallel sample distribution, high throughput, and multiplex analysis assays. This technology can regulate the penetration depth of melted wax printed on both sides of a paper substrate, resulting in multilayer patterned channels in the substrate. |
| Martinez et al. [190] | A novel family of point-of-care diagnostic devices is PADs. They are affordable, simple to operate, and particularly developed for usage in poor nations. When completely developed, they may deliver faster and less expensive bioanalyses. |
| Yamada et al. [191] | On microfluidic PADs, “distance-based” detection patterns provide quantitative analysis without the need of signal output tools. Quantitative analysis is enabled by the distance-based quantified signal and the strong batch-to-batch production repeatability based on printing processes. |
| Li and Liu [192] | A wax-printing process is used to create 3D microfluidic channels inside a single sheet of cellulose paper. It enabled the production of up to four layers of paper channels in a 315-micrometer-thick substrate surface without the need for process optimization. |
| Ardalan et al. [193] | The smart wearable sweat patch (SWSP) is a non-invasive and in situ multi-sensing sweat biomarkers sensor that measures glucose, lactate, pH, chloride, and volume. A smartphone app was also created to use a detection algorithm to estimate the quantity of biomarkers. |
| Arun et al. [194] | The capillary-driven fluid flow of a combination of fuel and electrolyte drives the capillary-driven fluid flow of a microfluidic fuel cell. Various pencils are used to produce the graphite electrodes to study their influence on fuel cell performance. To improve performance, the paper fuel cell was also manufactured in different diameters and coupled as cell stacks. |
| Yan et al. [148] | H2O2 is used as both fuel and oxidant in a paper-based microfluidic fuel cell for portable electronics. It does have a peak energy capacity of 0.88 mW·cm−2. The fuel cell does not require precious-metal catalysts, and the fuel utilized is carbon free and environmentally friendly. |
| del Torno-de Román et al. [195] | The power and output current of a paper-based enzyme glucose/O2 fuel cell can be enhanced by adopting a quasi-steady flow. The fuel cell’s anode and cathode are composed of display carbon electrodes that have been correctly functionalized with protease inks. |
| Jia et al. [196] | Because of its hydrophilic properties, cellulose paper has been widely employed in microfluidic devices. Cellulose is placed in paper at random, with no specific direction or pathways. White wood possesses natural microchannels as well as a quick and anisotropic liquid and big solid particle movement. |
| Cai et al. [90] | By silanizing filter cellulose using a paper mask, authors created a new, low-cost, and straightforward approach for fabricating PADs. This procedure requires no expensive equipment and may be carried out by inexperienced persons. |
| Murase et al. [197] | Cellulose nanofiber can be utilized as a component in PADs. The thixotropic characteristic of TEMPO-oxidized CNCs aqueous dispersion allowed for inkjet printability, which aided manufacturing. |
| Kumar et al. [198] | Cancer diagnostics are not currently prioritised in resource-limited settings. However, budget-friendly and targeted screening test and diagnostic tools are in great need. Multi-layer cellulose nanofibril-based coverings on expendable microfluidics were tuned for targeted capture and efficient release of target cells. |
| Choi et al. [199] | The microfluidic cellulose microfibre chip was prototyped by injecting 10 percent CM solutions onto CNC-milled substrates. It can identify exudative age-related macular degeneration in human aqueous sense organs. |
| Fu and Liu [200] | PADs are typically mounted on a cellulose paper substrate. Covalent bonds with the target biomolecule can be achieved by modifying chemicals. The optimum performance for biosensing applications comes from potassium periodate (KIO4)-modified cellulose paper. |
| Lu et al. [104] | Spider silks have amazing mechanical qualities; hence, one of the areas of research in biomimetic fibres was the construction of structures with high silk fibres as optical waveguides. The fibres might be useful in biological media, bio photonics, and central nervous system interfaces. |
| Bao et al. [201] | Transistors built of van der Waals materials are allowed by an all-cellulose paper with CNF on the upper surface, which results in outstanding surface roughness and electrolyte absorption. These planar transistors can be employed as sensors in PADs, together with other components. |
| Yadav et al. [202] | Microfluidics has the potential to revolutionise point-of-care detection in smart healthcare. Paper as a substrate aids in reducing existing stiff wastes and inevitable pollution. Flexible microfluidic technology hardcopy provides a low-cost technical foundation for next-generation intelligent sensors. |
| Solin et al. [203] | Point-of-care diagnosis can benefit from microfluidic technologies. Authors looked at the fluidic structure due to stencil painting on flexible surfaces. Combining minerals with cellulose fibrils resulted in optimal printability and flow profiles. The findings demonstrate the use of these pathways for drug and chemical analysis. |
As a recap of Table 2, PADs have been developed for sub-microliter surface area/volume analysis. The wax-printing technology that was previously used to design paper substrates has been improved to make high-resolution designs patterned in filter paper. In recent years, paper-based microfluidics used for analytical purposes, also known as PADs, have attracted a lot of interest for carrying out a variety of traditional analytical activities. PADs’ appealing characteristics are mostly due to them being made of paper (cellulose), which is inexpensive, readily disposable, and environmentally benign. Three-dimensional paper-based microfluidics with three layer channels made from a paper-made substrate demonstrates the enzymatic detection of biomarkers such as glucose, lactate and uric acid [192]. According to the ISI Web of Knowledge data collection, the market for these types of devices has been steadily growing, as seen by 942 publications published under the title microfluidic paper-based between the years 2018 and 2022. Clearly, the trend indicates the future growth of PADs.
Figure 3 depicts a brief overview of the use of cellulose in the creation of microfluidic chips of varying scale, size, shape, and design. The technique of transport depends on hydrophilic cellulose or nitrocellulose fibres to transfer liquid from an input guided through a porous medium via capillary action. The benefit of paper-based microfluidics is their passively controlled activity, which distinguishes them from more sophisticated microfluidic designs. The following regions are found in paper-based devices: an inlet in a substrate that is commonly constructed of cellulose where liquid is manually dispersed, a channel in which a hydrophilic network controls liquid transport, and a flow amplifier in which flow velocity is impacted to impart a controlled velocity to flowing liquid. A flow resistor is a capillary element that imparts a lowered flow velocity to control residence time, a barrier is a wall that prevents liquid from penetrating out of the channels, and an outlet is a location where a chemical or biological reaction occurs. For instance, in Figure 3a,c, the μPAD is divided into three parts: sensing, substrate, and water addition. The distance between the regions of addition of water and substrate was designed to be 12 mm, while the area of sensing was estimated to be 11 mm.
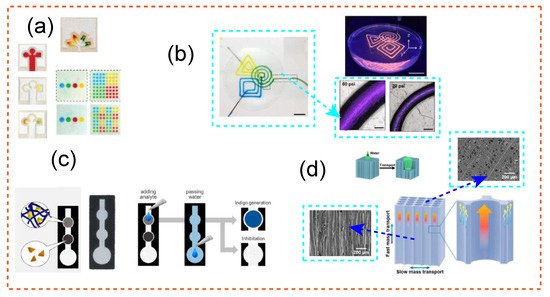
Figure 3. (a) Photolithographic devices for measuring glucose and protein. Adapted with permission from Ref. [190]. Copyright 2010 American Chemical Society. (b) As a supporting material embedding for the microchannels, cellulose nanofibrils hydrogel, a 3D structuring ultrathin film, was employed. Adapted with permission from Ref. [204]. Copyright 2017 American Chemical Society. (c) The μPAD is divided into three parts: sensing (6.5 mm diameter), substrate (6.5 mm diameter), and water addition (11 mm diameter). The distance between the regions of addition of water and substrate was calculated to be 12 mm, while the area of sensing was estimated to be 11 mm. Adapted with permission from Ref. [197]. Copyright 2018 American Chemical Society. (d) Scanning electron microscopy (SEM) photos of white wood microchannels are shown, as well as high magnification SEM photographs of individual microchannels, to demonstrate the presence of pits with an average diameter of 2.5 μm in addition to obstructed mass transmission that these designs can offer. Adapted with permissions from Ref. [196]. Copyright 2018 American Chemical Society.
