
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Eiji Nambara | + 1984 word(s) | 1984 | 2021-12-10 04:53:59 | | | |
| 2 | Lindsay Dong | + 97 word(s) | 2081 | 2022-02-09 03:47:33 | | |
Video Upload Options
Abscisic acid (ABA) regulates various aspects of plant physiology, including promoting seed dormancy and adaptive responses to abiotic and biotic stresses. Growth regulation by ABA is both promotive and inhibitive, depending on the context, such as concentrations, tissues, and environmental conditions.
1. Introduction
2. Role of ABA in Plant Growth
ABA-mediated growth regulation involves crosstalk with other hormones and nutritional signaling, to regulate various aspects of cellular growth, including cell division, enlargement, differentiation, and central metabolism. Our understanding of how ABA regulates cellular growth is still fragmental.
2.1. Growth Inhibition by Basal ABA
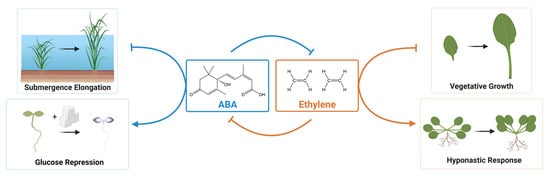
The outcome of basal ABA in growth inhibition should be seen as growth promotion in ABA-deficient and insensitive mutants. Growth promotion of these mutants is observed in local tissues or under particular conditions. Hyponastic growth is the upward bending of leaves that involves growth promotion of cells at the abaxial side. This growth is an adaptive strategy of plants to changing environments, such as submergence and shade avoidance [7]. In Arabidopsis, ABA-deficient (aba1, aba2, aba3) and ABA-insensitive (abi1, abi3) mutants show enhanced hyponastic growth of petioles, which are visible as increases in petiole angles [8]. The petiole angle of the wild type decreases when exogenous ABA is applied. Whereas the application of fluridone, an inhibitor of phytoene desaturases to block metabolite accumulation upstream of ABA biosynthesis, increases the petiole angle. The local growth inhibition by ABA at the petiole is antagonistic to ethylene, which promotes hyponastic growth [8] (Figure 1). It is noteworthy that different Arabidopsis accessions display differential growth responses to these hormones [8]. In Rumex, hyponastic growth is a critical avoidance response to complete submergence [9]. Submergence-induced ABA decreases are a critical step to elongate the shoot in flooding tolerance [10]. The decrease of ABA under submergence is a prerequisite for ethylene and gibberellin action [9]. The shoot growth of osaba1 is enhanced compared with wild type when rice seedlings are submerged [11]. Submergence-induced rapid decrease of ABA is associated with the induction of OsABA8ox1 encoding ABA 8′-hydroxylase [11]. Importantly, submergence-induced ABA decrease is ethylene dependent (Figure 1).
Some ABA-deficient and insensitive mutants of rice show enhanced growth. Rice osaba2 mutants defective in the xanthoxin dehydrogenase are viable in standard conditions. Interestingly, the plant heights of these mutants are taller than wild type, which is a typical and expected phenotype of mutants defective in growth inhibitors [12]. Although showing excessive growth, the osaba2 mutants do not appear to be healthy. These mutants were identified as a lesion mimic mutant showing spontaneous cell death with an overaccumulation of reactive oxygen species (ROS). The phenotype of osaba2 mutants indicates basal ABA plays a role in suppressing ROS production and growth under normal conditions. Some multigenic mutants of rice ABA receptors show increased growth compared to wild type [13].
One explanation for the stunted growth of ABA-related mutants is an increased sensitivity to environmental factors. Mutants are exposed to stresses more sensitively than wild type due to a lack of stress resistance mechanisms. ABA-deficient mutants of tomato grow faster and taller with an increased number of leaves when grown under mist [14]. Similarly, Arabidopsis ABA-deficient aba2 mutant and snrk2 triple mutants show enhanced growth on agar plates, which are relatively humid compared to standard soil conditions [15]. Excess growth of aba2 and snrk2 triple mutants is associated with increased respiration through the tricarboxylic acid cycle [15]. In ABA-deficient mutants of both tomato and Arabidopsis, a characteristic aspect of growth enhancement is the increase in the leaf numbers [15][14]. Initiation of leaf formation is a well-controlled process and used for determining biological time for flowering. It is particularly interesting to understand the mechanism of how basal ABA regulates leaf initiation. Interestingly, the increased leaf number was not observed in an ABA-insensitive quadruple areb mutant, suggesting that the ABA signaling to negatively regulate leaf initiation is SnRK2-dependent, but independent of AREB-mediated transcription [15].
