
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Marta Martínez-Avilés | + 2898 word(s) | 2898 | 2022-01-27 02:57:11 | | | |
| 2 | Bruce Ren | Meta information modification | 2898 | 2022-02-09 02:05:16 | | | | |
| 3 | Bruce Ren | Meta information modification | 2898 | 2022-02-09 02:06:09 | | | | |
| 4 | Bruce Ren | + 7 word(s) | 2905 | 2022-02-09 02:09:26 | | |
Video Upload Options
African swine fever (ASF) is currently the most threatening disease for domestic and wild pigs worldwide. Wild boar has been the main affected species in all EU countries except for Romania, where most notifications occur in domestic pigs. The spread of ASF in wild boar is challenging to control; risk factors are harder to identify and establish than in domestic pigs, which, together with an underestimation of the disease and the lack of treatment or an effective vaccine, are hindering control and eradication efforts. We distributed two online questionnaires, one for domestic pigs and one for wild boar, to experts of different background and countries in Europe, to explore risk factors in relation to ASF control connected to farming, hunting, trade, the environment, and domestic pig and wild boar populations.
1. Introduction
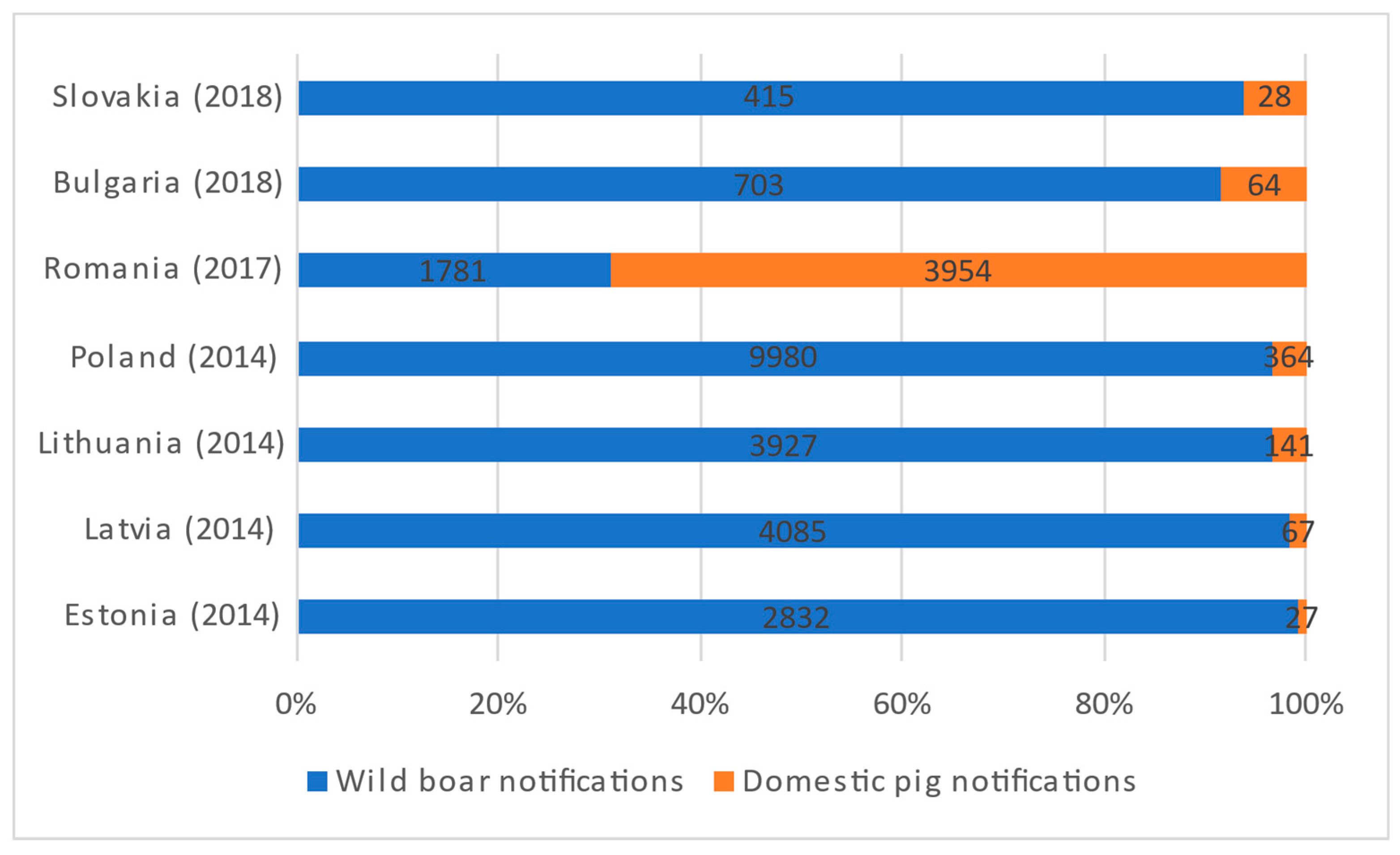
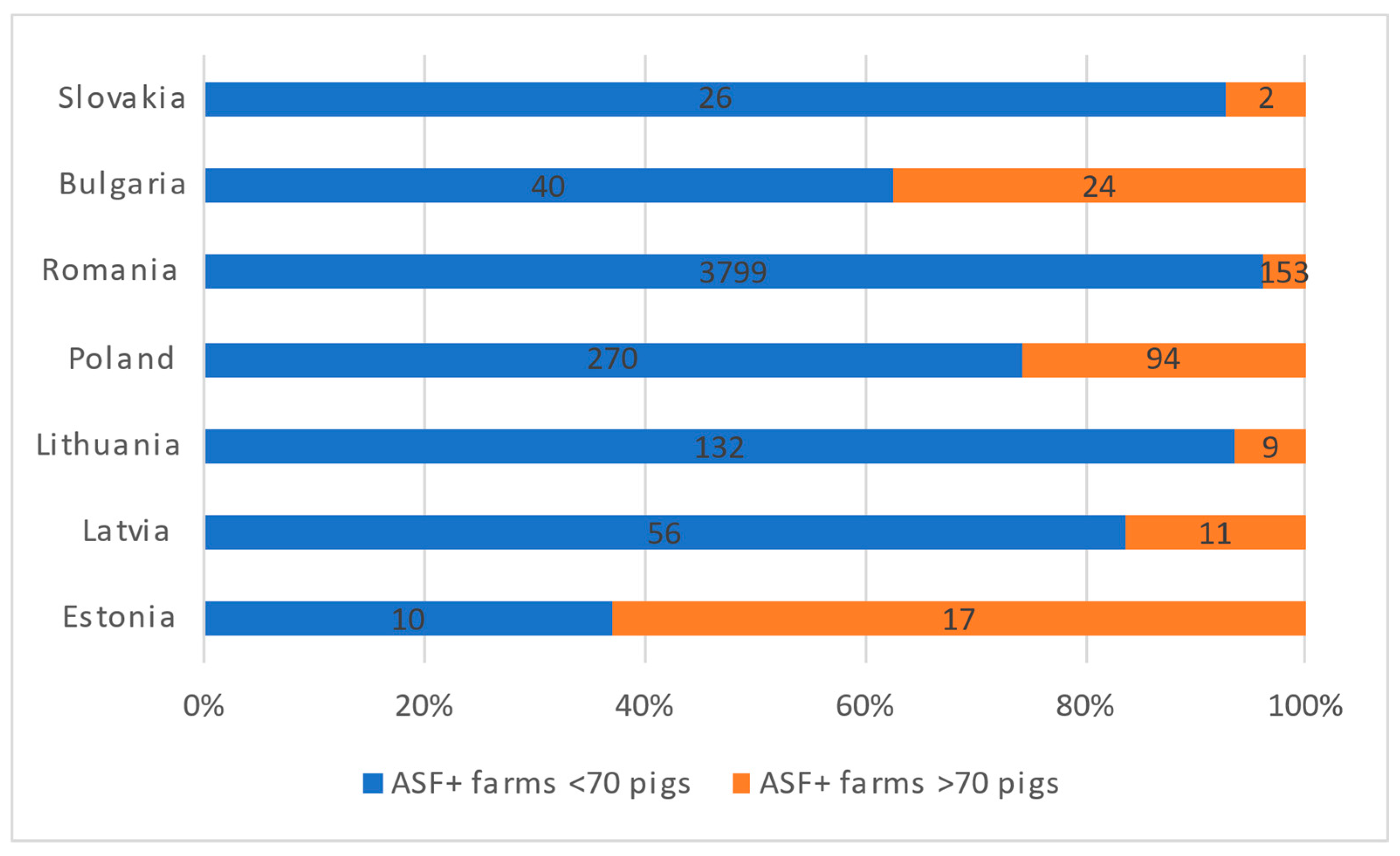
2. African Swine Fever Survey in a European Context
References
- Alkhamis, M.A.; Gallardo, C.; Jurado, C.; Soler, A.; Arias, M.; Sanchez-Vizcaino, J.M. Phylodynamics and evolutionary epidemiology of African swine fever p72-CVR genes in Eurasia and Africa. PLoS ONE 2018, 13, e0192565.