References
- Gahrooee, T.R.; Moud, A.A.; Danesh, M.; Hatzikiriakos, S.G. Rheological Characterization of CNC-CTAB Network below and above Critical Micelle Concentration (CMC). Carbohydr. Polym. 2021, 257, 117552.
- Moud, A.A.; Arjmand, M.; Yan, N.; Nezhad, A.S.; Hejazi, S.H. Colloidal behavior of cellulose nanocrystals in presence of sodium chloride. ChemistrySelect 2018, 3, 4969–4978.
- Moud, A.A.; Arjmand, M.; Liu, J.; Yang, Y.; Sanati-Nezhad, A.; Hejazi, S.H. Cellulose nanocrystal structure in the presence of salts. Cellulose 2019, 26, 9387–9401.
- Chen, Y.; Yu, H.-Y.; Li, Y. Highly Efficient and Superfast Cellulose Dissolution by Green Chloride Salts and Its Dissolution Mechanism. ACS Sustain. Chem. Eng. 2020, 8, 18446–18454.
- Thakur, M.; Sharma, A.; Ahlawat, V.; Bhattacharya, M.; Goswami, S. Process optimization for the production of cellulose nanocrystals from rice straw derived α-cellulose. Mater. Sci. Energy Technol. 2020, 3, 328–334.
- Xing, X.; Li, W.; Zhang, J.; Wu, H.; Guan, Y.; Gao, H. TEMPO-oxidized cellulose hydrogel for efficient adsorption of Cu2+ and Pb2+ modified by polyethyleneimine. Cellulose 2021, 28, 7953–7968.
- Ayouch, I.; Kassem, I.; Kassab, Z.; Barrak, I.; Barhoun, A.; Jacquemin, J.; Draoui, K.; El Achaby, M. Crosslinked carboxymethyl cellulose-hydroxyethyl cellulose hydrogel films for adsorption of cadmium and methylene blue from aqueous solutions. Surf. Interfaces 2021, 24, 101124.
- Bayramoglu, G.; Arica, M.Y. Grafting of regenerated cellulose films with fibrous polymer and modified into phosphate and sulfate groups: Application for removal of a model azo-dye. Colloids Surf. A Physicochem. Eng. Asp. 2021, 614, 126173.
- Zhao, H.; Ouyang, X.-K.; Yang, L.-Y. Adsorption of lead ions from aqueous solutions by porous cellulose nanofiber–sodium alginate hydrogel beads. J. Mol. Liq. 2021, 324, 115122.
- Duan, J.; He, X.; Zhang, L. Magnetic cellulose–TiO2 nanocomposite microspheres for highly selective enrichment of phosphopeptides. Chem. Commun. 2015, 51, 338–341.
- Laib, R.; Amokrane-Nibou, S.; Dahdouh, N.; Mansouri, T.E.M.; Rekhila, G.; Trari, M.; Nibou, D. Removal of the cationic textile dye by Recycled newspaper pulp and its cellulose microfibers extracted: Characterization, release, and adsorption studies. Iran. J. Chem. Chem. Eng. 2021, 40, 133–141.
- Lin, W.-H.; Jana, S.C. Analysis of porous structures of cellulose aerogel monoliths and microparticles. Microporous Mesoporous Mater. 2021, 310, 110625.
- Selman, M.H.; Hemayatkar, M.; Deelder, A.M.; Wuhrer, M. Cotton HILIC SPE microtips for microscale purification and enrichment of glycans and glycopeptides. Anal. Chem. 2011, 83, 2492–2499.
- Mwandira, W.; Nakashima, K.; Togo, Y.; Sato, T.; Kawasaki, S. Cellulose-metallothionein biosorbent for removal of Pb (II) and Zn (II) from polluted water. Chemosphere 2020, 246, 125733.
- Buruaga-Ramiro, C.; Valenzuela, S.V.; Valls, C.; Roncero, M.B.; Pastor, F.J.; Díaz, P.; Martínez, J. Bacterial cellulose matrices to develop enzymatically active paper. Cellulose 2020, 27, 3413–3426.
- Yeap, E.W.; Ng, D.Z.; Prhashanna, A.; Somasundar, A.; Acevedo, A.J.; Xu, Q.; Salahioglu, F.; Garland, M.V.; Khan, S.A. Bottom-up structural design of crystalline drug-excipient composite microparticles via microfluidic droplet-based processing. Cryst. Growth Des. 2017, 17, 3030–3039.
- Wsoo, M.A.; Shahir, S.; Bohari, S.P.M.; Nayan, N.H.M.; Abd Razak, S.I. A review on the properties of electrospun cellulose acetate and its application in drug delivery systems: A new perspective. Carbohydr. Res. 2020, 491, 107978.
- Khine, Y.Y.; Stenzel, M.H. Surface modified cellulose nanomaterials: A source of non-spherical nanoparticles for drug delivery. Mater. Horiz. 2020, 7, 1727–1758.
- Wei, S.; Ching, Y.C.; Chuah, C.H. Preparation of aerogel beads and microspheres based on chitosan and cellulose for drug delivery: A review. Int. J. Biol. Macromol. 2021, 170, 751–761.
- Herrick, F.W.; Casebier, R.L.; Hamilton, J.K.; Sandberg, K.R. Microfibrillated cellulose: Morphology and accessibility. In Journal of Applied Polymer Science: Applied Polymer Symposium (United States); ITT Rayonier Inc.: Shelton, WA, USA, 1983.
- Blok, A.E.; Bolhuis, D.P.; Kibbelaar, H.V.; Bonn, D.; Velikov, K.P.; Stieger, M. Comparing rheological, tribological and sensory properties of microfibrillated cellulose dispersions and xanthan gum solutions. Food Hydrocoll. 2021, 121, 107052.
- Ji, Q.; Yu, X.; Yagoub, A.E.-G.A.; Chen, L.; Zhou, C. Efficient cleavage of strong hydrogen bonds in sugarcane bagasse by ternary acidic deep eutectic solvent and ultrasonication to facile fabrication of cellulose nanofibers. Cellulose 2021, 28, 6159–6182.
- Wei, X.; Lin, T.; Duan, M.; Du, H.; Yin, X. Cellulose nanocrystal-based liquid crystal structures and the unique optical characteristics of cellulose nanocrystal films. BioResources 2021, 16, 2116.
- Teh, K.C.; Foo, M.L.; Ooi, C.W.; Chew, I.M.L. Sustainable and cost-effective approach for the synthesis of lignin-containing cellulose nanocrystals from oil palm empty fruit bunch. Chemosphere 2021, 267, 129277.
- Zhao, X.; Zhao, C.; Jiang, Y.; Ji, X.; Kong, F.; Lin, T.; Shao, H.; Han, W. Flexible cellulose nanofiber/Bi2Te3 composite film for wearable thermoelectric devices. J. Power Sources 2020, 479, 229044.
- Wang, Z.; Zhu, W.; Huang, R.; Zhang, Y.; Jia, C.; Zhao, H.; Chen, W.; Xue, Y. Fabrication and characterization of cellulose nanofiber aerogels prepared via two different drying techniques. Polymers 2020, 12, 2583.
- Wang, Y.; Huang, W.; Wang, Y.; Mu, X.; Ling, S.; Yu, H.; Chen, W.; Guo, C.; Watson, M.C.; Yu, Y. Stimuli-responsive composite biopolymer actuators with selective spatial deformation behavior. Proc. Natl. Acad. Sci. USA 2020, 117, 14602–14608.