High concentrations of exogenous ABA inhibit lateral root (LR) growth in Arabidopsis [16]. Ethylene and auxin act downstream of ABA in the regulation of Arabidopsis root elongation [16]. Arabidopsis ABI4 AP2 TFs play an important role in inhibiting LR growth [17]. The abi4 mutant enhanced LR formation and growth, which is characterized by increased LR density and elongated LRs. This mechanism involves counteracting ABA and CK action to regulate expression of ABI4, which in turn disturbs auxin transport [17]. As mentioned above, tomato sit and pea wilty mutants increase LR, indicating low concentrations of ABA can inhibit the formation of LR [18]. The mechanism for ABA-mediated inhibition of LR growth is dynamic and alters depending on time. The inhibition of wild-type LR growth is alleviated by a prolonged ABA treatment, while this recovery is delayed in the pyl8 mutant [19]. This indicates that PYL8 is required for the recovery of LR growth from ABA inhibition in a prolonged period. This involves the PYL8-MYB77 interaction to induce auxin-responsive genes independently of SnRK2 [19]. Consistent with this mechanism, application of auxin rescued the delayed recovery phenotype of the pyl8 mutant [19].
2.2. Growth Promotion by ABA
References
- Cutler, S.R.; Rodriguez, P.L.; Finkelstein, R.R.; Abrams, S.R. Abscisic acid: Emergence of a core signaling network. Annu. Rev. Plant Biol. 2010, 61, 651–679.
- Nambara, E.; Okamoto, M.; Tatematsu, K.; Yano, R.; Seo, M.; Kamiya, Y. Abscisic acid and the control of seed dormancy and germination. Seed Sci. Res. 2010, 20, 55–67.
- Sano, N.; Marion-Poll, A. ABA metabolism and homeostasis in seed dormancy and germination. Int. J. Mol. Sci. 2021, 22, 5069.
- Pan, W.; Liang, J.; Sui, J.; Li, J.; Liu, C.; Xin, Y.; Zhang, Y.; Wang, S.; Zhao, Y.; Zhang, J.; et al. ABA and bud dormancy in perennials: Current knowledge and future perspective. Genes 2021, 12, 1635.
- Humplik, J.F.; Bergougnoux, V.; van Volkenburgh, E. To stimulate or inhibit? That is the question for the function of abscisic acid. Trends Plant Sci. 2017, 22, 830–841.
- Yoshida, T.; Christmann, A.; Yamaguchi-Shinozaki, K.; Grill, E.; Fernie, A.R. Revisiting the basal role of ABA—Roles outside of stress. Trends Plant Sci. 2019, 24, 625–635.
- van Zanten, M.; Pons, T.L.; Janssen, J.A.M.; Voesenek, L.A.C.J.; Peeters, A.J.M. On the relevance and control of leaf angle. CRC Crit. Rev. Plant Sci. 2010, 29, 300–316.
- Benschop, J.J.; Millenaar, F.F.; Smeets, M.E.; van Zanten, M.; Voesenek, L.A.C.J.; Peeters, A.J.M. Abscisic acid antagonizes ethylene-induced hyponastic growth in Arabidopsis. Plant Physiol. 2007, 143, 1013–1023.
- Benschop, J.J.; Jackson, M.B.; Guhl, K.; Vreeburg, R.A.M.; Croker, S.J.; Peeters, A.J.M.; Voesenek, L.A.C.J. Contrasting interactions between ethylene and abscisic acid in Rumex species differing in submergence tolerance. Plant J. 2005, 44, 756–768.
- Chen, X.; Pierik, R.; Peeters, A.J.M.; Poorter, H.; Visser, E.J.W.; Huber, H.; de Kroon, H. Endogenous abscisic acid as a key switch for natural variation in flooding-induced shoot elongation. Plant Physiol. 2010, 154, 969–977.