- Blome, S.; Franzke, K.; Beer, M. African swine fever—A review of current knowledge. Virus Res. 2020, 287, 198099.
- European Union Animal Diseases Information System (ADIS). 2021. Available online: https://ec.europa.eu/food/animals/animal-diseases/animal-disease-information-system-adis_en (accessed on 1 December 2021).
- Sauter-Louis, C.; Schulz, K.; Richter, M.; Staubach, C.; Mettenleiter, T.C.; Conraths, F.J. African swine fever: Why the situation in Germany is not comparable to that in the Czech Republic or Belgium. Transbound. Emerg. Dis. 2021; Online ahead of print.
- Charvatova, P.; Wallo, R.; Satran, P. Lessons learned from successful eradication of ASF in the Czech Republic. In OIE Bulletin Panorama “African Swine Fever: Responding to the Global Threat”; OIE: Paris, France, 2020.
- Augère-Granier, M.-L. The EU Pig Meat Sector. 2020. Available online: https://www.europarl.europa.eu/RegData/etudes/BRIE/2020/652044/EPRS_BRI(2020)652044_EN.pdf (accessed on 1 December 2021).
- Bellini, S.; Casadei, G.; De Lorenzi, G.; Tamba, M. A Review of Risk Factors of African Swine Fever Incursion in Pig Farming within the European Union Scenario. Pathogens 2021, 10, 84.
- Jurado, C.; Martínez-Avilés, M.; De La Torre, A.; Štukelj, M.; de Carvalho Ferreira, H.C.; Cerioli, M.; Sánchez-Vizcaíno, J.M.; Bellini, S. Relevant Measures to Prevent the Spread of African Swine Fever in the European Union Domestic Pig Sector. Front. Vet. Sci. 2018, 5, 77.
- Gervasi, V.; Guberti, V. African swine fever endemic persistence in wild boar populations: Key mechanisms explored through modelling. Transbound. Emerg. Dis. 2021, 68, 2812–2825.
- O’Neill, X.; White, A.; Ruiz-Fons, F.; Gortázar, C. Modelling the transmission and persistence of African swine fever in wild boar in contrasting European scenarios. Sci. Rep. 2020, 10, 5895.
- Bergmann, H.; Schulz, K.; Conraths, F.J.; Sauter-Louis, C. A Review of Environmental Risk Factors for African Swine Fever in European Wild Boar. Animals 2021, 11, 2692.
- Miteva, A.; Papanikolaou, A.; Gogin, A.; Boklund, A.; Botner, A.; Linden, A.; Viltrop, A.; Schmidt, C.G.; Ivanciu, C.; Desmecht, D.; et al. Epidemiological analyses of African swine fever in the European Union (November 2018 to October 2019). EFSA J. 2020, 18, e05996.
- Mačiulskis, P.; Masiulis, M.; Pridotkas, G.; Buitkuvienė, J.; Jurgelevičius, V.; Jacevičienė, I.; Zagrabskaitė, R.; Zani, L.; Pilevičienė, S. The African Swine Fever Epidemic in Wild Boar (Sus scrofa) in Lithuania (2014–2018). Vet. Sci. 2020, 7, 15.
- Pautienius, A.; Grigas, J.; Pileviciene, S.; Zagrabskaite, R.; Buitkuviene, J.; Pridotkas, G.; Stankevicius, R.; Streimikyte, Z.; Salomskas, A.; Zienius, D.; et al. Prevalence and spatiotemporal distribution of African swine fever in Lithuania, 2014–2017. Virol. J. 2018, 15, 177.
- Petit, K.; Dunoyer, C.; Fischer, C.; Hars, J.; Baubet, E.; López-Olvera, J.R.; Rossi, S.; Collin, E.; Le Potier, M.; Belloc, C.; et al. Assessment of the impact of forestry and leisure activities on wild boar spatial disturbance with a potential application to ASF risk of spread. Transbound. Emerg. Dis. 2020, 67, 1164–1176.
- Podgórski, T.; Borowik, T.; Łyjak, M.; Woźniakowski, G. Spatial epidemiology of African swine fever: Host, landscape and anthropogenic drivers of disease occurrence in wild boar. Prev. Vet. Med. 2020, 177, 104691.
- Probst, C.; Gethmann, J.; Amendt, J.; Lutz, L.; Teifke, J.P.; Conraths, F.J. Estimating the Postmortem Interval of Wild Boar Carcasses. Vet. Sci. 2020, 7, 6.