- Yang, J.; Zhang, X.; Ma, M.; Xu, F. Modulation of assembly and dynamics in colloidal hydrogels via ionic bridge from cellulose nanofibrils and poly (ethylene glycol). ACS Macro Lett. 2015, 4, 829–833.
- Abbasi Moud, A. Gel Development Using Cellulose Nanocrystals. Ph.D. Thesis, Univeristy of Calgary, Calgary, AB, Canada, 2020.
- Moud, A.A.; Kamkar, M.; Sanati-Nezhad, A.; Hejazi, S.H.; Sundararaj, U. Nonlinear viscoelastic characterization of charged cellulose nanocrystal network structure in the presence of salt in aqueous media. Cellulose 2020, 27, 5729–5743.
- Moud, A.A.; Kamkar, M.; Sanati-Nezhad, A.; Hejazi, S.H.; Sundararaj, U. Viscoelastic properties of poly (vinyl alcohol) hydrogels with cellulose nanocrystals fabricated through sodium chloride addition: Rheological evidence of double network formation. Colloids Surf. A Physicochem. Eng. Asp. 2021, 609, 125577.
- Mao, H.; Wei, C.; Gong, Y.; Wang, S.; Ding, W. Mechanical and water-resistant properties of eco-friendly chitosan membrane reinforced with cellulose nanocrystals. Polymers 2019, 11, 166.
- Ferreira, F.; Pinheiro, I.; Gouveia, R.; Thim, G.; Lona, L. Functionalized cellulose nanocrystals as reinforcement in biodegradable polymer nanocomposites. Polym. Compos. 2018, 39, E9–E29.
- Xia, W.; Qin, X.; Zhang, Y.; Sinko, R.; Keten, S. Achieving enhanced interfacial adhesion and dispersion in cellulose nanocomposites via amorphous interfaces. Macromolecules 2018, 51, 10304–10311.
- Chowdhury, R.A.; Clarkson, C.M.; Shrestha, S.; El Awad Azrak, S.M.; Mavlan, M.; Youngblood, J.P. High-performance waterborne polyurethane coating based on a blocked isocyanate with cellulose nanocrystals (CNC) as the polyol. ACS Appl. Polym. Mater. 2019, 2, 385–393.
- Biswas, P.; Mamatha, S.; Naskar, S.; Rao, Y.S.; Johnson, R.; Padmanabham, G. 3D extrusion printing of magnesium aluminate spinel ceramic parts using thermally induced gelation of methyl cellulose. J. Alloy. Compd. 2019, 770, 419–423.
- Hudelja, H.; Konegger, T.; Wicklein, B.; Čretnik, J.; Akhtar, F.; Kocjan, A. Freeze-casting of highly porous cellulose-nanofiber-reinforced γ-Al2O3 monoliths. Open Ceram. 2021, 5, 100069.
- Yang, X.; Cranston, E.D. Chemically cross-linked cellulose nanocrystal aerogels with shape recovery and superabsorbent properties. Chem. Mater. 2014, 26, 6016–6025.
- de Morais Zanata, D.; Battirola, L.C.; do Carmo Gonçalves, M. Chemically cross-linked aerogels based on cellulose nanocrystals and polysilsesquioxane. Cellulose 2018, 25, 7225–7238.
- Zhu, H.; Yang, X.; Cranston, E.D.; Zhu, S. Flexible and porous nanocellulose aerogels with high loadings of metal–organic-framework particles for separations applications. Adv. Mater. 2016, 28, 7652–7657.
- Han, J.; Lei, T.; Wu, Q. Facile preparation of mouldable polyvinyl alcohol-borax hydrogels reinforced by well-dispersed cellulose nanoparticles: Physical, viscoelastic and mechanical properties. Cellulose 2013, 20, 2947–2958.
- Li, W.; Lan, Y.; Guo, R.; Zhang, Y.; Xue, W.; Zhang, Y. In vitro and in vivo evaluation of a novel collagen/cellulose nanocrystals scaffold for achieving the sustained release of basic fibroblast growth factor. J. Biomater. Appl. 2015, 29, 882–893.
- Park, J.H.; Noh, J.; Schütz, C.; Salazar-Alvarez, G.; Scalia, G.; Bergström, L.; Lagerwall, J. Macroscopic control of helix orientation in films dried from cholesteric liquid crystalline cellulose nanocrystal suspensions. Chemphyschem A Eur. J. Chem. Phys. Phys. Chem. 2014, 15, 1477–1484.
- Stephen, M.J.; Straley, J.P. Physics of liquid crystals. Rev. Mod. Phys. 1974, 46, 617.
- Honorato-Rios, C.; Lehr, C.; Schütz, C.; Sanctuary, R.; Osipov, M.A.; Baller, J.; Lagerwall, J.P. Fractionation of cellulose nanocrystals: Enhancing liquid crystal ordering without promoting gelation. NPG Asia Mater. 2018, 10, 455–465.
- De France, K.J.; Hoare, T.; Cranston, E.D. Review of hydrogels and aerogels containing nanocellulose. Chem. Mater. 2017, 29, 4609–4631.
- Siró, I.; Plackett, D. Microfibrillated cellulose and new nanocomposite materials: A review. Cellulose 2010, 17, 459–494.
- Lavoine, N.; Desloges, I.; Dufresne, A.; Bras, J. Microfibrillated cellulose–Its barrier properties and applications in cellulosic materials: A review. Carbohydr. Polym. 2012, 90, 735–764.
- Osong, S.H.; Norgren, S.; Engstrand, P. Processing of wood-based microfibrillated cellulose and nanofibrillated cellulose, and applications relating to papermaking: A review. Cellulose 2016, 23, 93–123.
- Sandquist, D. New horizons for microfibrillated cellulose. Appita Technol. Innov. Manuf. Environ. 2013, 66, 156–162.
- Vanderfleet, O.M.; Cranston, E.D. Production routes to tailor the performance of cellulose nanocrystals. Nat. Rev. Mater. 2021, 6, 124–144.
- Huang, C.; Yu, H.; Abdalkarim, S.Y.H.; Li, Y.; Chen, X.; Yang, X.; Zhou, Y.; Zhang, L. A comprehensive investigation on cellulose nanocrystals with different crystal structures from cotton via an efficient route. Carbohydr. Polym. 2021, 276, 118766.
- Shojaeiarani, J.; Bajwa, D.S.; Chanda, S. Cellulose Nanocrystal Based Composites: A Review. Compos. Part C Open Access 2021, 5, 100164.
- Miao, C.; Hamad, W.Y. Critical insights into the reinforcement potential of cellulose nanocrystals in polymer nanocomposites. Curr. Opin. Solid State Mater. Sci. 2019, 23, 100761.
- Chowdhury, R.A.; Nuruddin, M.; Clarkson, C.; Montes, F.; Howarter, J.; Youngblood, J.P. Cellulose nanocrystal (CNC) coatings with controlled anisotropy as high-performance gas barrier films. ACS Appl. Mater. Interfaces 2018, 11, 1376–1383.
- De La Cruz, J.A.; Liu, Q.; Senyuk, B.; Frazier, A.W.; Peddireddy, K.; Smalyukh, I.I. Cellulose-based reflective liquid crystal films as optical filters and solar gain regulators. ACS Photonics 2018, 5, 2468–2477.
- Giese, M.; Blusch, L.K.; Khan, M.K.; MacLachlan, M.J. Functional materials from cellulose-derived liquid-crystal templates. Angew. Chem. Int. Ed. 2015, 54, 2888–2910.
- Lagerwall, J.P.; Schütz, C.; Salajkova, M.; Noh, J.; Park, J.H.; Scalia, G.; Bergström, L. Cellulose nanocrystal-based materials: From liquid crystal self-assembly and glass formation to multifunctional thin films. NPG Asia Mater. 2014, 6, e80.