- Saika, H.; Okamoto, M.; Miyoshi, K.; Kushiro, T.; Shinoda, S.; Jikumaru, Y.; Fujimoto, M.; Arikawa, T.; Takahashi, H.; Ando, M.; et al. Ethylene promotes submergence-induced expression of OsABA8ox1, a gene that encodes ABA 8′-hydroxylase in rice. Plant Cell Physiol. 2007, 48, 287–298.
- Liao, Y.X.; Bai, Q.; Xu, P.Z.; Wu, T.K.; Guo, D.M.; Peng, Y.B.; Zhang, H.Y.; Deng, X.S.; Chen, X.Q.; Luo, M.; et al. Mutation in rice Abscisic Acid2 results in cell death, enhanced disease-resistance, altered seed dormancy and development. Front. Plant Sci. 2018, 9, 405.
- Miao, C.B.; Xiao, L.H.; Hua, K.; Zou, C.; Zhao, Y.; Bressan, R.A.; Zhu, J.K. Mutations in a subfamily of abscisic acid receptor genes promote rice growth and productivity. Proc. Natl. Acad. Sci. USA 2018, 115, 6058–6063.
- Jones, H.G.; Sharp, C.S.; Higgs, K.H. Growth and water relations of wilty mutants of tomato (Lycopersicon esculentum Mill.). J. Exp. Bot. 1987, 38, 1848–1856.
- Yoshida, T.; Obata, T.; Feil, R.; Lunn, J.E.; Fujita, Y.; Yamaguchi-Shinozaki, K.; Fernie, A.R. The role of abscisic acid signaling in maintaining the metabolic balance required for Arabidopsis growth under nonstress conditions. Plant Cell 2019, 31, 84–105.
- Emenecker, R.J.; Strader, L.C. Auxin-abscisic acid interactions in plant growth and development. Biomolecules 2020, 10, 281.
- Shkolnik-Inbar, D.; Bar-Zvi, D. ABI4 mediates abscisic acid and cytokinin inhibition of lateral root formation by reducing polar auxin transport in Arabidopsis. Plant Cell 2010, 22, 3560–3573.
- McAdam, S.A.M.; Brodribb, T.J.; Ross, J.J. Shoot-derived abscisic acid promotes root growth. Plant Cell Environ. 2016, 39, 652–659.
- Zhao, Y.; Xing, L.; Wang, X.; Hou, Y.J.; Gao, J.; Wang, P.; Duan, C.G.; Zhu, X.; Zhu, J.K. The ABA receptor PYL8 promotes lateral root growth by enhancing MYB77-dependent transcription of auxin-responsive genes. Sci. Signal. 2014, 7, ra53.
- Cheng, W.-H.; Endo, A.; Zhou, L.; Penney, J.; Chen, H.-C.; Arroyo, A.; Leon, P.; Nambara, E.; Asami, T.; Seo, M.; et al. A unique short-chain dehydrogenase/reductase in Arabidopsis glucose signaling and abscisic acid biosynthesis and functions. Plant Cell 2002, 14, 2723–2743.
- Sharp, R.E.; LeNoble, M.E.; Else, M.A.; Thorne, E.T.; and Gherardi, F. Endogenous ABA maintains shoot growth in tomato independently of effects on plant water balance: Evidence for an interaction with ethylene. J. Exp. Bot. 2000, 51, 1575–1584.
- Barrero, J.M.; Piqueras, P.; González-Guzmán, M.; Serrano, R.; Rodríguez, P.L.; Ponce, M.R.; Micol, J.L. A mutational analysis of the ABA1 gene of Arabidopsis thaliana highlights the involvement of ABA in vegetative development. J. Exp. Bot. 2005, 56, 2071–2083.
- Frey, A.; Effroy, D.; Lefebvre, V.; Seo, M.; Perreau, F.; Berger, A.; Sechet, J.; To, A.; North, H.M.; Marion-Poll, A. Epoxycarotenoid cleavage by NCED5 fine-tunes ABA accumulation and affects seed dormancy and drought tolerance with other NCED family members. Plant J. 2012, 70, 501–512.
- Léon-Kloosterziel, K.M.; Gil, M.A.; Ruijs, G.J.; Jacobsen, S.E.; Olszewski, N.E.; Schwartz, S.H.; Zeevaart, J.A.; Koornneef, M. Isolation and characterization of abscisic acid-deficient Arabidopsis mutants at two new loci. Plant J. 1996, 10, 655–661.