- Mauroy, A.; Depoorter, P.; Saegerman, C.; Cay, B.; De Regge, N.; Filippitzi, M.; Fischer, C.; Laitat, M.; Maes, D.; Morelle, K.; et al. Semi-quantitative risk assessment by expert elicitation of potential introduction routes of African swine fever from wild reservoir to domestic pig industry and subsequent spread during the Belgian outbreak (2018–2019). Transbound. Emerg. Dis. 2021, 68, 2761–2773.
- Martínez-Avilés, M.; Iglesias, I.; De La Torre, A. Evolution of the ASF Infection Stage in Wild Boar Within the EU (2014–2018). Front. Vet. Sci. 2020, 7, 155.
- Council Directive of the European Commission 2002/60/EC Laying Down Specific Provisions for the Control of African Swine Fever and Amending Directive 92/119/EEC as Regards Teschen Disease and African Swine Fever; 2002; pp. 27–46. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32002L0060&from=EN (accessed on 1 December 2021).
- Guberti, V.; Khomenko, S.; Masiulis, M.; Kerba, S. African Swine Fever in Wild Boar: Ecology and Biosecurity; FAO: Rome, Italy, 2019; p. 108.
- Muñoz-Pérez, C.; Jurado, C.; Sánchez-Vizcaíno, J.M. African swine fever vaccine: Turning a dream into reality. Transbound. Emerg. Dis. 2021, 68, 2657–2668.
- Stončiūtė, E.; Schulz, K.; Malakauskas, A.; Conraths, F.; Masiulis, M.; Sauter-Louis, C. What Do Lithuanian Hunters Think of African Swine Fever and Its Control—Perceptions. Animals 2021, 11, 525.
- Urner, N.; Mõtus, K.; Nurmoja, I.; Schulz, J.; Sauter-Louis, C.; Staubach, C.; Conraths, F.J.; Schulz, K. Hunters’ Acceptance of Measures against African Swine Fever in Wild Boar in Estonia. Prev. Vet. Med. 2020, 182, 105121.
- Urner, N.; Seržants, M.; Užule, M.; Sauter-Louis, C.; Staubach, C.; Lamberga, K.; Oļševskis, E.; Conraths, F.J.; Schulz, K. Hunters’ view on the control of African swine fever in wild boar. A participatory study in Latvia. Prev. Vet. Med. 2021, 186, 105229.
- Vergne, T.; Guinat, C.; Petkova, P.; Gogin, A.; Kolbasov, D.; Blome, S.; Molia, S.; Ferreira, J.P.; Wieland, B.; Nathues, H.; et al. Attitudes and Beliefs of Pig Farmers and Wild Boar Hunters Towards Reporting of African Swine Fever in Bulgaria, Germany and the Western Part of the Russian Federation. Transbound. Emerg. Dis. 2016, 63, e194–e204.
- De La Torre, A.; Bosch, J.; Iglesias, I.; Muñoz, M.J.; Mur, L.; Martinez-López, B.; Martínez, M.; Sánchez-Vizcaíno, J.M. Assessing the Risk of African Swine Fever Introduction into the European Union by Wild Boar. Transbound. Emerg. Dis. 2015, 62, 272–279.
- Bosch, J.; Rodríguez, A.; Iglesias, I.; Muñoz, M.J.; Jurado, C.; Sánchez-Vizcaíno, J.M.; De La Torre, A. Update on the Risk of Introduction of African Swine Fever by Wild Boar into Disease-Free European Union Countries. Transbound. Emerg. Dis. 2017, 64, 1424–1432.
- Mur, L.; Martínez-López, B.; Costard, S.; De La Torre, A.; Jones, B.A.; Martínez, M.; Sánchez-Vizcaíno, F.; Muñoz, M.J.; Pfeiffer, D.U.; Sánchez-Vizcaíno, J.M.; et al. Modular framework to assess the risk of African swine fever virus entry into the European Union. BMC Vet. Res. 2014, 10, 145.
- Bellini, S. The pig sector in the European Union. In Understanding and Combatting African Swine Fever—A European Perspective; Iacolina, L., Penrith, M.L., Bellini, S., Chenais, E., Jori, F., Montoya, M., Stahl, K., Gavier-Widen, D., Eds.; Wageningen Academic, Wageningen University and Research: Wageningen, The Netherlands, 2021; pp. 183–195.
- European Commission (EC) Regulation No 1069/2009 of the European Parliament and of the Council of 21 October 2009 Laying Down Health Rules as Regards Animal By-Products and Derived Products not Intended for Human Consumption and Repealing Regulation (EC) No 1774/2002 (Animal By-Products Regulation); 2009; pp. 1–33. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2009:300:0001:0033:EN:PDF (accessed on 1 December 2021).