- Zhang, Z.; Chen, Z.; Wang, Y.; Zhao, Y. Bioinspired conductive cellulose liquid-crystal hydrogels as multifunctional electrical skins. Proc. Natl. Acad. Sci. USA 2020, 117, 18310–18316.
- Syverud, K.; Pettersen, S.R.; Draget, K.; Chinga-Carrasco, G. Controlling the elastic modulus of cellulose nanofibril hydrogels—Scaffolds with potential in tissue engineering. Cellulose 2015, 22, 473–481.
- Huang, J.; Wang, S.; Lyu, S.; Fu, F. Preparation of a robust cellulose nanocrystal superhydrophobic coating for self-cleaning and oil-water separation only by spraying. Ind. Crops Prod. 2018, 122, 438–447.
- Gong, X.; Wang, Y.; Zeng, H.; Betti, M.; Chen, L. Highly porous, hydrophobic, and compressible cellulose nanocrystals/poly (vinyl alcohol) aerogels as recyclable absorbents for oil–water separation. ACS Sustain. Chem. Eng. 2019, 7, 11118–11128.
- Cheng, Q.-Y.; Guan, C.-S.; Wang, M.; Li, Y.-D.; Zeng, J.-B. Cellulose nanocrystal coated cotton fabric with superhydrophobicity for efficient oil/water separation. Carbohydr. Polym. 2018, 199, 390–396.
- Markstedt, K.; Escalante, A.; Toriz, G.; Gatenholm, P. Biomimetic inks based on cellulose nanofibrils and cross-linkable xylans for 3D printing. ACS Appl. Mater. Interfaces 2017, 9, 40878–40886.
- Li, D.; Yuan, J.; Cheng, Q.; Wei, P.; Cheng, G.J.; Chang, C. Additive printing of recyclable anti-counterfeiting patterns with sol–gel cellulose nanocrystal inks. Nanoscale 2021, 13, 11808–11816.
- Ebers, L.-S.; Laborie, M.-P. Direct ink writing of fully bio-based liquid crystalline lignin/hydroxypropyl cellulose aqueous inks: Optimization of formulations and printing parameters. ACS Appl. Bio. Mater. 2020, 3, 6897–6907.
- Li, H.; Zhou, J.; Zhao, J.; Li, Y.; Lu, K. Synthesis of cellulose nanocrystals-armored fluorinated polyacrylate latexes via Pickering emulsion polymerization and their film properties. Colloids Surf. B Biointerfaces 2020, 192, 111071.
- Hu, Z.; Ballinger, S.; Pelton, R.; Cranston, E.D. Surfactant-enhanced cellulose nanocrystal Pickering emulsions. J. Colloid Interface Sci. 2015, 439, 139–148.
- Wang, W.; Du, G.; Li, C.; Zhang, H.; Long, Y.; Ni, Y. Preparation of cellulose nanocrystals from asparagus (Asparagus officinalis L.) and their applications to palm oil/water Pickering emulsion. Carbohydr. Polym. 2016, 151, 1–8.
- Zhang, Y.; Cui, L.; Xu, H.; Feng, X.; Wang, B.; Pukánszky, B.; Mao, Z.; Sui, X. Poly (lactic acid)/cellulose nanocrystal composites via the Pickering emulsion approach: Rheological, thermal and mechanical properties. Int. J. Biol. Macromol. 2019, 137, 197–204.
- Jutakridsada, P.; Pimsawat, N.; Sillanpää, M.; Kamwilaisak, K. Olive oil stability in Pickering emulsion preparation from eucalyptus pulp and its rheology behaviour. Cellulose 2020, 27, 6189–6203.
- Kalashnikova, I.; Bizot, H.; Cathala, B.; Capron, I. New Pickering emulsions stabilized by bacterial cellulose nanocrystals. Langmuir 2011, 27, 7471–7479.
- Zhang, B.; Zhang, Z.; Kapar, S.; Ataeian, P.; da Silva Bernardes, J.; Berry, R.; Zhao, W.; Zhou, G.; Tam, K.C. Microencapsulation of phase change materials with polystyrene/cellulose nanocrystal hybrid shell via Pickering emulsion polymerization. ACS Sustain. Chem. Eng. 2019, 7, 17756–17767.
- Angkuratipakorn, T.; Sriprai, A.; Tantrawong, S.; Chaiyasit, W.; Singkhonrat, J. Fabrication and characterization of rice bran oil-in-water Pickering emulsion stabilized by cellulose nanocrystals. Colloids Surf. A Physicochem. Eng. Asp. 2017, 522, 310–319.
- Tang, J.; Lee, M.F.X.; Zhang, W.; Zhao, B.; Berry, R.M.; Tam, K.C. Dual responsive pickering emulsion stabilized by poly grafted cellulose nanocrystals. Biomacromolecules 2014, 15, 3052–3060.
- Deng, Z.; Jung, J.; Simonsen, J.; Zhao, Y. Cellulose nanocrystals Pickering emulsion incorporated chitosan coatings for improving storability of postharvest Bartlett pears (Pyrus communis) during long-term cold storage. Food Hydrocoll. 2018, 84, 229–237.
- Meirelles, A.A.D.; Costa, A.L.R.; Cunha, R.L. Cellulose nanocrystals from ultrasound process stabilizing O/W Pickering emulsion. Int. J. Biol. Macromol. 2020, 158, 75–84.
- Yu, H.; Huang, G.; Ma, Y.; Liu, Y.; Huang, X.; Zheng, Q.; Yue, P.; Yang, M. Cellulose nanocrystals based clove oil Pickering emulsion for enhanced antibacterial activity. Int. J. Biol. Macromol. 2021, 170, 24–32.
- Li, Y.; Liu, Y.; Liu, Y.; Lai, W.; Huang, F.; Ou, A.; Qin, R.; Liu, X.; Wang, X. Ester crosslinking enhanced hydrophilic cellulose nanofibrils aerogel. ACS Sustain. Chem. Eng. 2018, 6, 11979–11988.
- Nguyen, N.-T.; Wereley, S.T.; Shaegh, S.A.M. Fundamentals and Applications of Microfluidics; Artech House: Boston, MA, USA, 2019.
- Mark, D.; Haeberle, S.; Roth, G.; Von Stetten, F.; Zengerle, R. Microfluidic lab-on-a-chip platforms: Requirements, characteristics and applications. Microfluid. Based Microsyst. 2010, 305–376.
- Lebedev, A.; Miraghaie, R.; Kotta, K.; Ball, C.E.; Zhang, J.; Buchsbaum, M.S.; Kolb, H.C.; Elizarov, A. Batch-reactor microfluidic device: First human use of a microfluidically produced PET radiotracer. Lab Chip 2013, 13, 136–145.
- Yiotis, A.; Karadimitriou, N.; Zarikos, I.; Steeb, H. Pore-scale effects during the transition from capillary-to viscosity-dominated flow dynamics within microfluidic porous-like domains. Sci. Rep. 2021, 11, 3891.
- Ong, C.L.; Paredes, S.; Sridhar, A.; Michel, B.; Brunschwiler, T. Radial hierarchical microfluidic evaporative cooling for 3-d integrated microprocessors. In Proceedings of the 4th European Conference on Microfluidics, Limerick, Ireland, 10–12 December 2014.
- Liu, Z.; Liu, X.; Jiang, S.; Zhu, C.; Ma, Y.; Fu, T. Effects on droplet generation in step-emulsification microfluidic devices. Chem. Eng. Sci. 2021, 246, 116959.
- Guo, M.T.; Rotem, A.; Heyman, J.A.; Weitz, D.A. Droplet microfluidics for high-throughput biological assays. Lab Chip 2012, 12, 2146–2155.