- Nagel, O.W.; Konings, H.; Lambers, H. Growth rate, plant development and water relations of the ABA-deficient tomato mutant sitiens. Physiol Plant. 1994, 92, 102–108.
- Fujii, H.; Zhu, J.K. Arabidopsis mutant deficient in three abscisic acid-activated protein kinases reveals critical roles in growth, reproduction, and stress. Proc. Natl. Acad. Sci. USA 2009, 106, 8380–8385.
- Gonzalez-Guzman, M.; Pizzio, G.A.; Antoni, R.; Vera-Sirera, F.; Merilo, E.; Bassel, G.W.; Fernández, M.A.; Holdsworth, M.J.; Perez-Amador, M.A.; Kollist, H.; et al. Arabidopsis PYR/PYL/RCAR receptors play a major role in quantitative regulation of stomatal aperture and transcriptional response to abscisic acid. Plant Cell 2012, 24, 2483–2496.
- Zhao, Y.; Zhang, Z.J.; Gao, J.H.; Wang, P.C.; Hu, T.; Wang, Z.G.; Hou, Y.J.; Wan, Y.Z.; Liu, W.S.; Xie, S.J.; et al. Arabidopsis duodecuple mutant of PYL ABA receptors Reveals PYL repression of ABA-independent SnRK2 activity. Cell Rep. 2018, 23, 3340.
- Zheng, Z.F.; Xu, X.P.; Crosley, R.A.; Greenwalt, S.A.; Sun, Y.J.; Blakeslee, B.; Wang, L.Z.; Ni, W.T.; Sopko, M.S.; Yao, C.L.; et al. The protein kinase SnRK2.6 mediates the regulation of sucrose metabolism and plant growth in Arabidopsis. Plant Physiol. 2010, 153, 99–113.
- LeNoble, M.E.; Spollen, W.G.; Sharp, R.E. Maintenance of shoot growth by endogenous ABA: Genetic assessment of the involvement of ethylene suppression. J. Exp. Bot. 2004, 55, 237–245.
- Nitsch, L.; Kohen, W.; Oplaat, C.; Charnikhova, T.; Cristescu, S.; Michieli, P.; Wolters-Arts, M.; Bouwmeester, H.; Mariani, C.; Vriezen, W.H.; et al. ABA-deficiency results in reduced plant and fruit size in tomato. J. Plant Physiol. 2012, 169, 878–883.
- Spollen, W.G.; LeNoble, M.E.; Samuels, T.D.; Bernstein, N.; Sharp, R.E. Abscisic acid accumulation maintains maize primary root elongation at low water potentials by restricting ethylene production. Plant Physiol. 2000, 122, 967–976.
- Zhang, H.; Han, W.; De Smet, I.; Talboys, P.; Loya, R.; Hassan, A.; Rong, H.; Jurgens, G.; Paul Knox, J.; Wang, M.H. ABA promotes quiescence of the quiescent centre and suppresses stem cell differentiation in the Arabidopsis primary root meristem. Plant J. 2010, 64, 764–774.
- Li, X.; Chen, L.; Forde, B.G.; Davies, W.J. The biphasic root growth response to abscisic acid in Arabidopsis involves interaction with ethylene and auxin signalling pathways. Front. Plant Sci. 2017, 8, 1493.
- Gendreau, E.; Traas, J.; Desnos, T.; Grandjean, O.; Caboche, M.; Hofte, H. Cellular basis of hypocotyl growth in Arabidopsis thaliana. Plant Physiol. 1997, 114, 295–305.
- Humplik, J.F.; Bergougnoux, V.; Jandova, M.; Simura, J.; Pencik, A.; Tomanec, O.; Rolcik, J.; Novak, O.; Fellner, M. Endogenous abscisic acid promotes hypocotyl growth and affects endoreduplication during dark-Induced growth in tomato (Solanum lycopersicum L.). PLoS ONE 2015, 10, 0117793.
- Barrero, J.M.; Rodriguez, P.L.; Quesada, V.; Alabadi, D.; Blazquez, M.A.; Boutin, J.P.; Marion-Poll, A.; Ponce, M.R.; Micol, J.L. The ABA1 gene and carotenoid biosynthesis are required for late skotomorphogenic growth in Arabidopsis thaliana. Plant Cell Environ. 2008, 31, 227–234.