- Nurmoja, I.; Mõtus, K.; Kristian, M.; Niine, T.; Schulz, K.; Depner, K.; Viltrop, A. Epidemiological analysis of the 2015–2017 African swine fever outbreaks in Estonia. Prev. Vet. Med. 2020, 181, 104556.
- Lamberga, K.; Oļševskis, E.; Seržants, M.; Bērziņš, A.; Viltrop, A.; Depner, K. African Swine Fever in Two Large Commercial Pig Farms in LATVIA—Estimation of the High Risk Period and Virus Spread within the Farm. Vet. Sci. 2020, 7, 105.
- Zani, L.; Dietze, K.; Dimova, Z.; Forth, J.H.; Denev, D.; Depner, K.; Alexandrov, T. Zani African Swine Fever in a Bulgarian Backyard Farm—A Case Report. Vet. Sci. 2019, 6, 94.
- Mazur-Panasiuk, N.; Żmudzki, J.; Woźniakowski, G. African swine fever virus—persistence in different environmental conditions and the possibility of its indirect transmission. J. Vet. Res. 2019, 63, 303–310.
- Loyon, L. Overview of Animal Manure Management for Beef, Pig, and Poultry Farms in France. Front. Sustain. Food Syst. 2018, 2, 36.
- Desmecht, D.; Gerbier, G.; Schmidt, C.G.; Grigaliuniene, V.; Helyes, G.; Kantere, M.; Korytarova, D.; Linden, A.; Miteva, A.; Neghirla, I.; et al. Epidemiological analysis of African swine fever in the European Union (September 2019 to August 2020). EFSA J. 2021, 19, e06572.
- European Court of Auditors Audit Preview: Biodiversity in Farming. 2019. Available online: https://www.eca.europa.eu/lists/ecadocuments/ap19_09/ap_biodiversity_en.pdf (accessed on 1 December 2021).
- Zabel, F.; Delzeit, R.; Schneider, J.M.; Seppelt, R.; Mauser, W.; Václavík, T. Global impacts of future cropland expansion and intensification on agricultural markets and biodiversity. Nat. Commun. 2019, 10, 2844.
- EU Agricultural Outlook for Markets and Income, 2019–2030; European Commission, DG Agriculture and Rural Development, Publications Office of the EU: Brussels, Belgium, 2019; p. 96. Available online: https://ec.europa.eu/info/sites/default/files/food-farming-fisheries/farming/documents/agricultural-outlook-2019-report_en.pdf (accessed on 1 December 2021).
- Sanchez-Vizcaino, J.M.; Martinez-Lopez, B.; Martinez-Aviles, M.; Martins, C.; Boinas, F.; Vial, L.; Michaud, V.; Jori, F.; Etter, E.; Albina, E.; et al. Scientfic Review on African Swine Fever; EFSA: Parma, Italy, 2009; p. 142.
- Beltran-Alcrudo, D.; Falco, J.R.; Raizman, E.; Dietze, K. Transboundary spread of pig diseases: The role of international trade and travel. BMC Vet. Res. 2019, 15, 64.
- Probst, C.; Gethmann, J.; Amler, S.; Globig, A.; Knoll, B.; Conraths, F.J. The potential role of scavengers in spreading African swine fever among wild boar. Sci. Rep. 2019, 9, 11450.
- Arzumanyan, H.; Hakobyan, S.; Avagyan, H.; Izmailyan, R.; Nersisyan, N.; Karalyan, Z. Possibility of long-term surviv-al of African swine fever virus in natural conditions. Vet. World 2021, 14, 854–859.
- Cukor, J.; Linda, R.; Václavek, P.; Mahlerová, K.; Šatrán, P.; Havránek, F. Confirmed cannibalism in wild boar and its possible role in African swine fever transmission. Transbound. Emerg. Dis. 2020, 67, 1068–1073.
- Selva, N.; Jędrzejewska, B.; Jędrzejewski, W.; Wajrak, A. Factors affecting carcass use by a guild of scavengers in European temperate woodland. Can. J. Zool. 2005, 83, 1590–1601.
- Newsome, T.M.; Boitani, L.; Chapron, G.; Ciucci, P.; Dickman, C.R.; Dellinger, J.A.; Lopez-Bao, J.V.; Peterson, R.O.; Shores, C.R.; Wirsing, A.J.; et al. Food habits of the world’s grey wolves. Mammal. Rev. 2016, 46, 255–269.
- Mori, E.; Benatti, L.; Lovari, S.; Ferretti, F. What does the wild boar mean to the wolf? Eur. J. Wildl. Res. 2017, 63, 1–5.