- Ying, B.; Park, S.; Chen, L.; Dong, X.; Young, E.W.; Liu, X. NanoPADs and nanoFACEs: An optically transparent nanopaper-based device for biomedical applications. Lab Chip 2020, 20, 3322–3333.
- Markin, C.J.; Mokhtari, D.A.; Sunden, F.; Appel, M.J.; Akiva, E.; Longwell, S.; Sabatti, C.; Herschlag, D.; Fordyce, P.M. Revealing enzyme functional architecture via high-throughput microfluidic enzyme kinetics. Science 2021, 373, eabf8761.
- Baek, S.-Y.; Park, S.-Y. Highly-porous uniformly-sized amidoxime-functionalized cellulose beads prepared by microfluidics with N-methylmorpholine N-oxide. Cellulose 2021, 28, 5401–5419.
- Cai, L.; Wang, Y.; Wu, Y.; Xu, C.; Zhong, M.; Lai, H.; Huang, J. Fabrication of a microfluidic paper-based analytical device by silanization of filter cellulose using a paper mask for glucose assay. Analyst 2014, 139, 4593–4598.
- Pokhrel, P.; Jha, S.; Giri, B. Selection of appropriate protein assay method for a paper microfluidics platform. Pract. Lab. Med. 2020, 21, e00166.
- Song, J.; Babayekhorasani, F.; Spicer, P.T. Soft bacterial cellulose microcapsules with adaptable shapes. Biomacromolecules 2019, 20, 4437–4446.
- Lari, A.S.; Khatibi, A.; Zahedi, P.; Ghourchian, H. Microfluidic-assisted production of poly (ɛ-caprolactone) and cellulose acetate nanoparticles: Effects of polymers, surfactants, and flow rate ratios. Polym. Bull. 2020, 78, 5449–5466.
- Wise, H.G.; Takana, H.; Ohuchi, F.; Dichiara, A.B. Field-Assisted Alignment of Cellulose Nanofibrils in a Continuous Flow-Focusing System. ACS Appl. Mater. Interfaces 2020, 12, 28568–28575.
- Chen, J.; Wang, S.; Ke, H.; Zhou, M.; Li, X. Experimental investigation of annular two-phase flow splitting at a microimpacting T-junction. Chem. Eng. Sci. 2014, 118, 154–163.
- Lu, L.; Fan, S.; Niu, Q.; Peng, Q.; Geng, L.; Yang, G.; Shao, H.; Hsiao, B.S.; Zhang, Y. Strong silk fibers containing cellulose nanofibers generated by a bioinspired microfluidic chip. ACS Sustain. Chem. Eng. 2019, 7, 14765–14774.
- Zhang, M.; Guo, W.; Ren, M.; Ren, X. Fabrication of porous cellulose microspheres with controllable structures by microfluidic and flash freezing method. Mater. Lett. 2020, 262, 127193.
- Håkansson, K.M.; Fall, A.B.; Lundell, F.; Yu, S.; Krywka, C.; Roth, S.V.; Santoro, G.; Kvick, M.; Wittberg, L.P.; Wågberg, L. Hydrodynamic alignment and assembly of nanofibrils resulting in strong cellulose filaments. Nat. Commun. 2014, 5, 4018.
- Nechyporchuk, O.; Håkansson, K.M.; Gowda, V.K.; Lundell, F.; Hagström, B.; Köhnke, T. Continuous assembly of cellulose nanofibrils and nanocrystals into strong macrofibers through microfluidic spinning. Adv. Mater. Technol. 2019, 4, 1800557.
- Benvidi, A.; Banaei, M.; Tezerjani, M.D.; Molahosseini, H.; Jahanbani, S. Impedimetric PSA aptasensor based on the use of a glassy carbon electrode modified with titanium oxide nanoparticles and silk fibroin nanofibers. Microchim. Acta 2018, 185, 50.
- Hu, K.; Gupta, M.K.; Kulkarni, D.D.; Tsukruk, V.V. Ultra-robust graphene oxide-silk fibroin nanocomposite membranes. Adv. Mater. 2013, 25, 2301–2307.
- Steven, E.; Saleh, W.R.; Lebedev, V.; Acquah, S.F.; Laukhin, V.; Alamo, R.G.; Brooks, J.S. Carbon nanotubes on a spider silk scaffold. Nat. Commun. 2013, 4, 2435.
- Qiao, X.; Qian, Z.; Li, J.; Sun, H.; Han, Y.; Xia, X.; Zhou, J.; Wang, C.; Wang, Y.; Wang, C. Synthetic engineering of spider silk fiber as implantable optical waveguides for low-loss light guiding. ACS Appl. Mater. Interfaces 2017, 9, 14665–14676.
- Lu, L.; Fan, S.; Geng, L.; Yao, X.; Zhang, Y. Low-loss light-guiding, strong silk generated by a bioinspired microfluidic chip. Chem. Eng. J. 2021, 405, 126793.
- Kinahan, M.E.; Filippidi, E.; Köster, S.; Hu, X.; Evans, H.M.; Pfohl, T.; Kaplan, D.L.; Wong, J. Tunable silk: Using microfluidics to fabricate silk fibers with controllable properties. Biomacromolecules 2011, 12, 1504–1511.
- Konwarh, R.; Gupta, P.; Mandal, B.B. Silk-microfluidics for advanced biotechnological applications: A progressive review. Biotechnol. Adv. 2016, 34, 845–858.
- Peng, Q.; Zhang, Y.; Lu, L.; Shao, H.; Qin, K.; Hu, X.; Xia, X. Recombinant spider silk from aqueous solutions via a bio-inspired microfluidic chip. Sci. Rep. 2016, 6, 36473.
- Lu, L.; Fan, S.; Geng, L.; Lin, J.; Yao, X.; Zhang, Y. Flow Analysis of Regenerated Silk Fibroin/Cellulose Nanofiber Suspensions via a Bioinspired Microfluidic Chip. Adv. Mater. Technol. 2021, 6, 2100124.
- Rivera-Quintero, P.; Mercado, D.F.; Ballesteros-Rueda, L.M. Influence of the functionalization agent and crystalline phase of MnO2 Janus nanomaterials on the stability of aqueous nanofluids and its catalytic activity to promote asphaltene oxidation. Colloid Interface Sci. Commun. 2021, 45, 100525.
- Zúñiga, M.C.; Steitz, J.A. The nucleotide sequence of a major glycine transfer RNA from the posterior silk gland of Bombyx mori L. Nucleic Acids Res. 1977, 4, 4175–4196.
- Weitao, Z.; Jianxin, H.; Shan, D.; Shizhong, C.; Weidong, G. Electrospun silk fibroin/cellulose acetate blend nanofibres: Structure and properties. Iran. Polym. J. 2011, 20, 389–397.
- Zhou, W.; He, J.; Cui, S.; Gao, W. Preparation of electrospun silk fibroin/Cellulose Acetate blend nanofibers and their applications to heavy metal ions adsorption. Fibers Polym. 2011, 12, 431–437.
- Zhou, W.T.; He, J.X.; Cui, S.Z.; Gao, W.D. Nanofibrous membrane of silk fibroin/cellulose acetate blend for heavy metal ion adsorption. In Advanced Materials Research; Trans Tech Publications Ltd.: Freienbach, Switzerland, 2011; pp. 1431–1435.
- Du, S.; Zhang, J.; Zhou, W.T.; Li, Q.X.; Greene, G.W.; Zhu, H.J.; Li, J.L.; Wang, X.G. Interactions between fibroin and sericin proteins from Antheraea pernyi and Bombyx mori silk fibers. J. Colloid Interface Sci. 2016, 478, 316–323.