- Wang, H.; Qi, Q.; Schorr, P.; Cutler, A.J.; Crosby, W.L.; Fowke, L.C. ICK1, a cyclin-dependent protein kinase inhibitor from Arabidopsis thaliana interacts with both Cdc2a and CycD3, and Its expression Is induced by abscisic acid. Plant J. 1998, 15, 501–510.
- Bai, Q.; Huang, Y.; Shen, Y. The physiological and molecular mechanism of abscisic acid in regulation of freshy fruit ripening. Front. Plant Sci. 2021, 11, 619953.
- Kou, X.H.; Yang, S.; Chai, L.P.; Wu, C.E.; Zhou, J.Q.; Liu, Y.F.; Xue, Z.H. Abscisic acid and fruit ripening: Multifaceted analysis of the effect of abscisic acid on fleshy fruit ripening. Sci. Hortic. 2021, 281, 109999.
- Zhang, Y.S.; Li, Q.; Jiang, L.; Kai, W.B.; Liang, B.; Wang, J.; Du, Y.W.; Zhai, X.W.; Wang, J.L.; Zhang, Y.Q. Suppressing type 2C protein phosphatases alters fruit ripening and the stress response in tomato. Plant Cell Physiol. 2018, 59, 142–154.
- Kai, W.B.; Wang, J.; Liang, B.; Fu, Y.; Zheng, Y.; Zhang, W.B.; Li, Q.; Leng, P. PYL9 is involved in the regulation of ABA signaling during tomato fruit ripening. J. Exp. Bot. 2019, 70, 6305–6319.
- Zhang, M.; Yuan, B.; Leng, P. The role of ABA in triggering ethylene biosynthesis and ripening of tomato fruit. J. Exp. Bot. 2009, 60, 1579–1588.
- Leon, P.; Sheen, J. Sugar and hormone connections. Trends Plant Sci. 2003, 8, 110–116.
- Gazzarrini, S.; McCourt, P. Genetic interactions between ABA, ethylene and sugar signaling pathways. Curr. Opin. Plant Biol. 2001, 4, 387–391.
- Fujita, Y.; Fujita, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. ABA-mediated transcriptional regulation in response to osmotic stress in plants. J. Plant Res. 2011, 124, 509–525.
- Yamburenko, M.V.; Zubo, Y.O.; Borner, T. Abscisic acid affects transcription of chloroplast genes via protein phosphatase 2C-dependent activation of nuclear genes: Repression by guanosine-3-5-bisdiphosphate and activation by sigma factor 5. Plant J. 2015, 82, 1030–1041.
- Gibson, S.I. Sugar and phytohormone response pathways: Navigating a signaling network. J. Exp. Bot. 2004, 55, 253–264.
- Ma, Q.J.; Sun, M.H.; Lu, J.; Liu, Y.J.; Hu, D.G.; Hao, Y.J. Transcription factor AREB2 is involved in soluble sugar accumulation by activating sugar transporter and amylase genes. Plant Physiol. 2017, 174, 2348–2362.
- Poor, P.; Borbely, P.; Czekus, Z.; Takacs, Z.; Ordog, A.; Popovic, B.; Tari, I. Comparison of changes in water status and photosynthetic parameters in wild type and abscisic acid-deficient sitiens mutant of tomato (Solanum lycopersicum cv. Rheinlands Ruhm) exposed to sublethal and lethal salt stress. J. Plant Physiol. 2019, 232, 130–140.
- Innes, S.N.; Solhaug, K.A.; Torre, S.; Dodd, I.C. Different abscisic acid-deficient mutants show unique morphological and hydraulic responses to high air humidity. Physiol. Plant. 2021, 172, 1795–1807.
- Rock, C.D.; Bowlby, N.R.; Hoffmann-Benning, S.; Zeevaart, J.A.D. The aba mutant of Arabidopsis thaliana (L.) Heynh. has reduced chlorophyll fluorescence yields and reduced thylakoid stacking. Plant Physiol. 1992, 100, 1796–1801.
- Galpaz, N.; Wang, Q.; Menda, N.; Zamir, D.; Hirschberg, J. Abscisic acid deficiency in the tomato mutant high-pigment 3 leading to increased plastid number and higher fruit lycopene content. Plant J. 2008, 53, 717–730.