- Yi, S.; Wu, Y.; Zhang, Y.; Zou, Y.; Dai, F.; Si, Y. Antibacterial Activity of Photoactive Silk Fibroin/Cellulose Acetate Blend Nanofibrous Membranes against Escherichia coli. ACS Sustain. Chem. Eng. 2020, 8, 16775–16780.
- Wang, H.-Y.; Wei, Z.-G.; Zhang, Y.-Q. Dissolution and regeneration of silk from silkworm Bombyx mori in ionic liquids and its application to medical biomaterials. Int. J. Biol. Macromol. 2020, 143, 594–601.
- Rivera-Galletti, A.; Gough, C.R.; Kaleem, F.; Burch, M.; Ratcliffe, C.; Lu, P.; Salas-De la Cruz, D.; Hu, X. Silk-Cellulose Acetate Biocomposite Materials Regenerated from Ionic Liquid. Polymers 2021, 13, 2911.
- Arumugam, M.; Murugesan, B.; Pandiyan, N.; Chinnalagu, D.K.; Rangasamy, G.; Mahalingam, S. Electrospinning cellulose acetate/silk fibroin/Au-Ag hybrid composite nanofiber for enhanced biocidal activity against MCF-7 breast cancer cell. Mater. Sci. Eng. C 2021, 123, 112019.
- Pignon, F.; Challamel, M.; De Geyer, A.; Elchamaa, M.; Semeraro, E.F.; Hengl, N.; Jean, B.; Putaux, J.-L.; Gicquel, E.; Bras, J. Breakdown and buildup mechanisms of cellulose nanocrystal suspensions under shear and upon relaxation probed by SAXS and SALS. Carbohydr. Polym. 2021, 260, 117751.
- Wu, X.; Cao, J.; Bao, S.; Shao, G.; Wang, Z.; Qin, B.; Wang, T.; Fu, Y. Preparation and application of modified three-dimensional cellulose microspheres for paclitaxel targeted separation. J. Chromatogr. A 2021, 1655, 462487.
- Pepicelli, M.; Binelli, M.R.; Studart, A.R.; Rühs, P.A.; Fischer, P. Self-grown bacterial cellulose capsules made through emulsion templating. ACS Biomater. Sci. Eng. 2021, 7, 3221–3228.
- Duong, D.D.; Kwak, J.; Song, H.S.; Lee, N.Y. Construction of microfluidic blood–brain barrier model assisted by 3D coculture on cellulose fiber. Microsyst. Technol. 2021, 27, 3917–3926.
- Jayapiriya, U.; Goel, S. Influence of cellulose separators in coin-sized 3D printed paper-based microbial fuel cells. Sustain. Energy Technol. Assess. 2021, 47, 101535.
- Sharratt, W.N.; Lopez, C.G.; Sarkis, M.; Tyagi, G.; O’Connell, R.; Rogers, S.E.; Cabral, J.T. Ionotropic Gelation Fronts in Sodium Carboxymethyl Cellulose for Hydrogel Particle Formation. Gels 2021, 7, 44.
- Chen, C.; Wang, Y.; Zhang, D.; Wu, X.; Zhao, Y.; Shang, L.; Ren, J.; Zhao, Y. Natural polysaccharide based complex drug delivery system from microfluidic electrospray for wound healing. Appl. Mater. Today 2021, 23, 101000.
- Liu, L.; Yang, J.-P.; Ju, X.-J.; Xie, R.; Yang, L.; Liang, B.; Chu, L.-Y. Microfluidic preparation of monodisperse ethyl cellulose hollow microcapsules with non-toxic solvent. J. Colloid Interface Sci. 2009, 336, 100–106.
- Li, Y.; Wang, S.; Huang, R.; Huang, Z.; Hu, B.; Zheng, W.; Yang, G.; Jiang, X. Evaluation of the effect of the structure of bacterial cellulose on full thickness skin wound repair on a microfluidic chip. Biomacromolecules 2015, 16, 780–789.
- Zhao, D.; Zhu, Y.; Cheng, W.; Chen, W.; Wu, Y.; Yu, H. Cellulose-based flexible functional materials for emerging intelligent electronics. Adv. Mater. 2021, 33, 2000619.
- Mahapatra, S.; Srivastava, V.R.; Chandra, P. Nanobioengineered Sensing Technologies Based on Cellulose Matrices for Detection of Small Molecules, Macromolecules, and Cells. Biosensors 2021, 11, 168.
- Del Giudice, F.; Tassieri, M.; Oelschlaeger, C.; Shen, A.Q. When microrheology, bulk rheology, and microfluidics meet: Broadband rheology of hydroxyethyl cellulose water solutions. Macromolecules 2017, 50, 2951–2963.
- Zeng, J.; Hu, F.; Cheng, Z.; Wang, B.; Chen, K. Isolation and rheological characterization of cellulose nanofibrils (CNFs) produced by microfluidic homogenization, ball-milling, grinding and refining. Cellulose 2021, 28, 3389–3408.
- Wang, S.; Zeng, J.; Cheng, Z.; Yuan, Z.; Wang, X.; Wang, B. Precisely controlled preparation of uniform nanocrystalline cellulose via microfluidic technology. J. Ind. Eng. Chem. 2021.
- Carrick, C.; Larsson, P.A.; Brismar, H.; Aidun, C.; Wågberg, L. Native and functionalized micrometre-sized cellulose capsules prepared by microfluidic flow focusing. RSC Adv. 2014, 4, 19061–19067.
- Pei, Y.; Wang, X.; Huang, W.; Liu, P.; Zhang, L. Cellulose-based hydrogels with excellent microstructural replication ability and cytocompatibility for microfluidic devices. Cellulose 2013, 20, 1897–1909.
- Zhang, L.; Deraney, R.N.; Tripathi, A. Adsorption and isolation of nucleic acids on cellulose magnetic beads using a three-dimensional printed microfluidic chip. Biomicrofluidics 2015, 9, 064118.
- Wenzlik, D.; Ohm, C.; Serra, C.; Zentel, R. Preparation of cholesteric particles from cellulose derivatives in a microfluidic setup. Soft Matter 2011, 7, 2340–2344.
- Miyashita, Y.; Iwasaka, M.; Kimura, T. Microcrystal-like cellulose fibrils as the diamagnetic director for microfluidic systems. J. Appl. Phys. 2014, 115, 17B519.
- Chen, X.; Zhang, L.; Li, H.; Sun, J.; Cai, H.; Cui, D. Development of a multilayer microfluidic device integrated with a PDMS-cellulose composite film for sample pre-treatment and immunoassay. Sens. Actuators A Phys. 2013, 193, 54–58.
- Włodarczyk, E.; Zarzycki, P.K. Chromatographic behavior of selected dyes on silica and cellulose micro-TLC plates: Potential application as target substances for extraction, chromatographic, and/or microfluidic systems. J. Liq. Chromatogr. Relat. Technol. 2017, 40, 259–281.
- Ghorbani, M.; Aghdam, A.S.; Gevari, M.T.; Koşar, A.; Cebeci, F.Ç.; Grishenkov, D.; Svagan, A.J. Facile hydrodynamic cavitation ON CHIP via cellulose nanofibers stabilized perfluorodroplets inside layer-by-layer assembled SLIPS surfaces. Chem. Eng. J. 2020, 382, 122809.
- Park, J.-S.; Park, C.-W.; Han, S.-Y.; Lee, E.-A.; Cindradewi, A.W.; Kim, J.-K.; Kwon, G.-J.; Seo, Y.-H.; Youe, W.-J.; Gwon, J. Preparation and Properties of Wet-Spun Microcomposite Filaments from Cellulose Nanocrystals and Alginate Using a Microfluidic Device. BioResources 2021, 13, 1709.
- Grate, J.W.; Mo, K.-F.; Shin, Y.; Vasdekis, A.; Warner, M.G.; Kelly, R.T.; Orr, G.; Hu, D.; Dehoff, K.J.; Brockman, F.J. Alexa fluor-labeled fluorescent cellulose nanocrystals for bioimaging solid cellulose in spatially structured microenvironments. Bioconjugate Chem. 2015, 26, 593–601.
- Ke, Y.; Liu, G.; Wang, J.; Xue, W.; Du, C.; Wu, G. Preparation of carboxymethyl cellulose based microgels for cell encapsulation. Express Polym. Lett. 2014, 8, 841–849.
- Rao, L.T.; Dubey, S.K.; Javed, A.; Goel, S. Parametric Performance Investigation on Membraneless Microfluidic Paper Fuel Cell with Graphite Composed Pencil Stoke Electrodes. Int. J. Precis. Eng. Manuf. 2021, 22, 177–187.
- Shen, L.-L.; Zhang, G.-R.; Venter, T.; Biesalski, M.; Etzold, B.J. Towards best practices for improving paper-based microfluidic fuel cells. Electrochim. Acta 2019, 298, 389–399.
- Shefa, A.A.; Sultana, T.; Park, M.K.; Lee, S.Y.; Gwon, J.-G.; Lee, B.-T. Curcumin incorporation into an oxidized cellulose nanofiber-polyvinyl alcohol hydrogel system promotes wound healing. Mater. Des. 2020, 186, 108313.
- Chen, C.; Zhu, C.; Huang, Y.; Nie, Y.; Yang, J.; Shen, R.; Sun, D. Regenerated bacterial cellulose microfluidic column for glycoproteins separation. Carbohydr. Polym. 2016, 137, 271–276.
- Yan, X.; Xu, A.; Zeng, L.; Gao, P.; Zhao, T. A paper-based microfluidic fuel cell with hydrogen peroxide as fuel and oxidant. Energy Technol. 2018, 6, 140–143.
- Tzivelekis, C.; Selby, M.P.; Batet, A.; Madadi, H.; Dalgarno, K. Microfluidic chip fabrication and performance analysis of 3D printed material for use in microfluidic nucleic acid amplification applications. J. Micromech. Microeng. 2021, 31, 035005.
- Ren, K.; Zhou, J.; Wu, H. Materials for microfluidic chip fabrication. Acc. Chem. Res. 2013, 46, 2396–2406.
- Chen, X.; Mo, D.; Gong, M. A Flexible Method for Nanofiber-based 3D Microfluidic Device Fabrication for Water Quality Monitoring. Micromachines 2020, 11, 276.
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994.
- Martinez, A.W.; Phillips, S.T.; Whitesides, G.M. Three-dimensional microfluidic devices fabricated in layered paper and tape. Proc. Natl. Acad. Sci. USA 2008, 105, 19606–19611.
- Martinez, A.W.; Phillips, S.T.; Butte, M.J.; Whitesides, G.M. Patterned paper as a platform for inexpensive, low-volume, portable bioassays. Angew. Chem. 2007, 119, 1340–1342.
- Klasner, S.A.; Price, A.K.; Hoeman, K.W.; Wilson, R.S.; Bell, K.J.; Culbertson, C.T. Based microfluidic devices for analysis of clinically relevant analytes present in urine and saliva. Anal. Bioanal. Chem. 2010, 397, 1821–1829.
- Bruzewicz, D.A.; Reches, M.; Whitesides, G.M. Low-cost printing of poly (dimethylsiloxane) barriers to define microchannels in paper. Anal. Chem. 2008, 80, 3387–3392.
- Rousseau, D.; Amrouche, S.; Calafiura, P.; Estrade, V.; Farrell, S.; Germain, C.; Gligorov, V.; Golling, T.; Gray, H.; Guyon, I. The TrackML Particle Tracking Challenge. 2018. Available online: https://hal.inria.fr/hal-01680537v2/document (accessed on 26 December 2021).
- Abe, K.; Kotera, K.; Suzuki, K.; Citterio, D. Inkjet-printed paperfluidic immuno-chemical sensing device. Anal. Bioanal. Chem. 2010, 398, 885–893.
- Abe, K.; Suzuki, K.; Citterio, D. Inkjet-printed microfluidic multianalyte chemical sensing paper. Anal. Chem. 2008, 80, 6928–6934.
- Li, X.; Tian, J.; Shen, W. Progress in patterned paper sizing for fabrication of paper-based microfluidic sensors. Cellulose 2010, 17, 649–659.
- Wang, W.; Wu, W.-Y.; Zhu, J.-J. Tree-shaped paper strip for semiquantitative colorimetric detection of protein with self-calibration. J. Chromatogr. A 2010, 1217, 3896–3899.
- Fenton, E.M.; Mascarenas, M.R.; López, G.P.; Sibbett, S.S. Multiplex lateral-flow test strips fabricated by two-dimensional shaping. ACS Appl. Mater. Interfaces 2009, 1, 124–129.
- Lu, Y.; Shi, W.; Jiang, L.; Qin, J.; Lin, B. Rapid prototyping of paper-based microfluidics with wax for low-cost, portable bioassay. Electrophoresis 2009, 30, 1497–1500.
- Carrilho, E.; Martinez, A.W.; Whitesides, G.M. Understanding wax printing: A simple micropatterning process for paper-based microfluidics. Anal. Chem. 2009, 81, 7091–7095.
- Leung, V.; Shehata, A.-A.M.; Filipe, C.D.; Pelton, R. Streaming potential sensing in paper-based microfluidic channels. Colloids Surf. A Physicochem. Eng. Asp. 2010, 364, 16–18.
- Olkkonen, J.; Lehtinen, K.; Erho, T. Flexographically printed fluidic structures in paper. Anal. Chem. 2010, 82, 10246–10250.
- Dungchai, W.; Chailapakul, O.; Henry, C.S. A low-cost, simple, and rapid fabrication method for paper-based microfluidics using wax screen-printing. Analyst 2011, 136, 77–82.
- Chitnis, G.; Ding, Z.; Chang, C.-L.; Savran, C.A.; Ziaie, B. Laser-treated hydrophobic paper: An inexpensive microfluidic platform. Lab Chip 2011, 11, 1161–1165.
- Zargaryan, A.; Farhoudi, N.; Haworth, G.; Ashby, J.F.; Au, S.H. Hybrid 3D printed-paper microfluidics. Sci. Rep. 2020, 10, 18379.
- Zhang, Y.; Liu, J.; Wang, H.; Fan, Y. Laser-induced selective wax reflow for paper-based microfluidics. RSC Adv. 2019, 9, 11460–11464.
- Yamada, K.; Henares, T.G.; Suzuki, K.; Citterio, D. Paper-based inkjet-printed microfluidic analytical devices. Angew. Chem. Int. Ed. 2015, 54, 5294–5310.
- Olmos, C.M.; Vaca, A.; Rosero, G.; Peñaherrera, A.; Perez, C.; de Sá Carneiro, I.; Vizuete, K.; Arroyo, C.R.; Debut, A.; Pérez, M.S. Epoxy resin mold and PDMS microfluidic devices through photopolymer flexographic printing plate. Sens. Actuators B Chem. 2019, 288, 742–748.
- Xu, Y.; Liu, M.; Kong, N.; Liu, J. Lab-on-paper micro-and nano-analytical devices: Fabrication, modification, detection and emerging applications. Microchim. Acta 2016, 183, 1521–1542.
- Li, X.; Tian, J.; Nguyen, T.; Shen, W. based microfluidic devices by plasma treatment. Anal. Chem. 2008, 80, 9131–9134.
- Arce, C.; Llano, T.; García, P.; Alberto, C. Technical and environmental improvement of the bleaching sequence of dissolving pulp for fibre production. Cellulose 2020, 27, 4079–4090.
- Berlioz, S.; Molina-Boisseau, S.; Nishiyama, Y.; Heux, L. Gas-phase surface esterification of cellulose microfibrils and whiskers. Biomacromolecules 2009, 10, 2144–2151.
- Fox, S.C.; Li, B.; Xu, D.; Edgar, K.J. Regioselective esterification and etherification of cellulose: A review. Biomacromolecules 2011, 12, 1956–1972.
- Sen, S.; Martin, J.D.; Argyropoulos, D.S. Review of cellulose non-derivatizing solvent interactions with emphasis on activity in inorganic molten salt hydrates. ACS Sustain. Chem. Eng. 2013, 1, 858–870.
- Swatloski, R.P.; Spear, S.K.; Holbrey, J.D.; Rogers, R.D. Dissolution of cellose with ionic liquids. J. Am. Chem. Soc. 2002, 124, 4974–4975.
- Drofenik, J.; Gaberscek, M.; Dominko, R.; Poulsen, F.W.; Mogensen, M.; Pejovnik, S.; Jamnik, J. Cellulose as a binding material in graphitic anodes for Li ion batteries: A performance and degradation study. Electrochim. Acta 2003, 48, 883–889.
- Clasen, C.; Kulicke, W.-M. Determination of viscoelastic and rheo-optical material functions of water-soluble cellulose derivatives. Prog. Polym. Sci. 2001, 26, 1839–1919.
- Dai, L.; Cheng, T.; Duan, C.; Zhao, W.; Zhang, W.; Zou, X.; Aspler, J.; Ni, Y. 3D printing using plant-derived cellulose and its derivatives: A review. Carbohydr. Polym. 2019, 203, 71–86.
- Ma, T.; Hu, X.; Lu, S.; Liao, X.; Song, Y.; Hu, X. Nanocellulose: A promising green treasure from food wastes to available food materials. Crit. Rev. Food Sci. Nutr. 2020, 1–14.
- Fu, K.; Yao, Y.; Dai, J.; Hu, L. Progress in 3D printing of carbon materials for energy-related applications. Adv. Mater. 2017, 29, 1603486.
- Jungst, T.; Smolan, W.; Schacht, K.; Scheibel, T.; Groll, J.r. Strategies and molecular design criteria for 3D printable hydrogels. Chem. Rev. 2016, 116, 1496–1539.
- Izaguirre, A.; Lanas, J.; Álvarez, J. Behaviour of a starch as a viscosity modifier for aerial lime-based mortars. Carbohydr. Polym. 2010, 80, 222–228.
- Ma, B.; Peng, Y.; Tan, H.; Jian, S.; Zhi, Z.; Guo, Y.; Qi, H.; Zhang, T.; He, X. Effect of hydroxypropyl-methyl cellulose ether on rheology of cement paste plasticized by polycarboxylate superplasticizer. Constr. Build. Mater. 2018, 160, 341–350.
- Chatterjee, T.; O’Donnell, K.P.; Rickard, M.A.; Nickless, B.; Li, Y.; Ginzburg, V.V.; Sammler, R.L. Rheology of cellulose ether excipients designed for hot melt extrusion. Biomacromolecules 2018, 19, 4430–4441.
- Lin, C.-C.; Liao, C.-W.; Chao, Y.-C.; Kuo, C. Fabrication and characterization of asymmetric Janus and ternary particles. ACS Appl. Mater. Interfaces 2010, 2, 3185–3191.
- Martinez, A.W.; Phillips, S.T.; Whitesides, G.M.; Carrilho, E. Diagnostics for the developing world: Microfluidic paper-based analytical devices. Anal. Chem. 2010, 82, 3–10.
- Yamada, K.; Henares, T.G.; Suzuki, K.; Citterio, D. Distance-based tear lactoferrin assay on microfluidic paper device using interfacial interactions on surface-modified cellulose. ACS Appl. Mater. Interfaces 2015, 7, 24864–24875.
- Li, X.; Liu, X. Fabrication of three-dimensional microfluidic channels in a single layer of cellulose paper. Microfluid. Nanofluidics 2014, 16, 819–827.
- Ardalan, S.; Hosseinifard, M.; Vosough, M.; Golmohammadi, H. Towards smart personalized perspiration analysis: An IoT-integrated cellulose-based microfluidic wearable patch for smartphone fluorimetric multi-sensing of sweat biomarkers. Biosens. Bioelectron. 2020, 168, 112450.
- Arun, R.K.; Gupta, V.; Singh, P.; Biswas, G.; Chanda, N. Selection of graphite pencil grades for the design of suitable electrodes for stacking multiple single-inlet paper-pencil fuel cells. ChemistrySelect 2019, 4, 152–159.
- del Torno-de Román, L.; Navarro, M.; Hughes, G.; Esquivel, J.P.; Milton, R.D.; Minteer, S.D.; Sabaté, N. Improved performance of a paper-based glucose fuel cell by capillary induced flow. Electrochim. Acta 2018, 282, 336–342.
- Jia, C.; Jiang, F.; Hu, P.; Kuang, Y.; He, S.; Li, T.; Chen, C.; Murphy, A.; Yang, C.; Yao, Y. Anisotropic, mesoporous microfluidic frameworks with scalable, aligned cellulose nanofibers. ACS Appl. Mater. Interfaces 2018, 10, 7362–7370.
- Murase, R.; Kondo, S.; Kitamura, T.; Goi, Y.; Hashimoto, M.; Teramoto, Y. Cellulose nanofibers as a module for paper-based microfluidic analytical devices: Labile substance storage, processability, and reaction field provision and control. ACS Appl. Bio Mater. 2018, 1, 480–486.
- Kumar, T.; Soares, R.R.; Dholey, L.A.; Ramachandraiah, H.; Aval, N.A.; Aljadi, Z.; Pettersson, T.; Russom, A. Multi-layer assembly of cellulose nanofibrils in a microfluidic device for the selective capture and release of viable tumor cells from whole blood. Nanoscale 2020, 12, 21788–21797.
- Choi, S.; Moon, S.W.; Lee, S.H.; Kim, W.; Kim, S.; Kim, S.K.; Shin, J.-H.; Park, Y.-G.; Jin, K.-H.; Kim, T.G. A recyclable CNC-milled microfluidic platform for colorimetric assays and label-free aged-related macular degeneration detection. Sens. Actuators B Chem. 2019, 290, 484–492.
- Fu, H.; Liu, X. Experimental comparison of surface chemistries for biomolecule immobilization on paper-based microfluidic devices. J. Micromech. Microeng. 2019, 29, 124003.
- Bao, W.; Fang, Z.; Wan, J.; Dai, J.; Zhu, H.; Han, X.; Yang, X.; Preston, C.; Hu, L. Aqueous gating of van der waals materials on bilayer nanopaper. ACS Nano 2014, 8, 10606–10612.
- Yadav, S.; Kumar, M.; Singh, K.; Sharma, N.N.; Akhtar, J. Flexible Microfluidics Biosensor Technology. In Electrical and Electronic Devices, Circuits and Materials; CRC Press: Boca Raton, FL, USA, 2021; pp. 377–386.
- Solin, K.; Borghei, M.; Imani, M.; Kämäräinen, T.; Kiri, K.; Mäkelä, T.; Khakalo, A.; Orelma, H.; Gane, P.A.; Rojas, O.J. Bicomponent Cellulose Fibrils and Minerals Afford Wicking Channels Stencil-Printed on Paper for Rapid and Reliable Fluidic Platforms. ACS Appl. Polym. Mater. 2021, 3, 5536–5546.
- Shin, S.; Hyun, J. Matrix-assisted three-dimensional printing of cellulose nanofibers for paper microfluidics. ACS Appl. Mater. Interfaces 2017, 9, 26438–26446.
More
Information
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.0K
Revisions:
4 times
(View History)
Update Date:
10 Feb 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




